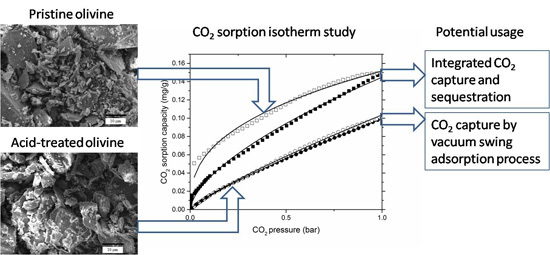
Carbon Dioxide Sorption Isotherm Study on Pristine and Acid-Treated Olivine and Its Application in the Vacuum Swing Adsorption Process
Abstract
:1. Introduction
2. Material and Experiments
2.1. Materials Preparation
2.2. Experimental Procedure
3. Results
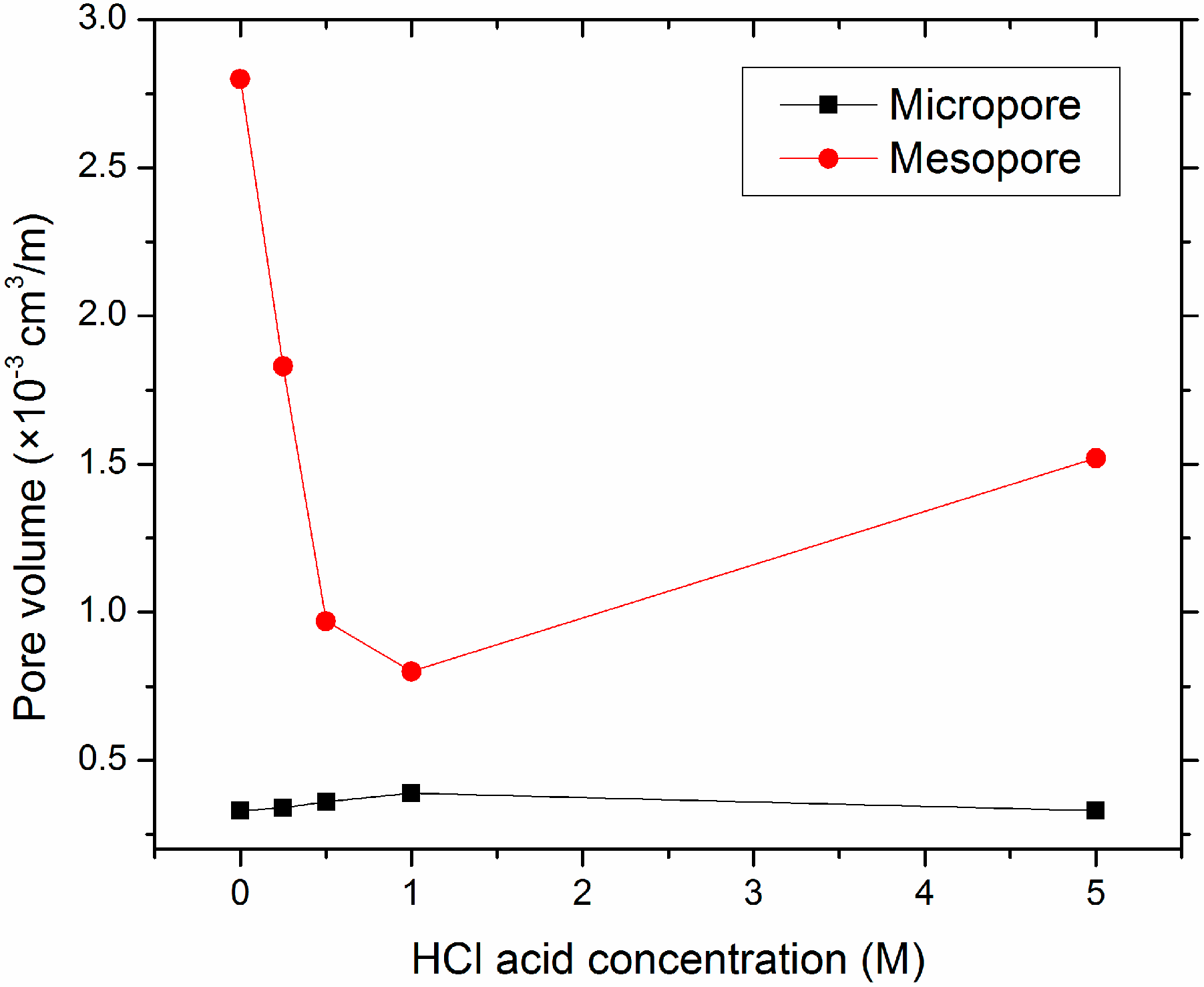
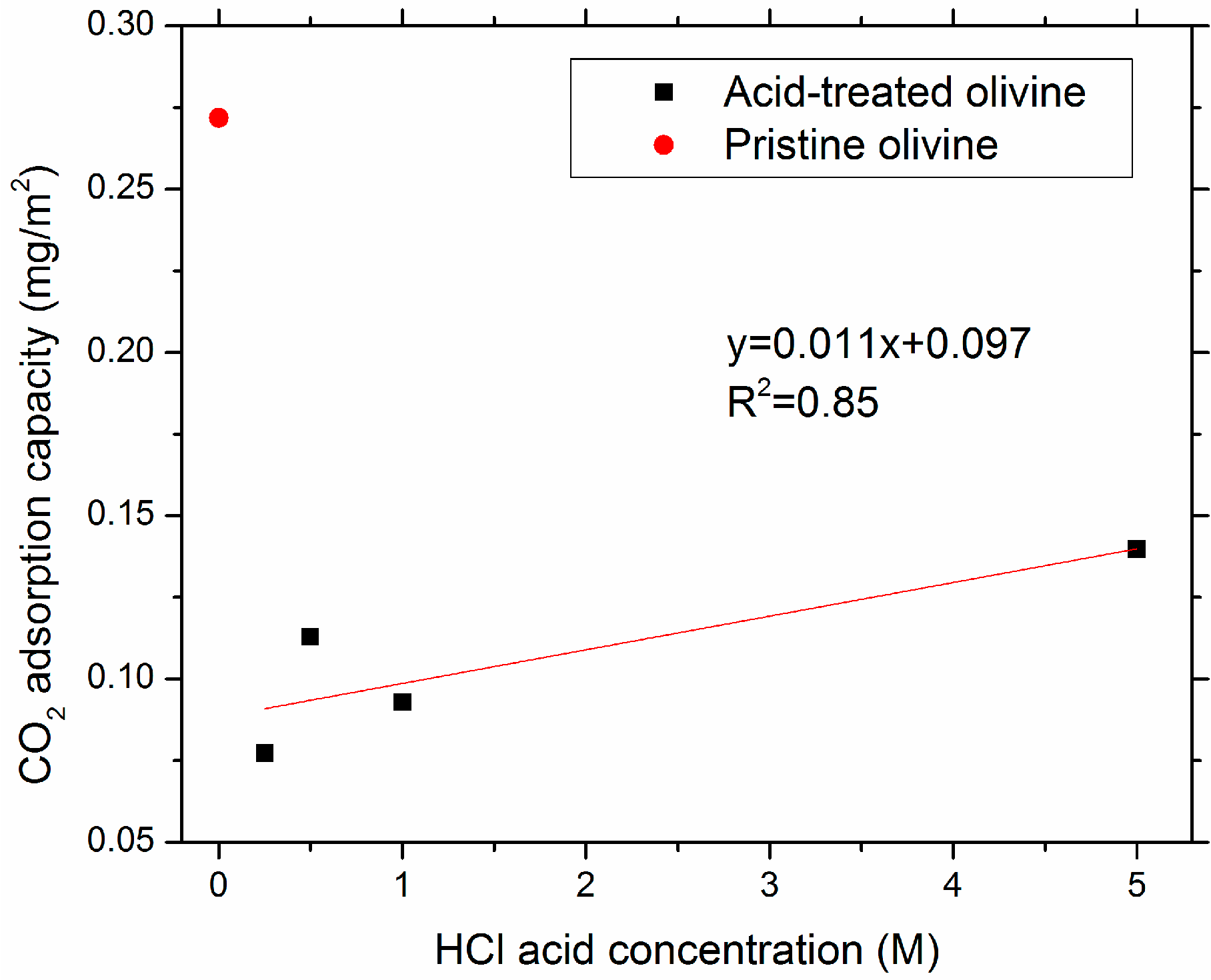
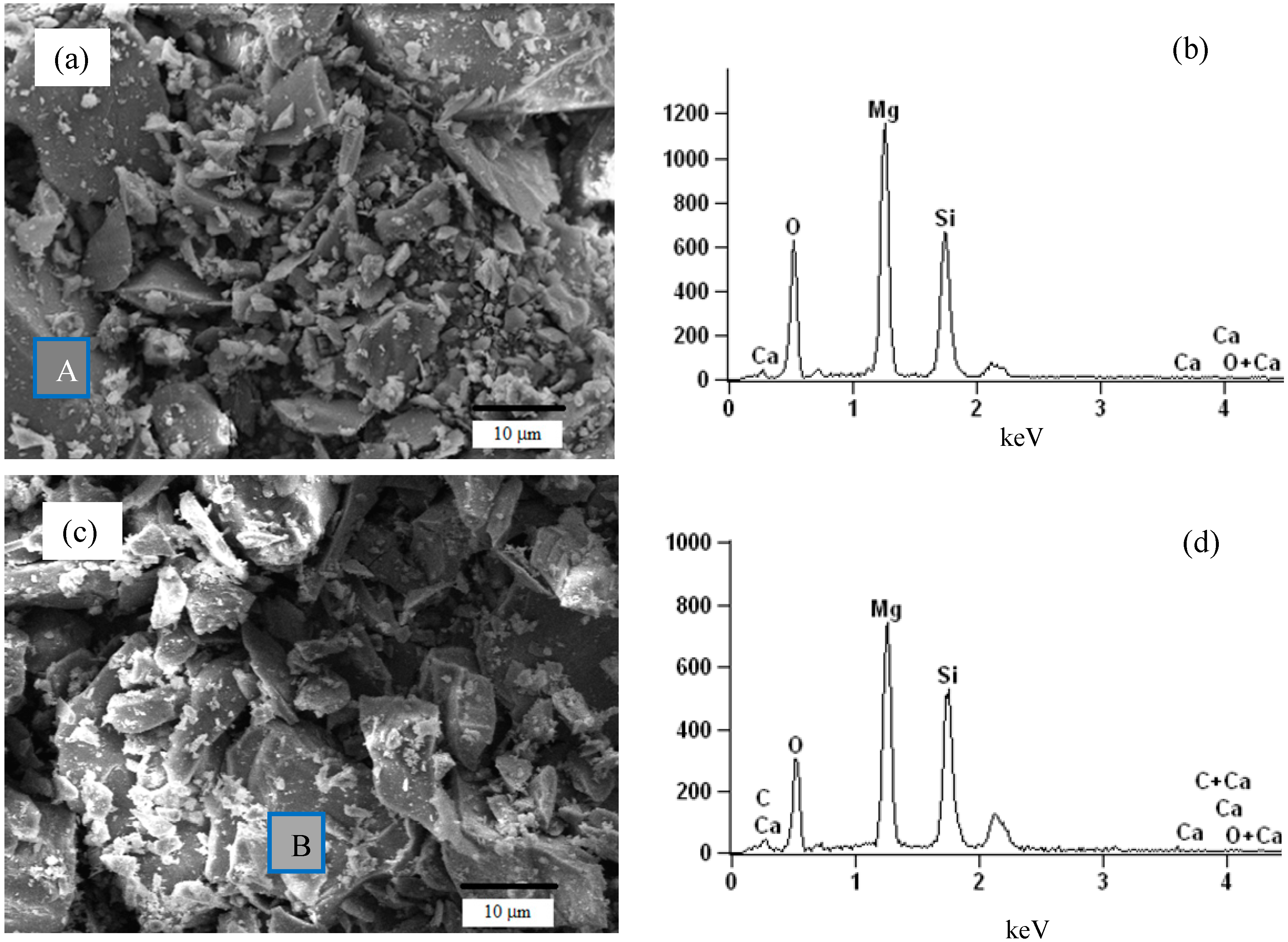
3.1. Characteristics of Pristine and Acid-Treated Olivine
| Material | CHCl | SBET | PV-micro | PV-meso | PV-total | ACO2a | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (M) | (m2/g) | (×10−3 cm3/g) | (×10−3 cm3/m2) | (×10−3 cm3/g) | (×10−3 cm3/m2) | (×10−3 cm3/g) | (×10−3 cm3/m2) | (mg/g) | (mg/m2) | |
| OL | 0 | 0.55 | 0.18 | 0.33 | 1.55 | 2.8 | 1.73 | 2.73 | 0.15 | 0.27 |
| OL-A0.25 | 0.25 | 1.29 | 0.44 | 0.34 | 2.36 | 1.83 | 2.80 | 2.02 | 0.10 | 0.08 |
| OL-A0.5 | 0.5 | 2.2 | 0.79 | 0.36 | 2.14 | 0.97 | 2.93 | 1.14 | 0.25 | 0.11 |
| OL-A1 | 1 | 2.79 | 1.10 | 0.39 | 2.23 | 0.8 | 3.33 | 1.08 | 0.26 | 0.09 |
| OL-A5 | 5 | 1.16 | 0. 38 | 0.33 | 1.76 | 1.52 | 2.14 | 1.72 | 0.16 | 0.14 |
3.2. CO2 Sorption Isotherm Profiles
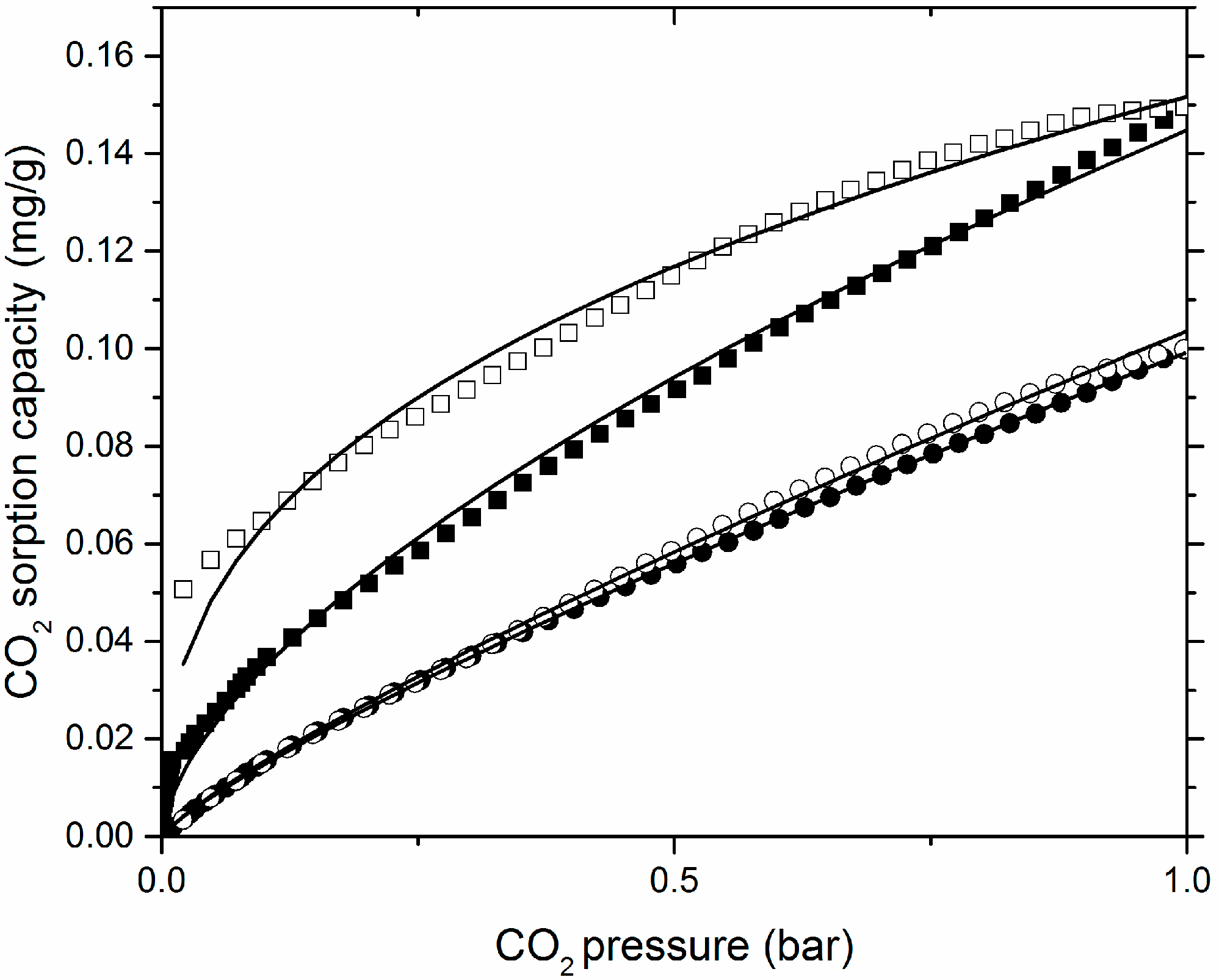
3.3. Mathematical Analysis of the CO2 Sorption Isotherms
3.3.1. CO2 Sorption Isotherm Models
| Isotherms | Models | Energy Parameters | Parameters in Equation |
|---|---|---|---|
| Langmiur | - | is the Langmuir adsorption constant; is the monolayer capacity (cc/g) | |
| Freundlich | - | is the parameters of Freundlich model; n is adsorption intensity | |
