Calcification and Diagenesis of Bacterial Colonies
Abstract
:1. Introduction
2. Material and Methods
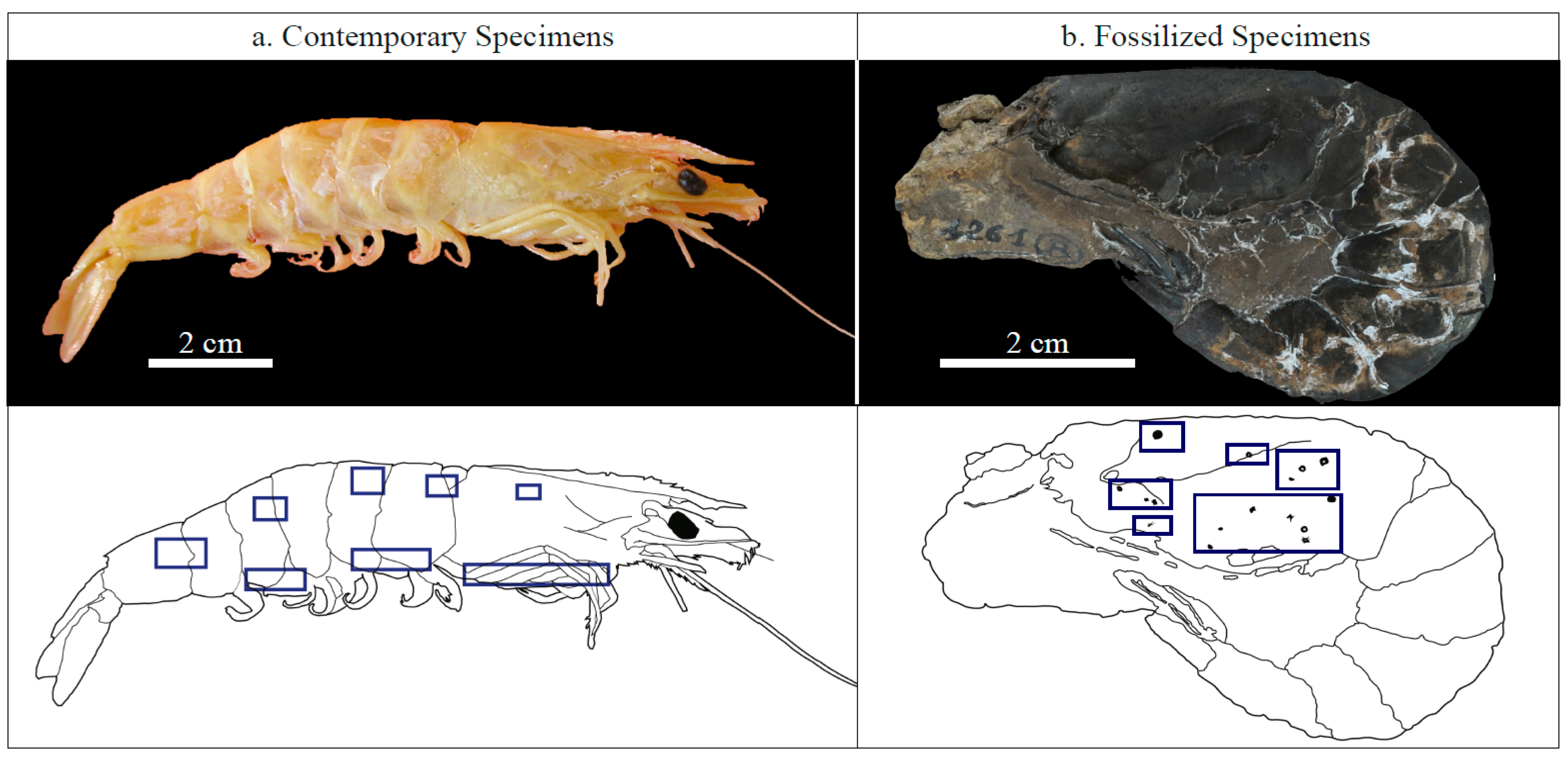
2.1. Investigated Specimens
2.2. X-Ray Diffraction
2.3. Scanning Electron Microscopy (SEM)
2.4. Focused Ion Beam (FIB) Milling
2.5. Transmission Electron Microscopy (TEM)
2.6. Scanning Transmission X-Ray Microscopy (STXM) and X-Ray Absorption Near Edge Structure (XANES) Spectroscopy
3. Results
3.1. Contemporary Epibiotic Organo-Mineral Structures
3.1.1. Morphology
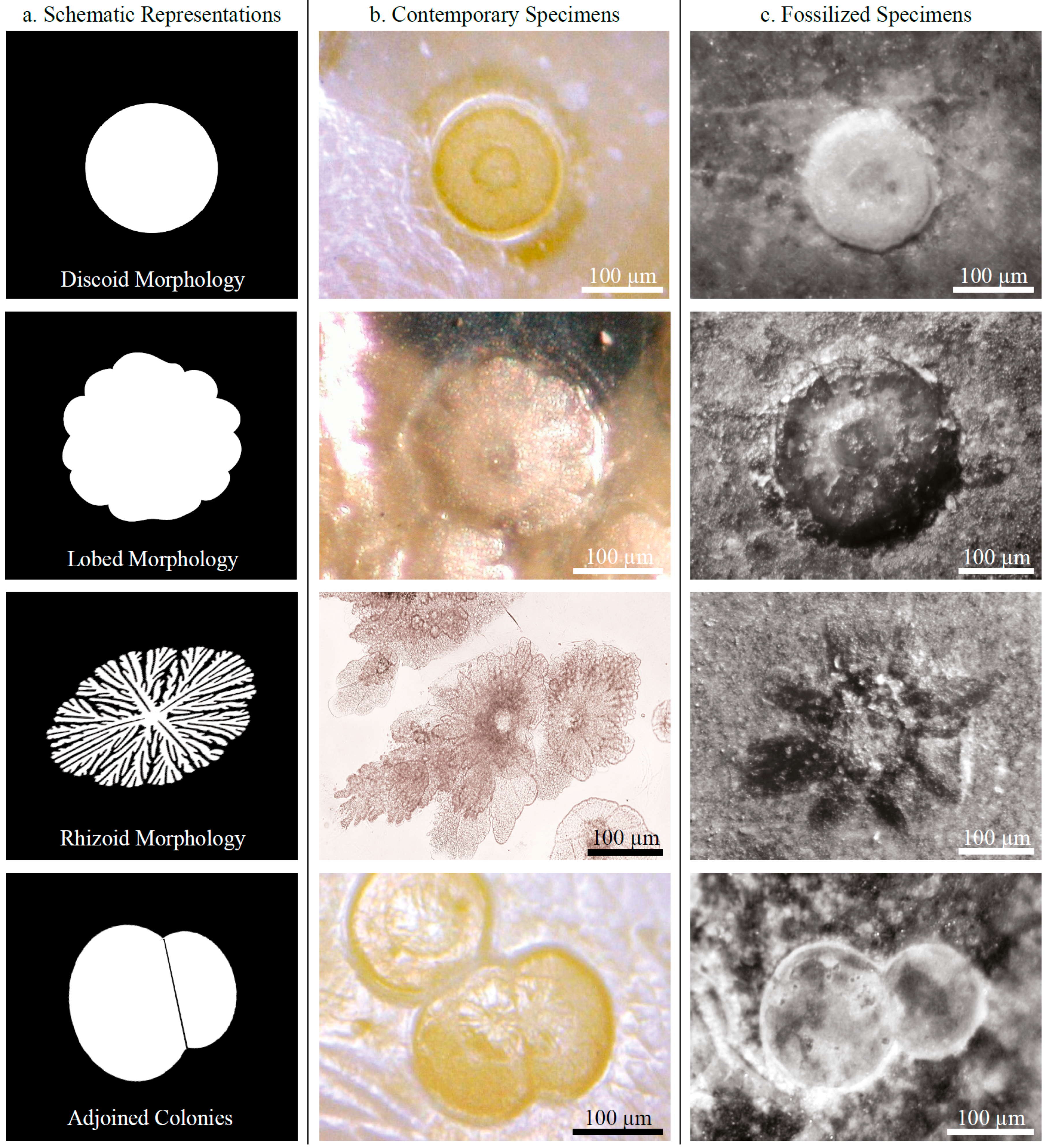
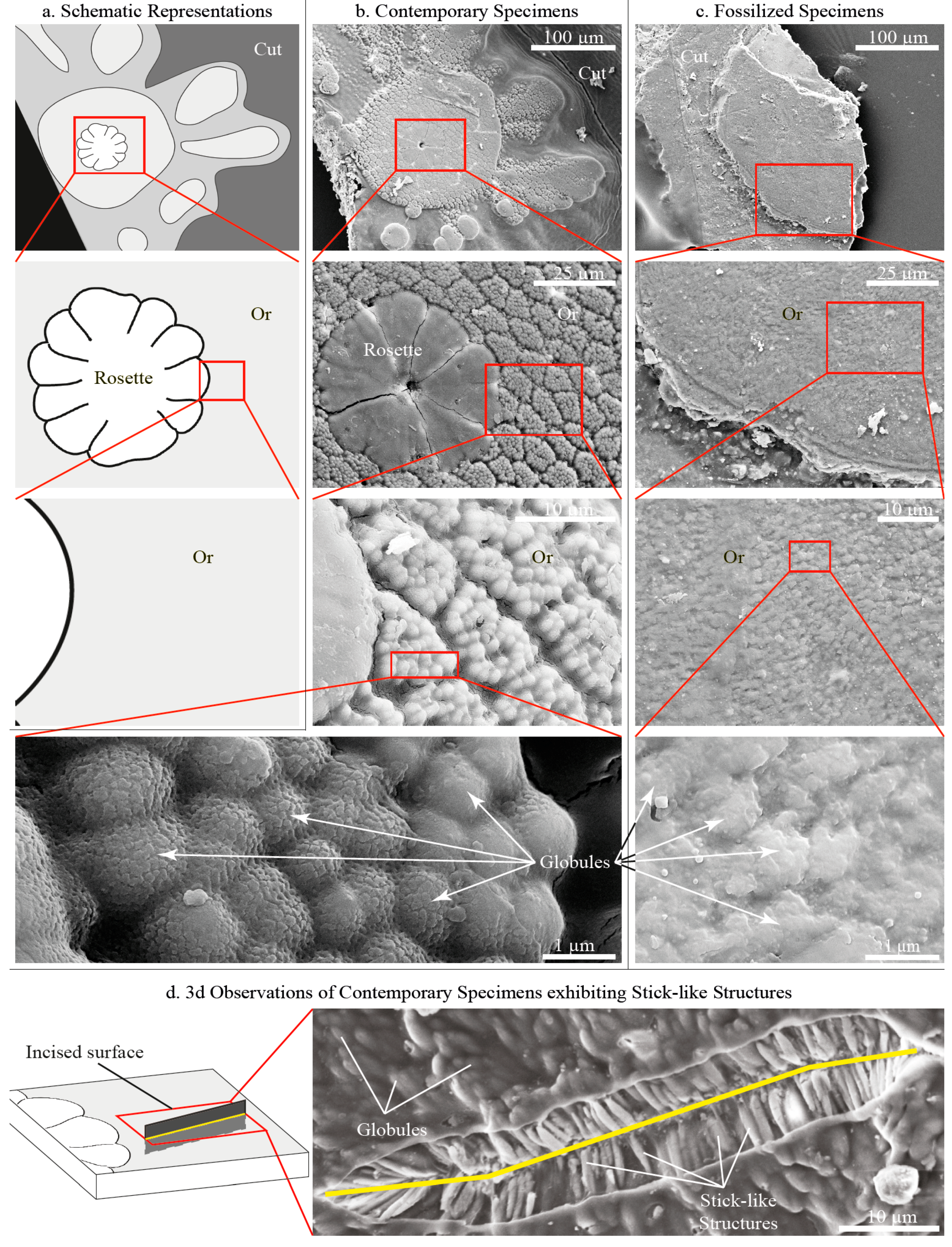
3.1.2. Composition
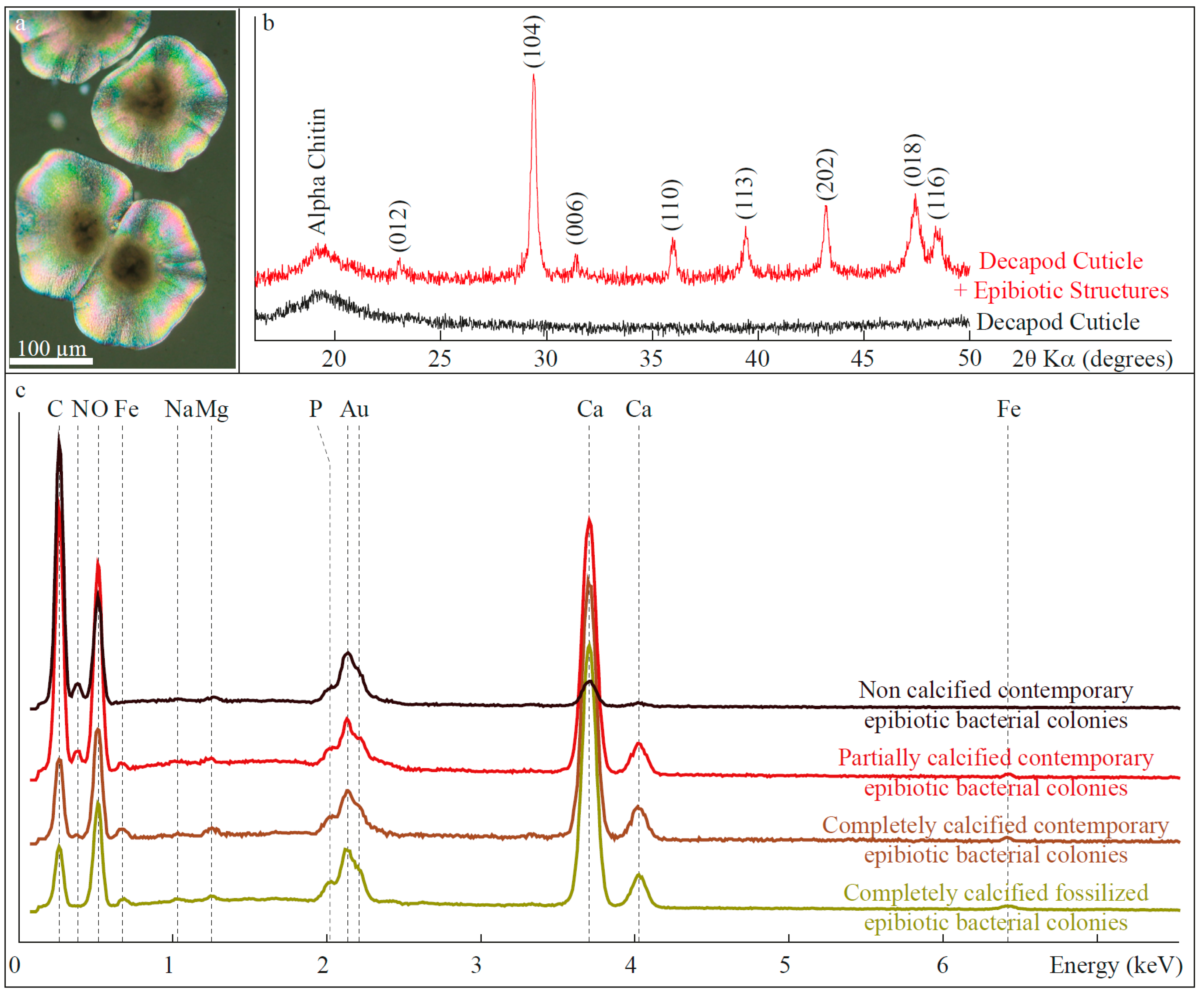
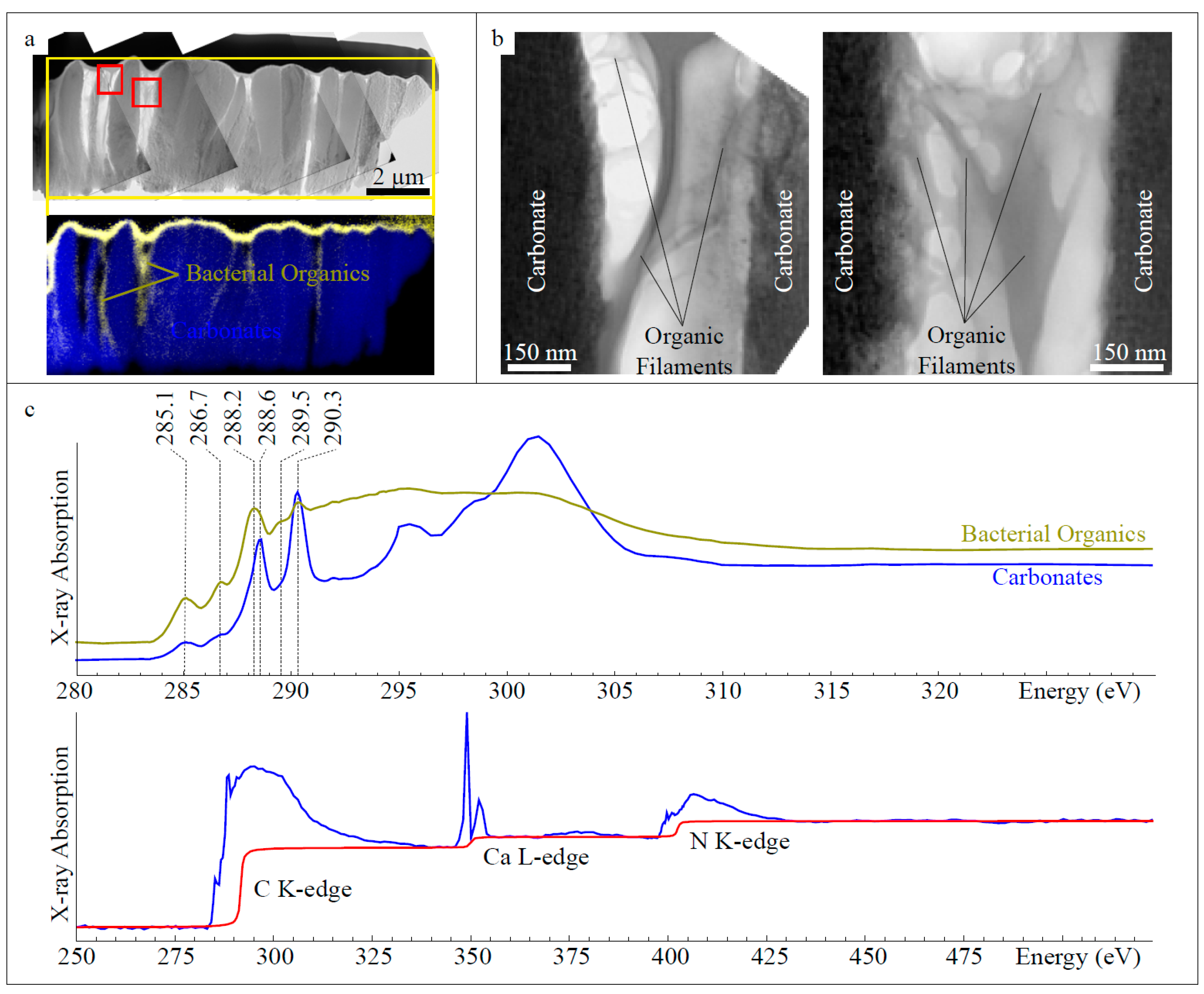
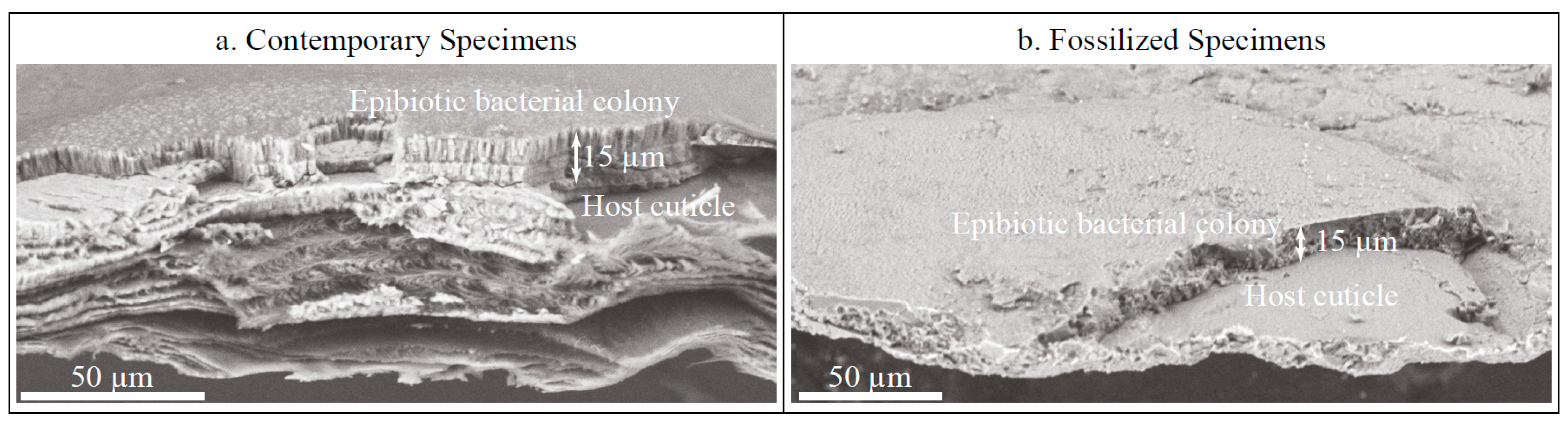
3.2. Fossilized Epibiotic Structures
4. Discussion
4.1. Biomineralization of Calcified Epibiotic Bacterial Colonies
4.2. Fossilization of Calcifying Bacterial Colonies
5. Concluding Remarks
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Fernandez-Leborans, G. Epibiosis in Crustacea: An overview. Crustaceana 2010, 83, 549–640. [Google Scholar] [CrossRef]
- Brandt, D.S. Epizoans on Flexicalymene (Trilobita) and implications for trilobite paleoecology. J. Paleontol. 1996, 70, 442–449. [Google Scholar]
- Waugh, D.A.; Feldmann, R.M.; Crawford, R.S.; Jakobsen, S.L.; Thomas, K.B. Epibiont preservational and observational bias in fossil marine decapods. J. Paleontol. 2004, 78, 761–767. [Google Scholar] [CrossRef]
- Galle, A.; Parsley, R.L. Epibiont relationships on hyolithids demonstrated by Ordovician trepostomes (Bryozoa) and Devonian tabulates (Anthozoa). Bull. Geosci. 2005, 80, 125–138. [Google Scholar]
- Engel, M.S. An Eocene ectoparasite of bees: The oldest definitive record of phoretic meloid triungulins (Coleoptera: Meloidae; Hymenoptera: Megachilidae). Acta Zool. Cracoviensia 2005, 48, 43–48. [Google Scholar] [CrossRef]
- Wisshak, M.; Neumann, C. A symbiotic association of a boring polychaete and an echinoid from the Late Cretaceous of Germany. Acta Palaeontol. Pol. 2006, 51, 589–597. [Google Scholar]
- Dunlop, J.A.; Kontschán, J.; Zwanzig, M. Fossil mesostigmatid mites (Mesostigmata: Gamasina, Microgyniina, Uropodina), associated with longhorn beetles (Coleoptera: Cerambycidae) in Baltic amber. Naturwissenschaften 2013, 100, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Petit, G.; Charbonnier, S. Fossil sponge gemmules, epibionts of Carpopenaeus garassinoi n. sp. (Crustacea, Decapoda) from the Sahel Alma Lagerstätte (Late Cretaceous, Lebanon). Geodiversitas 2012, 34, 359–372. [Google Scholar] [CrossRef]
