Mineralogical and Geochemical Compositions of the No. 5 Coal in Chuancaogedan Mine, Junger Coalfield, China
Abstract
:1. Introduction
2. Geological Setting
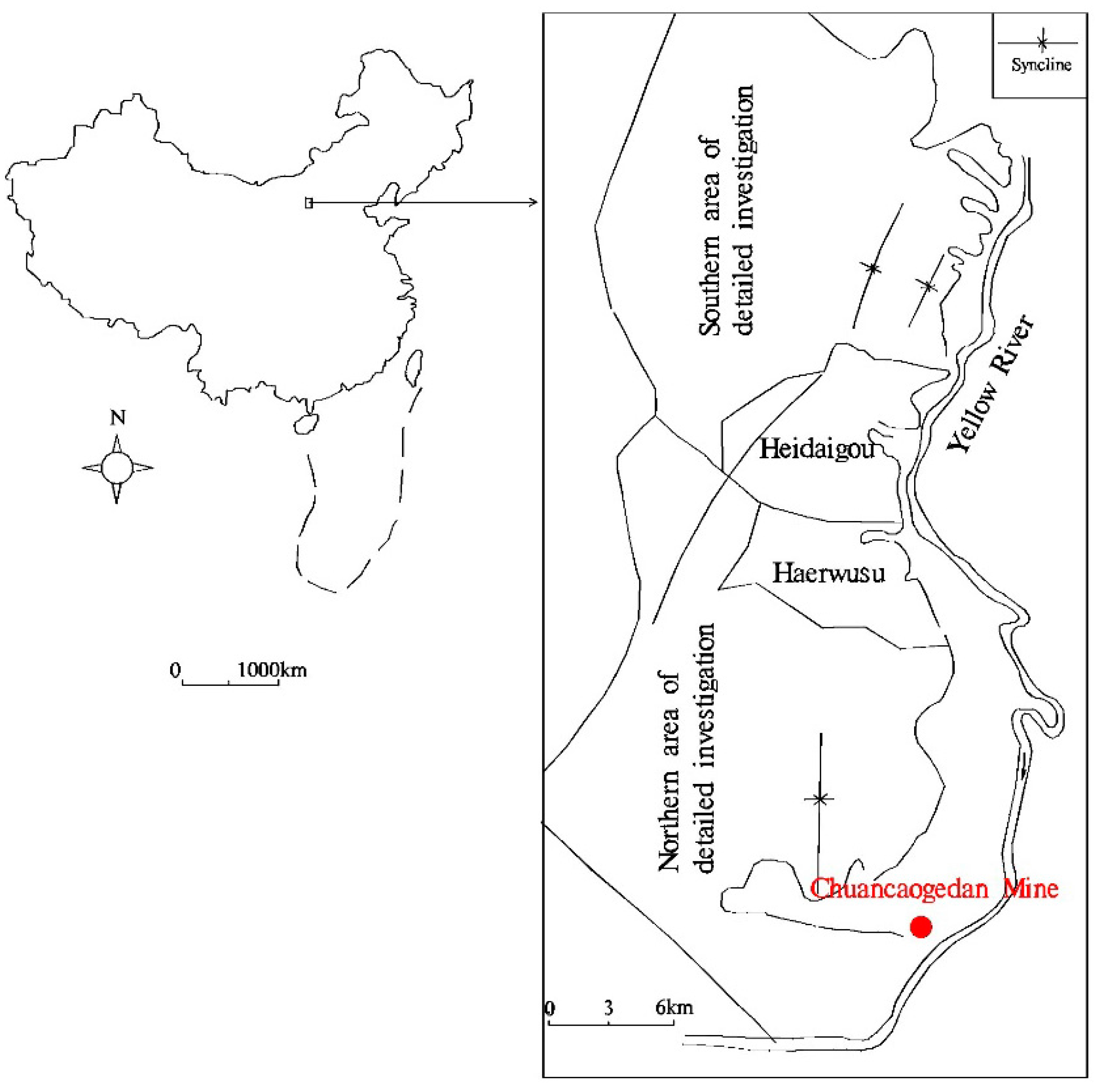
3. Samples and Analytical Procedures

4. Results and Discussion
4.1. Coal Chemistry
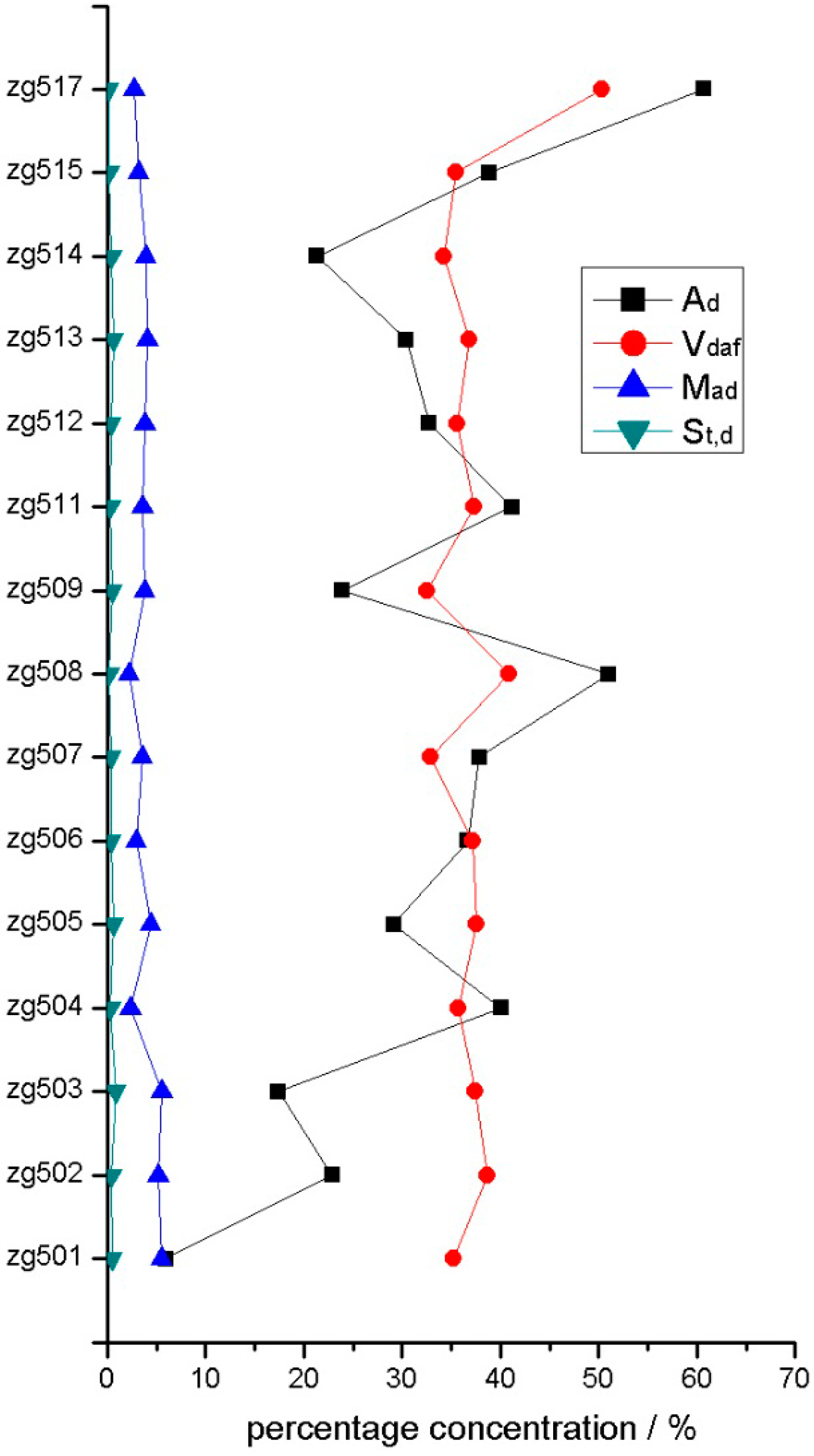
| Sample | Proximate Analysis | St,d | ||
|---|---|---|---|---|
| Mad | Vdaf | Ad | ||
| ZG517 | 2.78 | 50.3 | 60.7 | 0.12 |
| ZG515 | 3.19 | 35.52 | 38.9 | 0.18 |
| ZG514 | 3.91 | 34.3 | 21.38 | 0.40 |
| ZG513 | 4.14 | 36.9 | 30.46 | 0.61 |
| ZG512 | 3.86 | 35.59 | 32.79 | 0.35 |
| ZG511 | 3.64 | 37.39 | 41.16 | 0.30 |
| ZG509 | 3.82 | 32.57 | 23.9 | 0.47 |
| ZG508 | 2.22 | 40.84 | 51.02 | 0.21 |
| ZG507 | 3.54 | 32.91 | 37.88 | 0.36 |
| ZG506 | 2.94 | 37.23 | 36.66 | 0.36 |
| ZG505 | 4.39 | 37.61 | 29.17 | 0.64 |
| ZG504 | 2.42 | 35.75 | 40.04 | 0.29 |
| ZG503 | 5.61 | 37.47 | 17.41 | 0.83 |
| ZG502 | 5.2 | 38.63 | 22.89 | 0.46 |
| ZG501 | 5.52 | 35.29 | 5.95 | 0.48 |
| Average | 3.81 | 37.22 | 32.69 | 0.40 |
4.2. Minerals in the No. 5 Coal
| Samples | Kaolinite | Quartz | Magnetite | Pyrite | Gypsum | Calcite | Jarosite | I/S |
|---|---|---|---|---|---|---|---|---|
| ZG517 | 55.42 | 0.97 | 4.31 | - | - | - | - | - |
| ZG515 | 38.32 | - | 0.58 | - | - | - | - | - |
| ZG514 | 21.38 | - | - | - | - | - | - | - |
| ZG513 | 30.46 | - | - | - | - | - | - | - |
| ZG512 | 32.79 | - | - | - | - | - | - | - |
| ZG511 | 41.16 | - | - | - | - | - | - | - |
| ZG509 | 23.57 | 0.07 | - | 0.26 | - | - | - | - |
| ZG508 | 50.76 | - | 0.26 | - | - | - | - | - |
| ZG507 | 37.77 | 0.11 | - | - | - | - | - | |
| ZG506 | 36.22 | - | 0.44 | - | - | - | - | - |
| ZG505 | 29.11 | - | 0.06 | - | - | - | - | - |
| ZG504 | 39.72 | 0.08 | 0.24 | - | - | - | - | - |
| ZG503 | 15.32 | - | - | - | 1.15 | 0.33 | 0.61 | - |
| ZG502 | 22.07 | - | - | - | 0.82 | - | - | - |
| ZG501 | 5.89 | - | 0.05 | - | - | - | - | 0.01 |

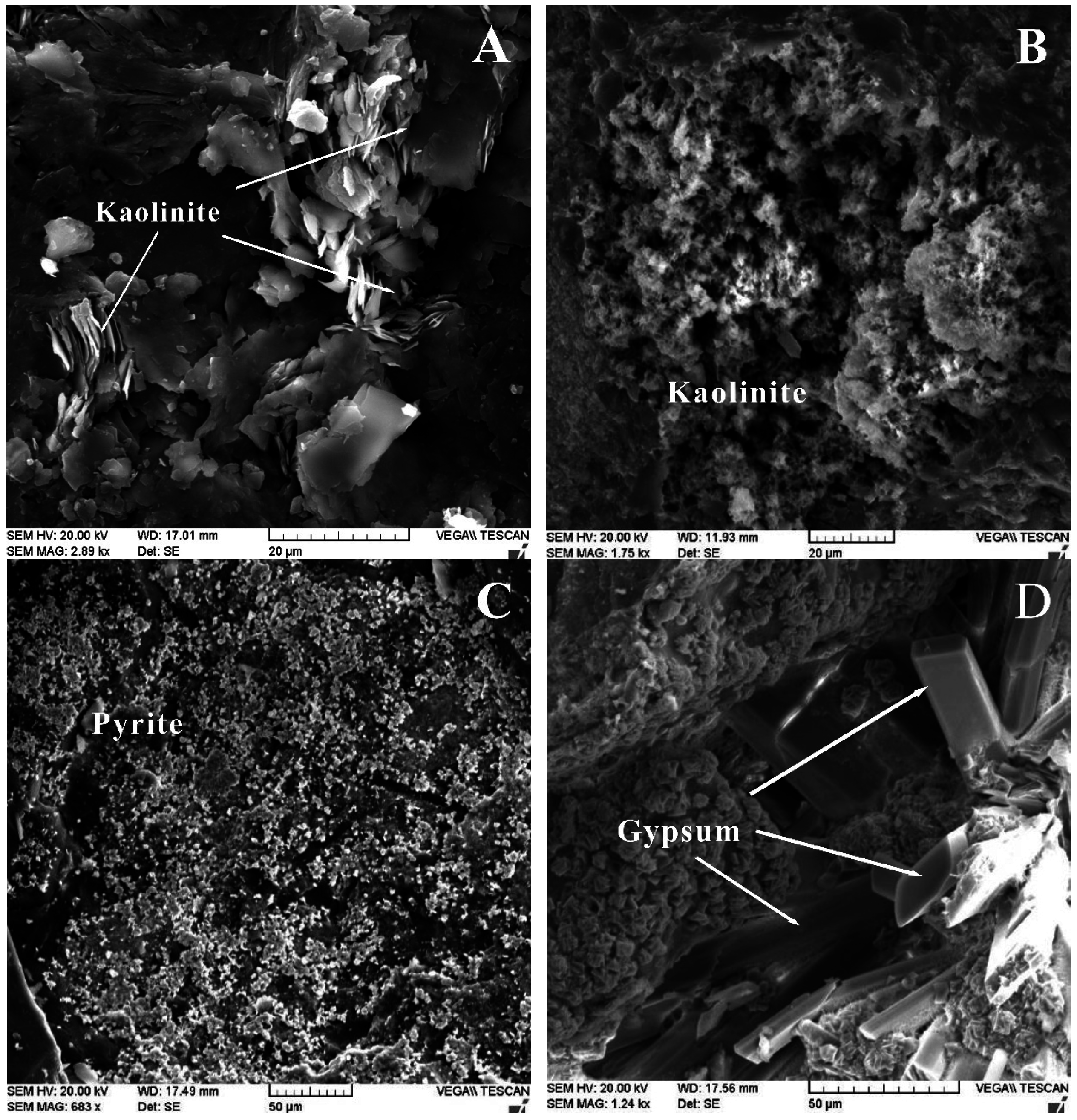
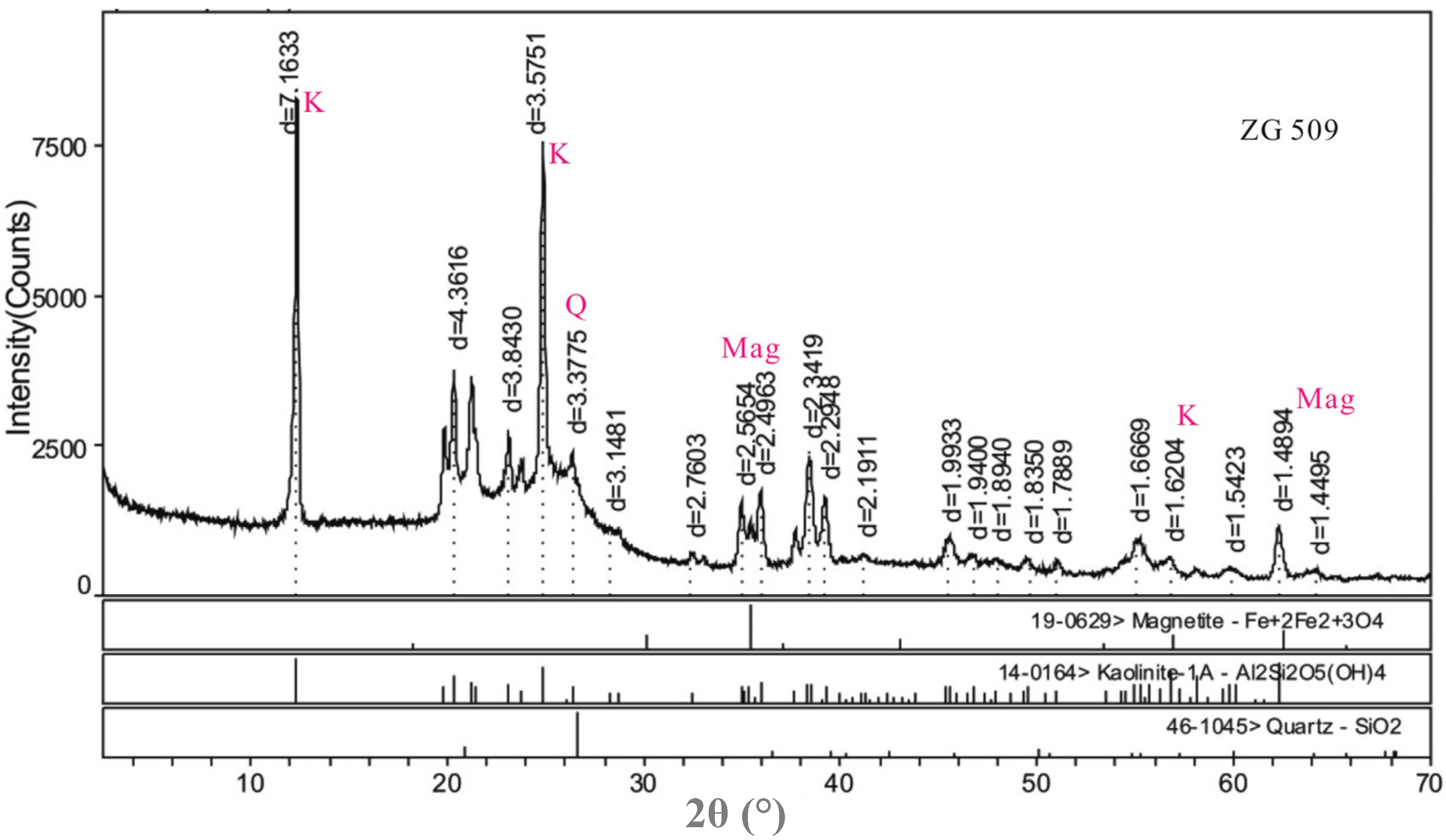
4.3. Geochemistry of the No. 5 Coals
4.3.1. Major Elements
4.3.2. Trace Elements
4.3.3. Evaluated Li, Ga, Se, Zr, Hf, As, and Ge in the No. 5 Coal
| Elemental Concentrations | Sample | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZG517 | ZG515 | ZG514 | ZG513 | ZG512 | ZG511 | ZG509 | ZG508 | ZG507 | ZG506 | ZG505 | ZG504 | ZG503 | ZG502 | ZG501 | Average | Coal a | |
| SiO2 | 32.55 | 20.7 | 11.13 | 15.98 | 17.15 | 21.82 | 12.32 | 27.33 | 19.57 | 19.55 | 15.1 | 20.24 | 7.94 | 11.63 | 2.98 | 16.9 | 8.47 |