| Dubinin-Radushkevich | is the number of moles of adsorbate required to fill the micropores (mg/g); is the D-R isotherm constant (mol2/kJ2) | ||
| Temkin | is the Temkin isotherm equilibrium binding constant (cc/g); is the Temkin isotherm constant |
3.3.2. Model Fitting of the CO2 Sorption Isotherm
| Isotherm Model | Parameter | OL | OL-A0.25 | ||
|---|---|---|---|---|---|
| CO2 Adsorption | CO2 Desorption | CO2 Adsorption | CO2 Desorption | ||
| Langmiur | am | 0.145 | 0.099 | 0.194 | 0.203 |
| kl | 1.182 | 4.296 | 0.388 | 0.386 | |
| R2 | 0.995 | 0.962 | 1.000 | 1.000 | |
| Freundlich | kf | 0.080 | 0.084 | 0.055 | 0.058 |
| n | 1.610 | 2.655 | 1.209 | 1.206 | |
| R2 | 0.999 | 0.992 | 1.000 | 0.999 | |
| Dubinin-Radushkevich | a0 | 0.085 | 0.084 | 0.064 | 0.069 |
| b | 0.328 | 0.169 | 0.519 | 0.554 | |
| E | 1.235 | 1.720 | 0.981 | 0.950 | |
| R2 | 0.984 | 0.933 | 0.992 | 0.990 | |
| Temkin | aT | 1.039 | 1.333 | 1.002 | 0.999 |
| bT | 21,079.803 | 23,903.631 | 31,779.675 | 30,402.608 | |
| B | 0.118 | 0.104 | 0.078 | 0.081 | |
| R2 | 0.995 | 0.996 | 1.000 | 1.000 | |
3.4. CO2 Expected Working Capacity
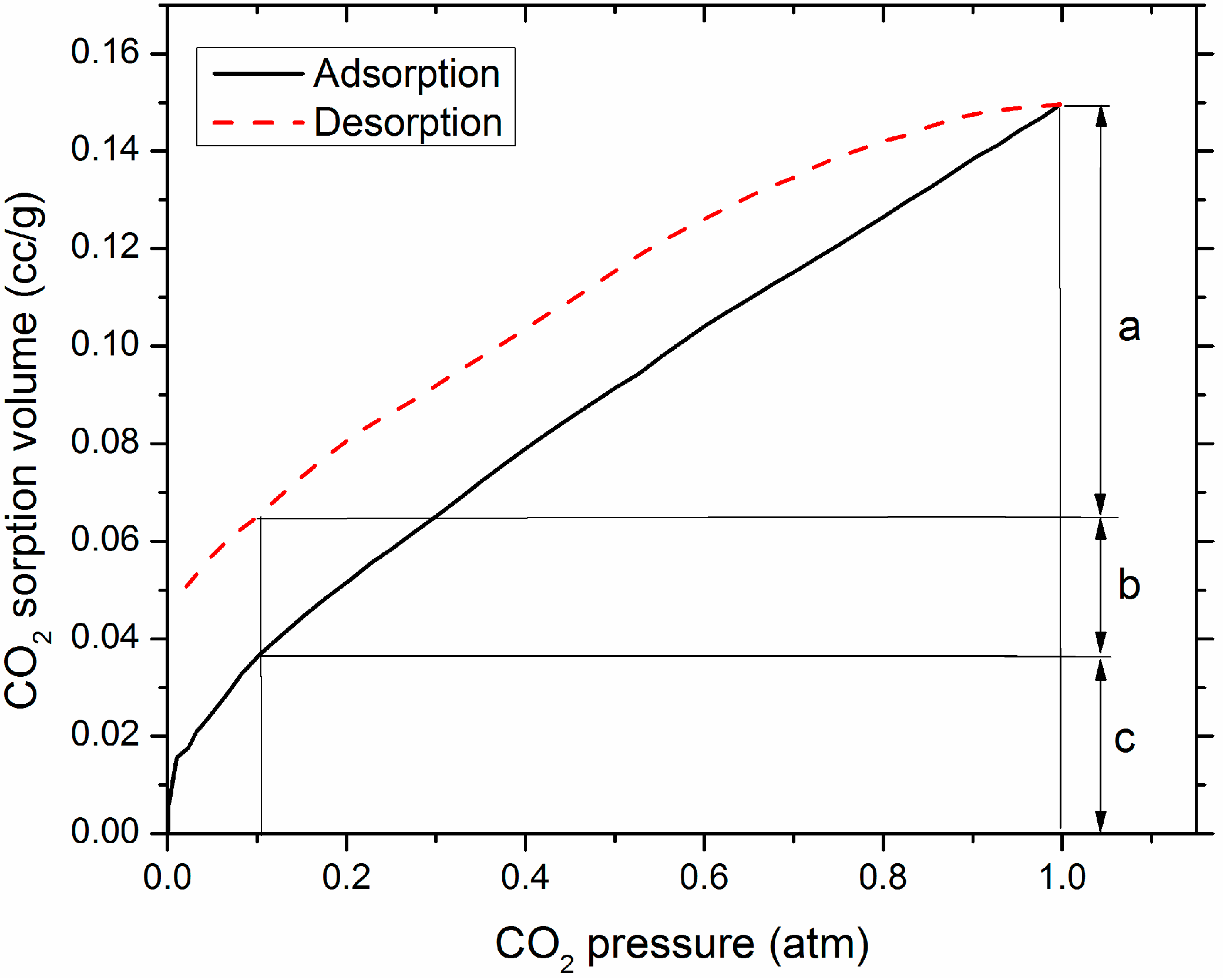
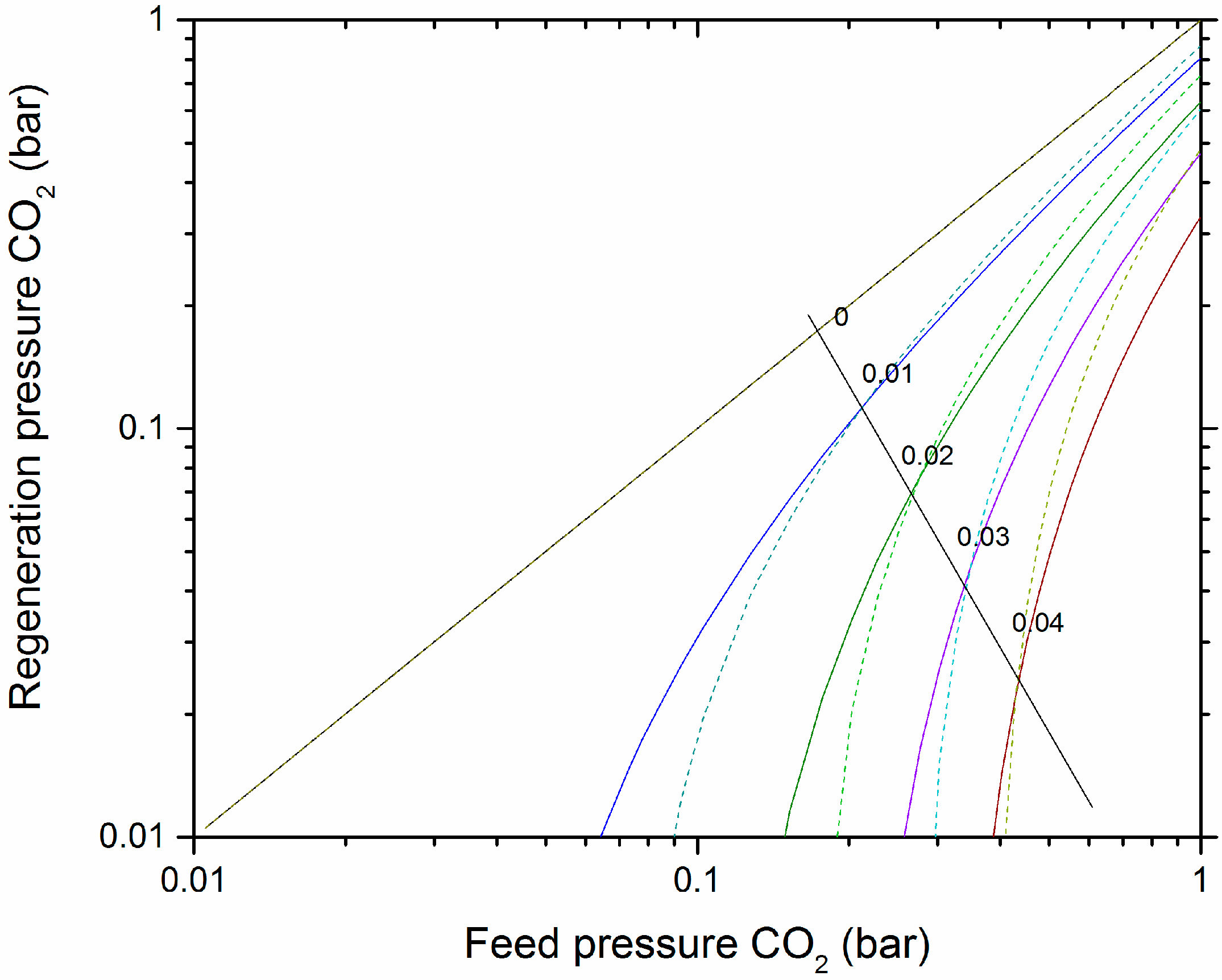
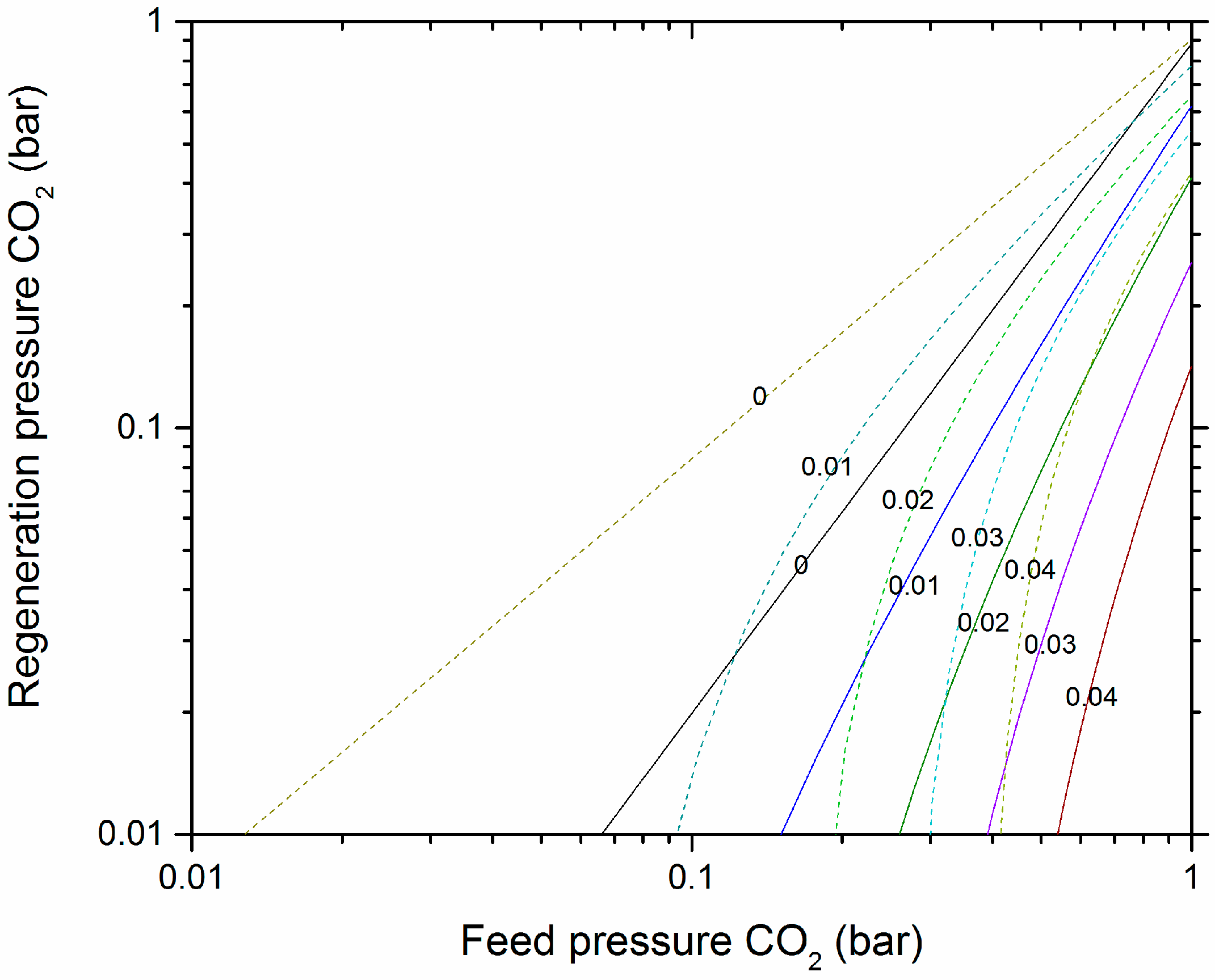
4. Discussion
4.1. The Effect of Leaching on Olivine Surface
4.2. VSA of CO2 on Pristine Olivine
4.3. VSA of CO2 on Acid-Treated Olivine
4.4. Comparison with Literature Data
| Sample Name | Type of Adsorbent | Surface Area (m2/g) | Temperature (°C) | ACO2a (mg/g) | EWC (mg/m2) | References |
|---|---|---|---|---|---|---|
| OL | Mineral | 0.55 | 298 | 0.041–0.088e | 0.078 | This work |