- Misaki, A.; Maeda, H.; Kumagae, T.; Ichida, M. Commensal anomiid bivalves on Late Cretaceous heteromorph ammonites from south-west Japan. Palaeontology 2014, 57, 77–95. [Google Scholar] [CrossRef]
- Robin, N.; Charbonnier, S.; Bartolini, A.; Petit, G. First occurrence of encrusting nubeculariids (Foraminifera) on a mobile host (Crustacea, Decapoda) from the Upper Jurassic Eichstätt Lagerstätte, Germany: A new possible relation of phoresy. Mar. Micropaleontol. 2013, 104, 44–52. [Google Scholar] [CrossRef]
- Robin, N.; Petit, G.; Charbonnier, S. A newly recognized Mesozoic-Recent interspecific association: Calcifying bacteria on decapod crustaceans. Lethaia 2015. [Google Scholar] [CrossRef]
- Siveter, D.J.; Briggs, D.E.; Siveter, D.J.; Sutton, M.D. A 425-Million-Year-Old Silurian Pentastomid Parasitic on Ostracods. Curr. Biol. 2015, 25, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Wacey, D.; McLoughlin, N.; Kilburn, M.R.; Saunders, M.; Cliff, J.B.; Kong, C.; Barley, M.E.; Brasier, M.D. Nanoscale analysis of pyritized microfossils reveals differential heterotrophic consumption in the ~1.9-Ga Gunflint chert. Proc. Natl. Acad. Sci. USA 2013, 110, 8020–8024. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Benzerara, K.; Beyssac, O.; Menguy, N.; Guyot, F.; Brown, G.E.; Goffe, B. Exceptional preservation of fossil plant spores in high-pressure metamorphic rocks. Earth Planet. Sci. Lett. 2007, 262, 257–272. [Google Scholar] [CrossRef]
- Bernard, S.; Benzerara, K.; Beyssac, O.; Brown, G.E.; Stamm, L.G.; Duringer, P. Ultrastructural and chemical study of modern and fossil sporoderms by Scanning Transmission X-ray Microscopy (STXM). Rev. Palaeobot. Palynol. 2009, 156, 248–261. [Google Scholar] [CrossRef]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.V.; Werner, C.; Schwille, P.; Geisler, T. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Zaton, M.; Bazhenov, V.V.; Behm, T.; Ehrlich, A.; Stelling, A.; Hog, M.; Ehrlich, H. Identification of chitin in 200-million-year-old gastropod egg capsules. Paleobiology 2014, 40, 529–540. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.M.; Carnerup, A.; Christy, A.G.; Welham, N.J.; Hyde, S.T. Morphology: An ambiguous indicator of biogenicity. Astrobiology 2002, 2, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W.; Kudryavtsev, A.B.; Sugitani, K.; Walter, M.R. Precambrian microbe-like pseudofossils: A promising solution to the problem. Precambrian Res. 2010, 179, 191–205. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Bernard, S.; Papineau, D. Graphitic carbons and biosignatures. Elements 2014, 10, 435–440. [Google Scholar] [CrossRef]