| Al2O3 | 25.71 | 16.72 | 9.08 | 13 | 13.97 | 17.8 | 10.2 | 22.47 | 16.28 | 16.03 | 12.37 | 17.3 | 6.55 | 9.45 | 2.5 | 13.87 | 5.98 |
| Fe2O3 | 1.66 | 0.33 | 0.27 | 0.56 | 0.31 | 0.34 | 0.46 | 0.37 | 0.51 | 0.34 | 0.75 | 0.42 | 1.3 | 0.86 | 0.22 | 0.7 | 4.85 |
| TiO2 | 1.11 | 0.73 | 0.52 | 0.45 | 0.96 | 0.76 | 0.5 | 0.47 | 0.73 | 0.36 | 0.45 | 0.77 | 0.24 | 0.2 | 0.07 | 0.55 | 0.33 |
| CaO | 0.33 | 0.14 | 0.12 | 0.13 | 0.11 | 0.16 | 0.12 | 0.09 | 0.17 | 0.1 | 0.12 | 0.2 | 0.69 | 0.37 | 0.08 | 0.26 | 1.23 |
| K2O | 0.14 | 0.05 | 0.02 | 0.04 | 0.03 | 0.04 | 0.02 | 0.08 | 0.09 | 0.06 | 0.09 | 0.17 | 0.04 | 0.03 | 0.01 | 0.06 | 0.19 |
| MgO | 0.1 | 0.04 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.04 | 0.05 | 0.03 | 0.04 | 0.06 | 0.06 | 0.04 | 0.01 | 0.04 | 0.22 |
| Na2O | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.03 | 0.03 | 0.02 | 0 | 0.02 | 0.16 |
| P2O5 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.18 | 0.01 | 0.02 | 0.47 | 0.01 | 0.01 | 0 | 0.05 | 0.09 |
| Li | 114.2 | 83.99 | 46.99 | 72.25 | 82.51 | 120.42 | 75.33 | 157.83 | 83.09 | 103.42 | 56.04 | 79.12 | 35.33 | 49.82 | 17.76 | 78.54 | 14 |
| Be | 7.75 | 12.28 | 6.43 | 4.16 | 2.75 | 1.7 | 1.97 | 1.56 | 3.42 | 1.41 | 1.07 | 2.79 | 1.78 | 2.31 | 3.65 | 3.67 | 2 |
| F | 345.88 | 251.06 | 156.83 | 193.14 | 214.15 | 263.93 | 159.81 | 279.3 | 291.42 | 208.39 | 204.84 | 385.27 | 137.27 | 124.31 | 59.85 | 218.37 | 140 |
| Sc | 12.79 | 7.62 | 4.61 | 4.57 | 6.87 | 9.09 | 3.62 | 12.95 | 14.82 | 11.47 | 9.23 | 10.36 | 6.51 | 5.74 | 3.75 | 8.27 | 3 |
| V | 64.73 | 32.79 | 31.86 | 28.11 | 37.3 | 39.77 | 44.09 | 19.05 | 30.68 | 27.24 | 23.07 | 31.78 | 11.74 | 10.8 | 11.35 | 29.63 | 21 |
| Cr | 18.61 | 8.41 | 12.87 | 7.89 | 10.26 | 7.7 | 10.48 | 3.83 | 8.86 | 6.49 | 7.66 | 13.05 | 4.43 | 2.64 | 1.65 | 8.32 | 12 |
| Co | 4.98 | 2.9 | 4.86 | 5.4 | 2.69 | 2.03 | 3.23 | 1.23 | 1.13 | 1.7 | 1.89 | 0.86 | 5.99 | 5.61 | 7.05 | 3.44 | 7 |
| Ni | 10.37 | 10.74 | 11.54 | 12.64 | 6.9 | 5.13 | 7.35 | 4.35 | 4.63 | 3.71 | 5.21 | 4.16 | 13.59 | 17.47 | 16.9 | 8.98 | 14 |
| Cu | 18.84 | 22.08 | 19.63 | 20.61 | 17.23 | 10.95 | 17.78 | 9.13 | 13.36 | 16.73 | 12.42 | 22.44 | 7.06 | 8.36 | 8 | 14.97 | 13 |
| Zn | 14.85 | 7.12 | 16.37 | 19.96 | 17.08 | 16.39 | 14.62 | 11.81 | 19.36 | 35.83 | 30.03 | 25.94 | 54.71 | 35.57 | 13.6 | 22.22 | 35 |
| Ga | 27.1 | 15.62 | 9.51 | 13.58 | 18.24 | 16.19 | 13.34 | 12.89 | 14.7 | 13.36 | 17.16 | 12.3 | 10.34 | 9.05 | 6.34 | 13.98 | 9 |