| 0.065–0.103f | 0.069 | This work | ||||
| OL-A5 | Mineral | 1.16 | 298 | 0.025–0.074 | 0.042 | This work |
| NaX/1 | Zeolite | -d | 298 | 123.9–171.2 | -d | [41] |
| Active carbon | Carbon | 1300 | 298 | 26.3–65.8 | 0.030 | [42] |
| Cu-BTC | MOFb | 692 | 298 | 21.9–87.8 | 0.095 | [43] |
| Ni/DOBDC | MOF | 1080 | 296 | 118.5–175.6 | 0.053 | [31] |
| Co/DOBDC | MOF | 1070 | 296 | 122.9–235.3 | 0.105 | [31] |
| Mg/DOBDC | MOF | 1495 | 296 | 235.3–298.5 | 0.042 | [31] |
| ZIF-78 | ZIFc | 620 | 298 | 33.8–60.0 | 0.042 | [44] |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK and New York, NY, USA, 2013. [Google Scholar]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Werner, M.; Hariharan, S.; Zingaretti, D.; Baciocchi, R.; Mazzotti, M. Dissolution of dehydroxylated lizardite at flue gas conditions: I. Experimental study. Chem. Eng. J. 2014, 241, 301–313. [Google Scholar] [CrossRef]
- Hariharan, S.; Werner, M.; Hänchen, M.; Mazzotti, M. Dissolution of dehydroxylated lizardite at flue gas conditions: II. Kinetic modeling. Chem. Eng. J. 2014, 241, 314–326. [Google Scholar] [CrossRef]
- Kwon, S.; Fan, M.; DaCosta, H.F.M.; Russell, A.G. Factors affecting the direct mineralization of CO2 with olivine. J. Environ. Sci. 2011, 23, 1233–1239. [Google Scholar] [CrossRef]
- Veetil, S.P.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. CO2 sequestration by direct dry gas-solid contact of serpentinite mining residues: A Solution for industrial CO2 emission. Int. J. Environ. Pollut. Remediat. 2014, 2, 52–59. [Google Scholar]
