Field Application of Accelerated Mineral Carbonation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Demonstration Scale Accelerated Mineral Carbonation Field Testing
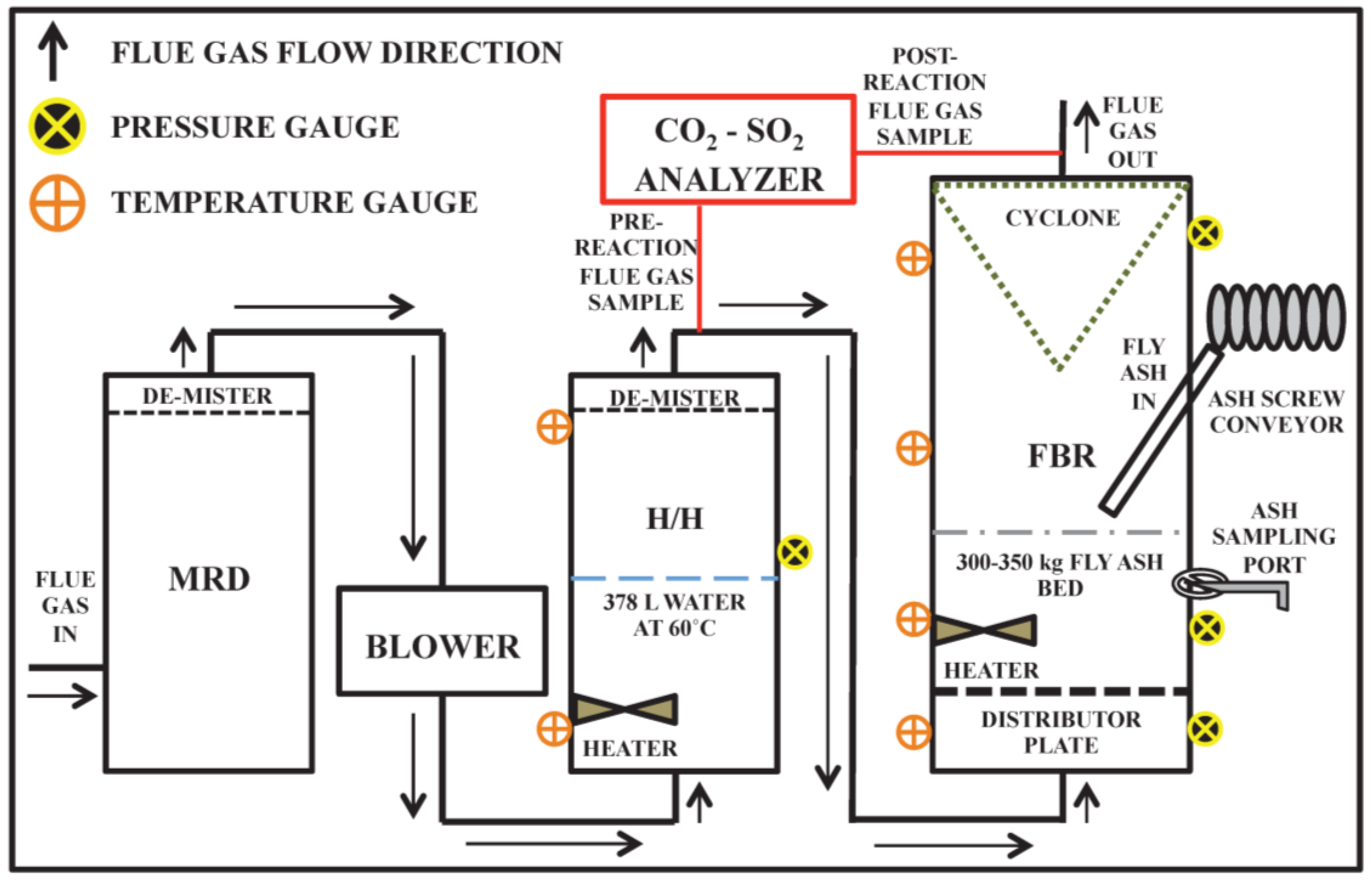
2.2. Total Carbon, Sulfur, and Mercury
2.3. Scanning Electron Microscopy-Energy Dispersive X-Ray Spectroscopy
2.4. Sequential Chemical Fractionation of Trace Elements
| Fraction | Extractant Fluid | Procedure |
|---|---|---|
| Exchangeable | 0.5 M Mg(NO3)2 | 30 min shaking at 120 rpm |
| Carbonate | 1 M NaOAc | 5 h shaking at 120 rpm |
| Oxide | 0.08 M NH2OH·HCl | 6 h shaking at 120 rpm |
| Organic Matter | 0.02 M HNO3 and H2O2 | 2 h shaking at 120 rpm at 85 °C |
| Residual | Concentrated HNO3 | 2 h shaking at 120 rpm |
2.5. Toxicity Characteristic Leaching Procedure (TCLP)
3. Results and Discussion
3.1. Elemental Composition and Distribution on Fly Ash Particles
| Component | wt % |
|---|---|
| SiO2 | 60.04 |
| Al2O3 | 19.67 |
| CaO | 5.86 |
| Fe2O3 | 4.66 |
| MgO | 3.85 |
| K2O | 2.00 |
| N2O | 1.00 |
| Loss on Ignition | 0.60 |
3.2. Demonstration Scale Studies
| Sample | CaCO3 (wt %) | SO42− (wt %) | Hg (mg/kg) |
|---|---|---|---|
| Control | 2.88 ±0.96 | 0.48 ±0.03 | 0.12 ±0.09 |
| 10 min | 3.11 ±1.57 | 1.21 ±0.19 | 0.27 ±0.12 |