| As | 0.64 | 0.42 | 0.36 | 0.62 | 0.23 | 0.18 | 0.42 | 0.15 | 0.24 | 0.19 | 0.41 | 0 | 0 | 0.31 | 0 | 0.28 | 5 |
| Se | 19.07 | 4.41 | 5.64 | 5.94 | 10.35 | 11.07 | 8.83 | 3.83 | 6.51 | 4.31 | 5.28 | 5.09 | 4.45 | 3.62 | 2.02 | 6.69 | 2 |
| Rb | 5.81 | 1.92 | 0.37 | 1.41 | 0.73 | 1 | 0.25 | 2.65 | 2.59 | 1.84 | 2.21 | 4.04 | 0.28 | 1.01 | 0.2 | 1.75 | 8 |
| Sr | 20.8 | 12.34 | 15.2 | 14.31 | 11.25 | 11.54 | 14.68 | 14.97 | 321.07 | 14.08 | 24.89 | 849.94 | 35.48 | 18.81 | 17.13 | 93.1 | 423 |
| Y | 0.19 | 0.22 | 0.29 | 0.2 | 0.27 | 0.18 | 0.38 | 0.21 | 0.28 | 0.08 | 0.12 | 0.3 | 3.77 | 0.27 | 5.43 | 0.81 | 20.76 |
| Zr | 450.76 | 241.43 | 165.49 | 292.81 | 303.95 | 354.05 | 272.13 | 221.16 | 270.52 | 262.09 | 326.43 | 202.89 | 139.76 | 150.53 | 34.28 | 245.89 | 52 |
| Ge | 1.41 | 1.15 | 3.49 | 4.21 | 2.74 | 1.72 | 1.66 | 0.77 | 0.43 | 1.39 | 1.67 | 0.35 | 1.33 | 1.4 | 2.4 | 1.74 | 2.78 |
| Mo | 1.45 | 1.77 | 2.11 | 2.31 | 2.72 | 1.6 | 1.77 | 0.72 | 1.52 | 1.69 | 3.14 | 1.69 | 3.05 | 1.9 | 3.13 | 2.04 | 4 |
| Cd | 0.35 | 0.17 | 0.12 | 0.21 | 0.19 | 0.22 | 0.18 | 0.32 | 0.37 | 0.39 | 0.63 | 0.29 | 0.22 | 0.21 | 0.06 | 0.26 | 0.2 |
| Sn | 5.21 | 1.11 | 0.06 | 0.77 | 1.02 | 1.75 | 0 | 2.98 | 3.01 | 2.29 | 1.94 | 3.08 | 1.17 | 2.84 | 0.63 | 1.86 | 2 |
| Sb | 0.34 | 0.28 | 0.49 | 0.52 | 0.38 | 0.31 | 0.4 | 0.22 | 0.15 | 0.3 | 0.64 | 0.13 | 0.2 | 0.57 | 0.43 | 0.36 | 2 |
| Cs | 0.72 | 0.25 | 0.07 | 0.31 | 0.13 | 0.15 | 0.06 | 0.31 | 0.25 | 0.14 | 0.23 | 0.3 | 0.04 | 0.09 | 0.02 | 0.2 | 1 |
| Ba | 424.52 | 13.3 | 11.1 | 18.23 | 17.36 | 9.69 | 14.36 | 11.76 | 38.57 | 7.68 | 23.79 | 235.07 | 20.29 | 10.21 | 12.67 | 57.91 | 56.03 |
| La | 0.11 | 0.16 | 0.73 | 0.64 | 0.57 | 0.22 | 0.63 | 0.28 | 0.76 | 0.05 | 0.08 | 2.7 | 4.87 | 1.22 | 4.6 | 1.17 | 25.78 |
| Ce | 1.5 | 3.96 | 20.77 | 17.15 | 8.1 | 2.46 | 8.88 | 2.57 | 12.33 | 1.25 | 2.33 | 41.7 | 25.28 | 13.79 | 20.11 | 12.15 | 49.11 |
| Nd | 0.11 | 0.2 | 1.1 | 0.66 | 0.58 | 0.15 | 0.75 | 0.19 | 0.5 | 0.06 | 0.15 | 1.75 | 6.42 | 1.03 | 4.95 | 1.24 | 21.5 |
| Sm | 0.02 | 0.04 | 0.2 | 0.1 | 0.09 | 0.03 | 0.14 | 0.03 | 0.09 | 0.01 | 0.03 | 0.36 | 1.33 | 0.13 | 1.02 | 0.24 | 4.3 |
| Eu | 0.05 | 0 | 0.02 | 0.01 | 0.01 | 0 | 0.02 | 0.01 | 0.02 | 0 | 0.01 | 0.07 | 0.24 | 0.02 | 0.21 | 0.05 | 0.87 |
| Yb | 0.04 | 0.03 | 0.05 | 0.02 | 0.03 | 0.02 | 0.05 | 0.03 | 0.04 | 0.02 | 0.02 | 0.04 | 0.45 | 0.04 | 0.59 | 0.1 | 2.12 |
| Hf | 13.5 | 6.96 | 4.41 | 7.82 | 8.16 | 10.04 | 7.51 | 6.61 | 7.54 | 8.11 | 8.94 | 5.88 | 3.43 | 4.03 | 1.01 | 6.93 | 2.4 |