- Butterfield, N.J.; Balthasar, U.; Wilson, L.A. Fossil diagenesis in the Burgess Shale. Palaeontology 2007, 50, 537–543. [Google Scholar] [CrossRef]
- Schiffbauer, J.D.; Yin, L.M.; Bodnar, R.J.; Kaufman, A.J.; Meng, F.W.; Hu, J.; Shen, B.; Yuan, X.L.; Bao, H.M.; Xiao, S.H. Ultrastructural and geochemical characterization of Archean-Paleoproterozoic graphite particles: Implications for recognizing traces of life in highly metamorphosed rocks. Astrobiology 2007, 7, 684–704. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Benzerara, K.; Beyssac, O.; Brown, G.E., Jr. Multiscale characterization of pyritized plant tissues in blueschist facies metamorphic rocks. Geochim. Cosmochim. Acta 2010, 74, 5054–5068. [Google Scholar] [CrossRef]
- Galvez, M.E.; Beyssac, O.; Benzerara, K.; Bernard, S.; Menguy, N.; Cox, S.C.; Martinez, I.; Johnston, M.R.; Brown, G.E., Jr. Morphological preservation of carbonaceous plant fossils in blueschist metamorphic rocks from New Zealand. Geobiology 2012, 10, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; MacLellan, K.; Benzerara, K.; Boisset, N. Preservation of protein globules and peptidoglycan in the mineralized cell wall of nitrate-reducing, iron (II)-oxidizing bacteria: A cryo-electron microscopy study. Geobiology 2011, 9, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Kremer, B.; Kazmierczak, J.; Lukomska-Kowalczyk, M.; Kempe, S. Calcification and silicification: Fossilization potential of cyanobacteria from stromatolites of Niuafo‘ou’s caldera lakes (Tonga) and implications for the early fossil record. Astrobiology 2012, 12, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Benzerara, K.; Bernard, S.; Beyssac, O. The link between biomineralization and fossilization of bacteria: Insights from field and experimental studies. Chem. Geol. 2013, 359, 49–69. [Google Scholar] [CrossRef]
- Li, J.; Bernard, S.; Benzerara, K.; Beyssac, O.; Allard, T.; Cosmidis, J.; Mousou, J. Impact of biomineralization on the preservation of microorganisms during fossilization: An experimental perspective. Earth Planet. Sci. Lett. 2014, 400, 113–122. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial-algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Burns, B.P.; Goh, F.; Allen, M.; Nellan, B.A. Microbial diversity of extant stromatolites in the hypersaline marine environment of Shark Bay, Australia. Environ. Microbiol. 2004, 6, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Couradeau, E.; Benzerara, K.; Gérard, E.; Estève, I.; Moreira, D.; Tavera, R.; Lopez-Garcia, P. Cyanobacterial calcification in modern microbialites at the submicrometer scale. Biogeosciences 2013, 10, 5255–5266. [Google Scholar] [CrossRef] [Green Version]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Charbonnier, S.; Vannier, J.; Hantzpergue, P.; Gaillard, C. Ecological significance of the arthropod fauna from the Jurassic (Callovian) La Voulte Lagerstätte. Acta Palaeontol. Pol. 2010, 55, 111–132. [Google Scholar] [CrossRef]