| 30 min | 3.67 ±1.53 | 1.35 ±0.13 | 0.25 ±0.08 |
| Final | 3.86 ±1.28 | 1.42 ±0.14 | 0.30 ±0.11 |
3.3. SEM/EDS

3.4. Sequential Chemical Fractionation of Trace Elements

3.5. Toxicity Characteristic Leaching Procedure
| TCLP (mg/L) | As | Ba | Cd | Cr | Hg | Pb | Se | Ag |
|---|---|---|---|---|---|---|---|---|
| Reporting Limit | 5.0 | 100 | 1.0 | 5.0 | 0.2 | 5.0 | 1.0 | 5.0 |
| Control | * | * | * | * | * | * | 0.21 | * |
| 30 min | * | * | * | * | * | * | 0.18 | * |
| Final | * | * | * | * | * | * | 0.18 | * |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- U.S. Energy Information Administration. Energy in Brief: What is the Role of Coal in the United States? Available online: http://www.eia.gov/energy_in_brief/article/role_coal_us.cfm (accessed on 21 September 2013).
- U.S. Energy Information Administration. China. Available online: http://www.eia.gov/countries/cab.cfm?fips=CH (accessed on 21 September 2013).
- U.S. Energy Information Administration. India. Available online: http://www.eia.gov/countries/cab.cfm?fips=IN (accessed on 21 September 2013).
- U.S. Energy Information Administration. Monthly Energy Review October 2013. Available online: http://www.eia.gov/totalenergy/data/monthly/pdf/sec12_9.pdf (accessed on 5 October 2013).