| Ta | 3.86 | 0.95 | 0.5 | 0.73 | 0.97 | 1.21 | 0.48 | 0.77 | 0.86 | 0.61 | 0.38 | 0.72 | 0.46 | 0.82 | 0.11 | 0.89 | 0.7 |
| W | 2.5 | 1.42 | 0.7 | 0.65 | 1.69 | 1.43 | 0.67 | 0.9 | 1.16 | 0.42 | 0.02 | 1.21 | 0.66 | 0.61 | 1.2 | 1.02 | 2 |
| Hg | 29 | 20 | 44 | 54 | 81 | 17 | 129 | 38 | 45 | 90 | 145 | 52 | 87 | 83 | 66 | 65.42 | 15 |
| Tl | 0.37 | 0.36 | 0.45 | 0.28 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.11 | 0.27 | 0.05 | 0.13 | 0.14 | 0.14 | 0.16 | 0.4 |
| Pb | 55.82 | 52.08 | 42.78 | 40.99 | 57.31 | 55.41 | 54.81 | 36.74 | 38.99 | 32.3 | 37.3 | 30.22 | 20.5 | 20.03 | 8.95 | 38.95 | 13 |
| Bi | 0.77 | 0.66 | 0.36 | 0.44 | 0.5 | 0.51 | 0.39 | 0.51 | 0.74 | 0.42 | 0.37 | 0.56 | 0.36 | 0.33 | 0.1 | 0.47 | 0.8 |
| Th | 1.71 | 1.32 | 1.54 | 1.06 | 1.09 | 0.79 | 2.02 | 1.17 | 1.1 | 0.81 | 1.41 | 0.67 | 2.51 | 0.65 | 0.29 | 1.21 | 6 |
| U | 5.93 | 18.41 | 17.64 | 22.3 | 8.55 | 4.8 | 6.16 | 4.92 | 8.73 | 5.32 | 5.15 | 5.1 | 1.75 | 2.1 | 0.91 | 7.85 | 3 |
| Ad | SiO2 | Al2O3 | TiO2 | Fe2O3 | CaO | K2O | MgO | Na2O | P2O5 | Li | Ga | Se | Zr | Hf | As | Ge | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad | 1 | ||||||||||||||||
| SiO2 | 0.66 ** | 1 | |||||||||||||||
| Al2O3 | 0.51 | 0.89 ** | 1 | ||||||||||||||
| TiO2 | 0.09 | 0.15 | 0.12 | 1 | |||||||||||||
| Fe2O3 | −0.66 ** | −0.92 ** | −0.93 ** | −0.37 | 1 | ||||||||||||
| CaO | −0.52 * | −0.92 ** | −0.96 ** | −0.29 | 0.96 ** | 1 | |||||||||||
| K2O | 0.22 | −0.30 | −0.13 | −0.15 | 0.14 | 0.14 | 1 | ||||||||||
| MgO | −0.51 | −0.91 ** | −0.91 ** | −0.27 | 0.92 ** | 0.92 ** | 0.33 | 1 | |||||||||
| Na2O | −0.47 | −0.95 ** | −0.91 ** | −0.34 | 0.93 ** | 0.96 ** | 0.34 | 0.91 ** | 1 | ||||||||
| P2O5 | 0.15 | −0.19 | 0.15 | 0.16 | −0.14 | −0.09 | 0.74 ** | 0.05 | 0.11 | 1 | |||||||
| Li | 0.88 ** | 0.69 ** | 0.62 * | −0.02 | −0.67 ** | −0.56 * | −0.07 | −0.66 ** | −0.51 * | −0.01 | 1 | ||||||
| Ga | 0.78 ** | 0.51 | 0.24 | 0.37 | −0.47 | −0.39 | 0.13 | −0.30 | −0.41 | −0.10 | 0.55 * | 1 | |||||
| Se | 0.60 * | 0.37 | 0.11 | 0.48 | −0.37 | −0.24 | −0.07 | −0.18 | −0.30 | −0.12 | 0.41 | 0.87 ** | 1 | ||||
| Zr | 0.76 ** | 0.59 * | 0.36 | 0.35 | −0.55 * | −0.51 | 0.05 | −0.47 | −0.50 | −0.11 | 0.62 * | 0.93 ** | 0.81 ** | 1 | |||
| Hf | 0.81 ** | 0.64 * | 0.41 | 0.29 | −0.59 * | −0.53 * | 0.07 | −0.49 | −0.52 * | −0.10 | 0.67 ** | 0.94 ** | 0.81 ** | 0.99 ** | 1 | ||
| As | 0.34 | 0.54 * | 0.22 | 0.20 | −0.36 | −0.41 | −0.23 | −0.32 | −0.51 | −0.41 | 0.16 | 0.58 * | 0.51 | 0.63 * | 0.61 * | 1 | |