- Becker, K.; Wahl, M. Behaviour patterns as natural antifouling mechanisms of tropical marine crabs. J. Exp. Mar. Biol. Ecol. 1996, 203, 245–258. [Google Scholar] [CrossRef]
- Goffredi, S.K.; Jones, W.J.; Erhlich, H.; Springer, A.; Vrijenhoek, R.C. Epibiotic bacteria associated with the recently discovered Yeti Crab, Kiwa hirsuta. Environ. Microbiol. 2008, 10, 2623–2634. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Suzuki, Y.; Fujiwara, Y.; Kawato, M.; Uematsu, K.; Yamanaka, T.; Yamamoto, H. Epibiotic association between filamentous bacteria and the vent-associated galatheid crab, Shinkaia crosnieri (Decapoda: Anomura). J. Mar. Biol. Assoc. UK 2010, 91, 23–32. [Google Scholar] [CrossRef]
- Fernandez-Leborans, G.; Tato-Porto, M.L. A review of the species of protozoan epibionts on crustaceans. I. Peritrich ciliates. Crustaceana 2013, 73, 643–683. [Google Scholar] [CrossRef]
- Wilby, P.R.; Briggs, D.E.G.; Riou, B. Mineralization of soft-bodied invertebrates in a Jurassic metalliferous deposit. Geology 1996, 24, 847–850. [Google Scholar] [CrossRef]
- Heaney, P.J.; Vicenzi, E.P.; Giannuzzi, L.A.; Livi, K.J.T. Focused ion beam milling: A method of site-specific sample extraction for microanalysis of Earth and planetary materials. Am. Mineral. 2001, 86, 1094–1099. [Google Scholar]
- Langford, R.M. Focused ion beams techniques for nanomaterials characterization. Microsc. Res. Tech. 2006, 69, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Drobne, D.; Milani, M.; Leser, V.; Tatti, F. Surface damage induced by FIB milling and imaging of biological samples is controllable. Microsc. Res. Tech. 2007, 70, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Munroe, P.R.; Tran, T.; Wainwright, M.S. FIB preparation of a sensitive porous catalyst for TEM elemental mapping at high magnifications. J. Mater. Sci. 2001, 36, 3519–3524. [Google Scholar] [CrossRef]
- Thompson, L.E.; Rice, P.M.; Delenia, E.; Lee, V.Y.; Brock, P.J.; Magbitang, T.P.; Dubois, G.; Volksen, W.; Miller, R.D.; Kim, H.-C. Imaging Thin Films of Nanoporous Low-k Dielectrics: Comparison between Ultramicrotomy and Focused Ion Beam Preparations for Transmission Electron Microscopy. Microsc. Microanal. 2006, 12, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Rubanov, S.; Munroe, P.R. FIB-induced damage in silicon. J. Microsc. 2004, 214, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Obst, M.; Gasser, P.; Mavrocordatos, D.; Dittrich, M. TEM-specimen preparation of cell/mineral interfaces by Focused Ion Beam milling. Am. Mineral. 2005, 90, 1270–1277. [Google Scholar] [CrossRef]