- Kohlmann, J.; Zevenhoven, R. The removal of CO2 from Flue gases using magnesium silicates, in Finland. In Proceedings of the 11th International Conference on Coal Science, San Francisco, CA, USA, 29 September–5 October 2001.
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon mineralization: From natural analogues to engineered systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Kohlmann, J. Dry Mineral Carbonation for CO2 Emissions Reduction in Finland. In Proceedings of the 27th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FL, USA, 4–7 March 2002.
- Kwon, S.; Fan, M.; Dacosta, H.F.M.; Russell, A.G.; Berchtold, K.A.; Dubey, M.K. CO2 Sorption. In Coal Gasification and Its Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 293–339. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size, and Densit; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; p. 347. [Google Scholar]
- Guthrie, G.D.; Carey, J.W.; Bergfeld, D.; Byler, D.; Chipera, S.; Ziock, H.; Lackner, K.S. Geochemical Aspects of the Carbonation of Magnesium Silicates in an Aqueious Medium; Los Alamos National Laboratory: Los Alamos, NM, USA, 1999; p. 14. [Google Scholar]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the cost of CO2 capture from flue gases using pressure swing adsorption. Ind. Eng. Chem. Res. 2008, 47, 4883–4890. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerrero, R. Flue gas treatment via CO2 adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Maring, B.J.; Webley, P.A. A new simplified pressure/vacuum swing adsorption model for rapid adsorbent screening for CO2 capture applications. Int. J. Greenh. Gas Control 2013, 15, 16–31. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Lackner, K.S.; Butt, D.P.; Wendt, C.H.; Ziock, H. Mineral Carbonates as Carbon Dioxide Sinks; Los Alamos National Laboratory: Los Alamos, NM, USA, 1999; p. 10. [Google Scholar]
- Hitch, M.; Ballantyne, S.M.; Hindle, S.R. Revaluing mine waste rock for carbon capture and storage. Int. J. Min. Reclam. Environ. 2010, 24, 64–79. [Google Scholar] [CrossRef]
- Kwon, S. Mineralization for CO2 Sequstration Using Olivine Sorbent in the Presence of Water Vapor; Georgia Institute of Technology: Atlanta, GA, USA, 2011; p. 181. [Google Scholar]
- Alexander, G.; Maroto-Valer, M.M.; Gafarova-Aksoy, P. Evaluation of reaction variables in the dissolution of serpentine for mineral carbonation. Fuel 2007, 86, 273–281. [Google Scholar] [CrossRef]
- Maroto-valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andre, J.M. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Gadikota, G.; Natali, C.; Boschi, C.; Park, A.-H.A. Morphological changes during enhanced carbonation of asbestos containing material and its comparison to magnesium silicate minerals. J. Hazard. Mater. 2014, 264, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Gadikota, G.; Swanson, E.J.; Zhao, H.; Park, A.-H.A. Experimental design and data analysis for accurate estimation of reaction kinetics and conversion for carbon mineralization. Ind. Eng. Chem. Res. 2014, 53, 6664–6676. [Google Scholar] [CrossRef]