| Ge | −0.40 | 0.09 | −0.06 | 0.26 | 0.01 | −0.06 | −0.53 * | −0.09 | −0.22 | −0.47 | −0.35 | −0.13 | 0.03 | −0.04 | −0.10 | 0.40 | 1 |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, S.F.; Zou, J.H.; Jiang, Y.F.; Ward, C.R.; Wang, X.B.; Li, T.; Xue, W.F.; Liu, S.D.; Tian, H.M.; Sun, X.H.; et al. Mineralogical and geochemical compositions of the Pennsylvanian coal in the Adaohai Mine, Daqingshan Coalfield, Inner Mongolia, China: Modes of occurrence and origin of diaspore, gorceixite, and ammonian illite. Int. J. Coal Geol. 2012, 94, 250–270. [Google Scholar] [CrossRef]
- Dai, S.F.; Luo, Y.B.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Zhao, L.; Liu, S.D.; Zhao, C.L.; Tian, H.M.; Zou, J.H. Revisiting the late Permian coal from the Huayingshan, Sichuan, southwestern China: Enrichment and occurrence modes of minerals and trace elements. Int. J. Coal Geol. 2014, 122, 110–128. [Google Scholar] [CrossRef]
- Gürdal, G. Abundances and modes of occurrence of trace elements in the Çan coals (Miocene), Çanakkale-Turkey. Int. J. Coal Geol. 2011, 87, 157–173. [Google Scholar] [CrossRef]
- Yang, J.Y. Concentrations and modes of occurrence of trace elements in the Late Permian coals from the Puan Coalfield, southwestern Guizhou, China. Environ. Geochem. Health 2006, 28, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B. Geochemistry of Late Triassic coals in the Changhe Mine, Sichuan Basin, southwestern China: Evidence for authigenic lanthanide enrichment. Int. J. Coal Geol. 2009, 80, 167–174. [Google Scholar] [CrossRef]
- Kolker, A. Minor element distribution in iron disulfides in coal: A geochemical review. Int. J. Coal Geol. 2012, 94, 32–43. [Google Scholar] [CrossRef]
- Finkelman, R.B. Modes of occurrence of potentially hazardous elements in coals: Levels of confidence. Fuel Process. Technol. 1994, 39, 21–34. [Google Scholar] [CrossRef]
- Tang, S.S.; Sun, S.L.; Qin, Y.; Jiang, Y.F.; Wang, W.F. Distribution characteristics of sulfur and the main harmful trace elements in China’s coal. Acta Geol. Sin. Engl. Ed. 2008, 82, 722–730. [Google Scholar]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Li, S.S.; Jiang, Y.F. Mineralogy and geochemistry of the No. 6 Coal (Pennsylvanian) in the Junger Coalfield, Ordos Basin, China. Int. J. Coal Geol. 2006, 66, 253–270. [Google Scholar] [CrossRef]