- Mayer, J.; Giannuzzi, L.A.; Kamino, T.; Michael, J. TEM sample preparation and FIB-induced damage. MRS Bull. 2007, 32, 400–407. [Google Scholar] [CrossRef]
- Bassim, N.D.; de Gregorio, B.T.; Kylcoyne, A.L.D.; Scott, K.; Chou, T.; Wirick, S.; Cody, G.D.; Stroud, R.M. Minimizing damage during FIB sample preparation of soft materials. J. Microsc. 2012, 245, 288–301. [Google Scholar] [CrossRef]
- Kilcoyne, A.L.D.; Tyliszczak, T.; Steele, W.F.; Fakra, S.; Hitchcock, P.; Franck, K.; Anderson, E.; Harteneck, B.; Rightor, E.G.; Mitchell, G.E.; Hitchcock, A.P.; Yang, L.; Warwick, T.; Ade, H. Interferometer-Controlled Scanning Transmission X-ray Microscopes at the Advanced Light Source. J. Synchrotron Radiat. 2003, 10, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Rightor, E.G.; Hitchcock, A.P.; Ade, H.; Leapman, R.D.; Urquhart, S.G.; Smith, A.P.; Mitchell, G.; Fischer, D.; Shin, H.J.; Warwick, T. Spectromicroscopy of poly(ethylene terephthalate): Comparison of spectra and radiation damage rates in X-ray absorption and electron energy loss. J. Phys. Chem. B 1997, 101, 1950–1960. [Google Scholar] [CrossRef]
- Braun, A.; Huggins, F.E.; Shah, N.; Chen, Y.; Wiric, S.; Mun, S.B.; Jacobsen, C.; Huffman, G.P. Advantages of soft X-ray absorption over TEM-EELS for solid carbon studies: A comparative study on diesel soot with EELS and NEXAFS. Carbon 2005, 43, 117–124. [Google Scholar] [CrossRef]
- Hitchcock, A.P.; Dynes, J.J.; Johansson, G.; Wang, J.; Botton, G. Comparison of NEXAFS microscopy and TEM-EELS for studies of soft matter. Micron 2008, 39, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Kubatova, A.; Wirick, S.; Mun, S. Radiation damage from EELS and NEXAFS in diesel soot and diesel soot extracts. J. Electron Spectrosc. 2009, 170, 42–48. [Google Scholar] [CrossRef]
- Wang, J.; Morin, C.; Li, L.; Hitchcock, A.; Scholl, A.; Doran, A. Radiation damage in soft X-ray microscopy. J. Electron Spectrosc. Relat. Phenom. 2009, 170, 25–36. [Google Scholar] [CrossRef]
- Ben-Jacob, E.; Schochet, O.; Tenenbaum, A.; Cohen, I.; Czirok, A.; Vicsek, T. Generic modelling of cooperative growth patterns in bacterial colonies. Nature 1994, 368, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Lega, J.; Passot, T. Hydrodynamics of bacterial colonies. Nonlinearity 2007, 20. [Google Scholar] [CrossRef]