- Gadikota, G.; Kelemen, P.; Matter, J.; Park, A.-H.A. Chemical and morphological changes during olivine carbonation for CO2 Storage in the presence of NaCl and NaHCO3. Phys. Chem. Chem. Phys. 2014, 16, 4679–4693. [Google Scholar] [CrossRef] [PubMed]
- Gadikota, G.; Park, A.-H.A. Accelerated carbonation of Ca- and Mg-bearing minerals and industrial wastes using CO2. In Carbon Dioxide Utilization: Closing the Carbon Cycle; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Hänchen, M.; Prigiobbe, V.; Storti, G.; Seward, T.M.; Mazzotti, M. Dissolution kinetics of fosteritic olivine at 90–150 °C including effects of the presence of CO2. Geochim. Cosmochim. Acta 2006, 70, 4403–4416. [Google Scholar] [CrossRef]
- Soares, J.L.; Casarin, G.L.; Jose, H.J.; Moreira, R.D.; Rodrigues, A.E. Experimental and Theoretical Analysis for the CO2 Adsorption on Hydrotalcite. Adsorption 2005, 11, 237–241. [Google Scholar] [CrossRef]
- Raudsepp, M.; Pani, E. Application of Rietveld analysis to environmental mineralogy. In Environmental Mineralogy of Mine Wastes, Mineralogical Association of Canada Short Course; Jambor, J.L., Blowes, D.W., Ritchie, A.I.M., Eds.; Mineralogical Association of Canada: Ottawa, ON, Canada, 2003; pp. 165–180. [Google Scholar]
- Presnali, D.C.; Walter, M.J. Melting of forsterite, Mg2SiO4,From 9.7 to 16.5 GPa. J. Geophys. Res. 1993, 98, 19777–19783. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, B. Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. J. Hazard. Mater. 2006, 129, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Itodo, A.U.; Itodo, H.U. Sorption energies estimation using Dubinin-Radushkevich and Temkin adsorption isotherm. Life Sci. J. 2010, 7, 31–39. [Google Scholar]