- Dai, S.F.; Li, D.; Chou, C.L.; Zhao, L.; Zhang, Y.; Ren, D.Y.; Ma, Y.W.; Sun, Y.Y. Mineralogy and geochemistry of boehmite-rich coals: New insights from the Haerwusu Surface Mine, Jungar Coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2008, 74, 185–202. [Google Scholar] [CrossRef]
- Dai, S.F.; Jiang, Y.F.; Ward, C.R.; Gu, L.; Seredin, V.V.; Liu, H.D.; Zhou, D.; Wang, X.B.; Sun, Y.Z.; Zou, J.H.; et al. Mineralogical and geochemical compositions of the coal in the Guanbanwusu Mine, Inner Mongolia, China: Further evidence for the existence of an Al (Ga and REE) ore deposit in the Jungar Coalfield. Int. J. Coal Geol. 2012, 98, 10–40. [Google Scholar] [CrossRef]
- Wang, X.B.; Dai, S.F.; Sun, Y.Y.; Li, D.; Zhang, W.G.; Zhang, Y.; Luo, Y.B. Modes of occurrence of fluorine in the Late Paleozoic No. 6 coal from the Haerwusu Surface Mine, Inner Mongolia, China. Fuel 2011, 90, 248–254. [Google Scholar] [CrossRef]
- China Coal Research Institute. GB/T 482-2008. Sampling of Coal Seams; Chinese National Standard; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008. (In Chinese)
- American Society for Testing and Materials (ASTM) International. Test Method for Moisture in the Analysis Sample of Coal and Coke; ASTM D3173-11; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. Test Method for Volatile Matter in the Analysis Sample of Coal and Coke; ASTM D3175-11; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. Test Method for Ash in the Analysis Sample of Coal and Coke from Coal; ASTM D3174-11; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- American Society for Testing and Materials (ASTM) International. Test Methods for Total Sulfur in the Analysis Sample of Coal and Coke; ASTM D3177-02; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Dai, S.F.; Yang, J.Y.; Ward, C.R.; Hower, J.C.; Liu, H.D.; Garrison, T.M.; French, D.; O’Keefe, J.M.K. Geochemical and mineralogical evidence for a coal-hosted uranium deposit in the Yili Basin, Xinjiang, northwestern China. Ore Geol. Rev. 2015, 70, 1–30. [Google Scholar] [CrossRef]
- Dai, S.F.; Li, T.J.; Jiang, Y.F.; Ward, C.R.; Hower, J.C.; Sun, J.H.; Liu, J.J.; Song, H.J.; Wei, J.P.; Li, Q.Q.; et al. Mineralogical and geochemical compositions of the Pennsylvanian coal in the Hailiushu Mine, Daqingshan Coalfield, Inner Mongolia, China: Implications of sediment-source region and acid hydrothermal solutions. Int. J. Coal Geol. 2015, 137, 92–110. [Google Scholar] [CrossRef]
- Wang, X.B.; Wang, R.X.; Wei, Q.; Wang, P.P.; Wei, J.P. Mineralogical and geochemical characteristics of late Permian coals from the Mahe Mine, Zhaotong Coalfield, Northeastern Yunnan, China. Minerals 2015, 5, 380–396. [Google Scholar] [CrossRef]