- Brandes, J.A.; Wirick, S.; Jacobsen, C. Carbon K-edge spectra of carbonate minerals. J. Synchrotron Rad. 2010, 17, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.; Lehmann, J.; Kinyangi, J.; Liang, B.Q.; Heymann, K.; Dathe, L.; Hanley, K.; Wirick, S.; Jacobsen, C. Carbon (1s) NEXAFS Spectroscopy of Biogeochemically Relevant Reference Organic Compounds. Soil Sci. Soc. Am. J. 2009, 73, 1817–1830. [Google Scholar] [CrossRef]
- Benzerara, K.; Yoon, T.H.; Tyliszczak, T.; Constantz, B.; Sporman, A.M.; Brown, G.E. Scanning transmission X-ray microscopy study of microbial calcification. Geobiology 2004, 2, 249–259. [Google Scholar] [CrossRef]
- Benzerara, K.; Menguy, N.; Guyot, F.; Skouri, F.; de Luca, G.; Barakat, M.; Heulin, T. Biologically controlled precipitation of calcium phosphate by Ramlibacter tataouinesis. Earth Planet. Sci. Lett. 2004, 228, 439–449. [Google Scholar] [CrossRef]
- Benzerara, K.; Menguy, N.; Lopez-Garcia, P.; Yoon, T.H.; Kazmierczak, J.; Tyliszczak, T.; Guyot, F.; Brown, G.E., Jr. Nanoscale detection of organic signatures in carbonate microbialites. Proc. Natl. Acad. Sci. USA 2006, 103, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
- Bontognali, T.R.R.; Vasconcelos, C.; Whartmann, R.J.; Dupraz, C.; Bernasconi, S.M.; McKenzie, J.A. Microbes produce nanobacteria-like structures, avoirding cell entombment. Geology 2008, 36, 663–666. [Google Scholar] [CrossRef]
- Spadafora, A.; Perri, E.; McKenzie, J.A.; Vasconcelos, C. Microbial biomineralization processes forming modern Ca:Mg carbonate stromatolites. Sedimentology 2010, 57, 27–40. [Google Scholar] [CrossRef]
- Rusznyak, A.; Akob, D.M.; Nletzsche, S.; Eusterhues, K.; Totsche, K.U.; Neu, T.R.; Frosch, T.; Popp, J.; Keiner, R.; Geletneky, J.; Katzschmann, L.; Schulze, E.D.; Kusel, K. Calcite biomineralization by bacterial isolates from the recently discovered pristine karstic Herrenberg cave. Appl. Environ. Microbiol. 2011, 78, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; Benzerara, K.; Morin, G.; Kappler, A.; Bernard, S.; Obst, M.; Férard, C.; Skouri-Panet, F.; Guigner, J.M.; Posth, N.; Galvez, M.; Brown, G.E., Jr. Iron biomineralization by anaerobic neutrophilic iron-oxidizing bacteria. Geochim. Cosmochim. Acta 2009, 73, 696–711. [Google Scholar] [CrossRef]
- Leinweber, P.; Kruse, J.; Walley, F.L.; Gillespie, A.; Eckhardt, K.U.; Blyth, R.I.; Regier, T. Nitrogen K-edge XANES-an overview of reference compounds used to identify unknown organic nitrogen in environmental samples. J. Synchrotron Radiat. 2007, 14, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Cody, G.; Alexander, C.; Yabuta, H.; Kilcoyne, A.A.; Ade, H.; Dera, P.; Fogel, M.; Militzer, B.; Myse, B. Organic thermometry for chondritic parent bodies. Earth Planet. Sci. Lett. 2008, 272, 446–455. [Google Scholar] [CrossRef]