- Harlick, P.J.E.; Tezel, F.H. An experimental adsorbent screening study for CO2 removal from N2. Microporous Mesoporous Mater. 2004, 76, 71–79. [Google Scholar] [CrossRef]
- Haug, T. Dissolution and Carbonation of Mechanically Activated Olivine; Norwegian University of Science and Technology: Trondheim, Norway, 2010; p. 243. [Google Scholar]
- Béarat, H.; McKelvy, M.J.; Chizmeshya, A.V.G.; Gormley, D.; Nunez, R.; Carpenter, R.W.; Squires, K.; Wolf, G.H. Carbon sequestration via aqueous olivine mineral carbonation: role of passivating layer formation. Environ. Sci. Technol. 2006, 40, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Rimstidt, J.D.; Brantley, S.L.; Olsen, A.A. Systematic review of forsterite dissolution rate data. Geochim. Cosmochim. Acta 2012, 99, 159–178. [Google Scholar] [CrossRef]
- Zhang, Q.; Sugiyama, K.; Saito, F. Enhancement of acid extraction of magnesium and silicon from serpentine by mechanochemical treatment. Hydrometallurgy 1997, 45, 323–331. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, J., II; Lee, S.G.; Jang, S.S. A density functional theory (DFT) study of CO2 adsorption on Mg-rich minerals by enhanced charge distribution. Comput. Mater. Sci. 2014, 95, 181–186. [Google Scholar] [CrossRef]
- Keeling, R. Scripps Institution of Oceanography. Available online: http://scrippsco2.ucsd.edu/ (accessed on 20 December 2014).