- Dai, S.F.; Wang, X.B.; Zhou, Y.P.; Hower, J.C.; Li, D.H.; Chen, W.M.; Zhu, X.W.; Zou, J.H. Chemical and mineralogical compositions of silicic, mafic, and alkali tonsteins in the late Permian coals from the Songzao Coalfield, Chongqing, Southwest China. Chem. Geol. 2011, 282, 29–44. [Google Scholar] [CrossRef]
- Li, X.; Dai, S.F.; Zhang, W.G.; Li, T.; Zheng, X.; Chen, W. Determination of As and Se in coal and coal combustion products using closed vessel microwave digestion and collision/reaction cell technology (CCT) of inductively coupled plasma mass spectrometry (ICP-MS). Int. J. Coal Geol. 2014, 124, 1–4. [Google Scholar] [CrossRef]
- China Coal Research Institute. GB/T 15224.1-2004, Classification for Quality of Coal—Part 1: Ash; Chinese National Standard; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2004. (In Chinese)
- China Coal Research Institute. MT/T849-2000, Classification for Volatile Matter of Coal; Chinese National Standard; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2000. (In Chinese)
- China Coal Science Research Institute Beijing Coal Chemical Research Branch. MT/T850-2000, Classification for Total Moisture in Coal; Chinese National Standard; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2000. (In Chinese)
- China Coal Research Institute Beijing Coal Chemical Research Branch. GB/T 15224.2-2004, Classification for Coal Quality—Part 2: Sulfur Content; Chinese National Standard; General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China: Beijing, China, 2004. (In Chinese)
- Dai, S.F.; Wang, X.B.; Seredin, V.V.; Hower, J.C.; Ward, C.R.; O’Keefe, J.M.K.; Huang, W.H.; Li, T.; Li, X.; Liu, H.D.; et al. Petrology, mineralogy, and geochemistry of the Ge-rich coal from the Wulantuga Ge ore deposit, Inner Mongolia, China: New data and genetic implications. Int. J. Coal Geol. 2012, 90–91, 72–99. [Google Scholar] [CrossRef]
- Dai, S.F.; Li, T.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Zhou, Y.P.; Zhang, M.Q.; Song, X.L.; Song, W.J.; Zhao, C.L. Origin of minerals and elements in the late Permian coals, tonsteins, and host rocks of the Xinde Mine, Xuanwei, eastern Yunnan, China. Int. J. Coal Geol. 2014, 121, 53–78. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Chou, C.-L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y.P. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Dai, S.F.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.W.; Zhang, W.G.; Song, W.J.; Wang, P.P. Enrichment of U–Se–Mo–Re–V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Min. Deposita 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Wang, S. (Ed.) Coal Accumulation and Coal Resources Evaluation of Ordos Basin, China; China Coal Industry Publishing House: Beijing, China, 1996; p. 437. (In Chinese)