- Nuevo, M.; Milam, S.; Sandford, S.; de Gregorio, B.; Cody, G.; Kilcoyne, A. XANES analysis of organic residues produced from the UV irradiation of astrophysical ice analogs. Adv. Space Res. 2011, 48, 1126–1135. [Google Scholar] [CrossRef]
- Stylo, M.; Alessi, D.S.; Shao, P.P.; Lezama-Pacheco, J.S.; Bergar, J.R.; Bernier-Latmani, R. Biogeochemical controls on the product of microbial U(VI) reduction. Envriron. Sci. Technol. 2013, 47, 12351–12358. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.; McKenzie, J.A.; Bernasconi, S.; Grujic, D.; Tiens, A.J. Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 1995, 377, 220–222. [Google Scholar] [CrossRef]
- Dupraz, S.; Parmentier, M.; Ménez, B.; Guyot, F. Experimental and numerical modeling of bacterially induced pH increase and calcite precipitation in saline aquifers. Chem. Geol. 2009, 265, 44–53. [Google Scholar] [CrossRef]
- Couradeau, E.; Benzerara, K.; Gerard, E.; Moreira, D.; Bernard, S.; Brown, G.E.; Lopez-Garcia, P. An early-branching microbialite cyanobacterium forms intracellular carbonates. Science 2012, 336, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, C.; Fowler, A.; Tobias, C.; Visscher, P.T. Stromatolitic knobs in Storr’s Lake (San Salvador, Bahamas): A model system for formation and alteration of laminae. Geobiology 2013, 11, 527–548. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially induced mineralization of calcium carbonate in terrestrial environments: The role of exopolysaccharides and amino acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; McKenzie, J.A. Microbial fossilization in carbonate sediments: A result of the bacterial surface involvement in dolomite precipitation. Sedimentology 2003, 50, 237–245. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- Obst, M.; Dyes, J.J.; Lawrence, J.R.; Swerhone, G.D.W.; Benzerara, K.; Karunakaran, C.; Kaznatcheev, K.; Tyliszczak, T.; Hitchcock, A.P. Precipitation of amorphous CaCO3 (aragonite-like) by cyanobacteria: A STXM study of the influence of EPS on the nucleation process. Geochim. Cosmochim. Acta 2009, 73, 4180–4198. [Google Scholar]
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-induced biofilm calcification and calcium concentrations in phanerozoic oceans. Science 2001, 292, 1701–1704. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.; Warthmann, R.; McKenzie, J.A.; Viwsscher, P.T.; Bittermann, A.G.; van Lith, Y. Lithifying microbial mats in Lagoa Vermelha, Brazil: Modern precambrian relics? Sediment. Geol. 2006, 185, 175–183. [Google Scholar] [CrossRef]