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolites 13X at high pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Na, B.K.; Koo, I.K.; Eum, H.M.; Lee, H.; Song, H.K. CO2 recovery from flue gas by PSA process using activated carbon, Korean. J. Chem. Eng. 2001, 18, 220–227. [Google Scholar]
- Yang, Q.; Xue, C.; Zhong, C.; Chen, J.F. Molecular simulation of separation of CO2 from flue gas in Cu-BTC metal-organic framework. AIChE J. 2007, 53, 2832–2840. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.; Shopska, M.; Paneva, D.; Kadinov, G.; Kostova, N.; Turianicová, E.; Briančin, J.; Mitov, I.; Kleiv, R.A.; Baláž, P. The influence of attrition milling on carbon dioxide sequestration on magnesium–iron silicate. Miner. Eng. 2010, 23, 616–620. [Google Scholar] [CrossRef]
- Hangx, S.J.T.; Spiers, C.J. Coastal spreading of olivine to control atmospheric CO2 concentrations: A critical analysis of viability. Int. J. Greenh. Gas Control 2009, 3, 757–767. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hitch, M. Carbon Dioxide Sorption Isotherm Study on Pristine and Acid-Treated Olivine and Its Application in the Vacuum Swing Adsorption Process. Minerals 2015, 5, 259-275. https://doi.org/10.3390/min5020259
Li J, Hitch M. Carbon Dioxide Sorption Isotherm Study on Pristine and Acid-Treated Olivine and Its Application in the Vacuum Swing Adsorption Process. Minerals. 2015; 5(2):259-275. https://doi.org/10.3390/min5020259
Chicago/Turabian StyleLi, Jiajie, and Michael Hitch. 2015. "Carbon Dioxide Sorption Isotherm Study on Pristine and Acid-Treated Olivine and Its Application in the Vacuum Swing Adsorption Process" Minerals 5, no. 2: 259-275. https://doi.org/10.3390/min5020259