- Chou, C.-L. Abundances of sulfur, chlorine, and trace elements in Illinois Basin coals, USA. In Proceedings of the 14th Annual International Pittsburgh Coal Conference & Workshop, Taiyuan, China, 23–27 September 1997; Section 1. pp. 76–87.
- Belkin, H.E.; Zheng, B.S.; Finkelman, R.B. Geochemistry of Coals Causing Arsenismin Southwest China. In 4th International Symposium on Environmental Geochemistry; US Geological Survey Open-File Report; U.S. Geological Survey: Reston, VA, USA, 1997. [Google Scholar]
- Ding, Z.H.; Zheng, B.S.; Long, J.P.; Belkin, H.E.; Finkelman, R.B.; Chen, C.G.; Zhou, D.; Zhou, Y. Geological and geochemical characteristics of high arsenic coals from endemic arsenosis areas in southwestern Guizhou Province. Appl. Geochem. 2001, 16, 1353–1360. [Google Scholar] [CrossRef]
- Chen, J.; Chen, P.; Yao, D.X.; Liu, Z.; Wu, Y.S.; Liu, W.Z.; Hu, Y.B. Mineralogy and geochemistry of late Permian coals from the Donglin Coal Mine in the Nantong coalfield in Chongqing, southwestern China. Int. J. Coal Geol. 2015, 149, 24–40. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, X.G.; Querol, X.; Font, O.; Izquierdo, M.; Wang, Z.M. New data on mineralogy and geochemistry of high-Ge coals in the Yimin coalfield, Inner Mongolia, China. Int. J. Coal Geol. 2014, 125, 10–21. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of clarkes for carbonaceous biolithes: World average for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Tang, S.; Zhang, S.; Chen, Y. Mineralogical and Geochemical Compositions of the No. 5 Coal in Chuancaogedan Mine, Junger Coalfield, China. Minerals 2015, 5, 788-800. https://doi.org/10.3390/min5040525
Yang N, Tang S, Zhang S, Chen Y. Mineralogical and Geochemical Compositions of the No. 5 Coal in Chuancaogedan Mine, Junger Coalfield, China. Minerals. 2015; 5(4):788-800. https://doi.org/10.3390/min5040525
Chicago/Turabian StyleYang, Ning, Shuheng Tang, Songhang Zhang, and Yunyun Chen. 2015. "Mineralogical and Geochemical Compositions of the No. 5 Coal in Chuancaogedan Mine, Junger Coalfield, China" Minerals 5, no. 4: 788-800. https://doi.org/10.3390/min5040525