- Bosak, T.; Greene, S.E.; Newman, D.K. A likely role for anoxygenic photosynthetic microbes in the formation of ancient stromatolites. Geobiology 2007, 5, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the alkalinity engine: The role of electron donors in the organoomineralization potential of sulfate-reducing bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Benzerara, K.; Skouri-Panet, F.; Li, J.; Férard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N.; Margaret-Olivier, I.; Tavera, R.; Lopez-Garcia, P.; Moreira, D. Intracellular Ca-carbonate biomineralisation is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef] [PubMed]
- Walsby, A.E.; Hayes, P.K.; Boje, R. The gas vesicles, buoyancy and vertical distribution of cyanobacteria in the Baltic Sea. Eur. J. Phycol. 1995, 30, 87–94. [Google Scholar] [CrossRef]
- Lawrence, J.R.; Swerhone, G.D.W.; Leppard, G.G.; Araki, T.; Zhang, X.; West, M.M.; Hitchcock, A.P. Scanning Transmission X-ray, laser scanning and transmission electron microscopy mapping of the exopolymeric patrix of microbial biofilms. Appl. Environ. Microbiol. 2003, 69, 5543–5554. [Google Scholar] [CrossRef] [PubMed]
- Cosmidis, J.; Benzerara, K.; Gheerbrant, E.; Esteve, I.; Bouya, B.; Amaghzaz, M. Nanometer-scale characterization of exceptionally preserved bacterial fossils in Paleocene phosphorites from Ouled-Abdoun (Morocco). Geobiology 2013, 11, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; Recham, N.; Larcher, D.; Guyot, F.; Brest, J.; Tarascon, J.M. Biomineralized-Fe2O3: Texture and electrochemical reaction with Li. Energy Environ. Sci. 2014, 7, 451–460. [Google Scholar] [CrossRef]
- Lepot, K.; Benzerara, K.; Brown, G.E., Jr.; Philippot, P. Microbially influenced formation of 2,724-million-year-old stromatolites. Nat. Geosci. 2008, 1, 118–121. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robin, N.; Bernard, S.; Miot, J.; Blanc-Valleron, M.-M.; Charbonnier, S.; Petit, G. Calcification and Diagenesis of Bacterial Colonies. Minerals 2015, 5, 488-506. https://doi.org/10.3390/min5030488
Robin N, Bernard S, Miot J, Blanc-Valleron M-M, Charbonnier S, Petit G. Calcification and Diagenesis of Bacterial Colonies. Minerals. 2015; 5(3):488-506. https://doi.org/10.3390/min5030488
Chicago/Turabian StyleRobin, Ninon, Sylvain Bernard, Jennyfer Miot, Marie-Madeleine Blanc-Valleron, Sylvain Charbonnier, and Gilles Petit. 2015. "Calcification and Diagenesis of Bacterial Colonies" Minerals 5, no. 3: 488-506. https://doi.org/10.3390/min5030488