- Asokan, P.; Saxena, M.; Asolekar, S. Coal combustion residues-environmental implications and recycling potentials. Resour. Conserv. Recycl. 2005, 43, 239–262. [Google Scholar] [CrossRef]
- Pei-Wei, G.; Xiao-Lin, L.; Hui, L.; Xiaoyan, L.; Jie, H. Effects of fly ash on the properties of environmentally friendly dam concrete. Fuel 2007, 86, 1208–1211. [Google Scholar] [CrossRef]
- Barnes, D.; Sear, L. Ash Utilisation from Coal-Based Power Plants; Report COAL-R-274 for UK Quality Ash Association; Hatterrall Associates: Cheltenham, UK, 2004. [Google Scholar]
- Mardon, S.; Hower, J. Impact of coal properties on coal combustion by-product quality: Examples from a Kentucky power plant. Int. J. Coal Geol. 2004, 59, 153–169. [Google Scholar] [CrossRef]
- Seames, W. An initial study of the fine fragmentation fly ash particle mode generated during pulverized coal combustion. Fuel Process. Technol. 2003, 81, 109–125. [Google Scholar] [CrossRef]
- Coles, D.; Ragaini, R.; Ondov, J.; Fisher, G.; Silberman, D.; Prentice, B. Chemical studies of stack fly ash from a coal-fired power plant. Environ. Sci. Technol. 1979, 13, 455–459. [Google Scholar] [CrossRef]
- Hower, J. Petrographic examination of coal-combustion fly ash. Int. J. Coal Geol. 2013, 92, 90–97. [Google Scholar] [CrossRef]
- Davison, R.; Natusch, D.; Wallace, J.; Evans, C. Trace elements in fly ash. Dependence on concentration of particle size. Environ. Sci. Technol. 1974, 8, 1107–1113. [Google Scholar] [CrossRef]
- Galbreath, K.; Zygarlicke, C. Mercury speciation in coal combustion and gasification flue gases. Environ. Sci. Technol. 1996, 30, 2421–2426. [Google Scholar] [CrossRef]
- Resource Conservation and Recovery Act (Public Law 94-580). Code of Federal Regulations, Chapter 139, Title 42. 1980.
- Ruhl, L.; Vengosh, A.; Dwyer, G.; Hsu-Kim, H.; Deonarine, A. Environmental impacts of the coal ash spill in Kingston, Tennessee: An 18-month survey. Environ. Sci. Technol. 2010, 44, 9272–9278. [Google Scholar] [CrossRef]
- Talbot, R.; Anderson, M.; Andren, A. Qualitative model of heterogeneous equilibria in a fly ash pond. Environ. Sci. Technol. 1978, 12, 1057–1062. [Google Scholar]
- Dudas, M. Long-term leachability of selected elements from fly ash. Environ. Sci. Technol. 1981, 15, 840–843. [Google Scholar] [CrossRef]
- Wadge, A.; Hutton, M. The leachability and chemical speciations of selected trace elements in fly ash and coal combustion refuse incineration. Environ. Pollut. 1987, 48, 85–99. [Google Scholar] [CrossRef]
- Fernandez-Turiel, J.; de Carvalho, W.; Cabanas, M.; Querol, X.; Lopez-Soler, A. Mobility of heavy metals from coal fly ash. Environ. Geol. 1994, 23, 264–270. [Google Scholar]
- Querol, X.; Umana, J.; Alastuey, A.; Ayora, C.; Lopez-Soler, A.; Plana, F. Extraction of soluble major and trace elements from fly ash in open and closed leaching systems. Fuel 2001, 80, 801–813. [Google Scholar] [CrossRef]
- Nugteren, H.; Janssen-Jurkovicova, M.; Scarlett, B. Removal of heavy metals from fly ash and the impact on its quality. J. Chem. Technol. Biotechnol. 2002, 77, 389–395. [Google Scholar] [CrossRef]
- Smeda, A.; Zyrnicki, W. Application of sequential extraction and the ICP-AES method for study of the portioning of metals in fly ashes. Microchem. J. 2002, 72, 9–16. [Google Scholar] [CrossRef]
- Kim, A.; Kazonich, G. Relative solubility of cations in Class F fly ash. Environ. Sci. Technol. 2003, 37, 4507–4511. [Google Scholar] [CrossRef]
- Kim, A.; Kazonich, G. The silicate/non-silicate distribution of metals in fly ash and its effect on solubility. Fuel 2004, 83, 2285–2292. [Google Scholar] [CrossRef]
- Kim, A. The effect of alkalinity of Class F PC fly ash on metal release. Fuel 2006, 85, 1403–1410. [Google Scholar] [CrossRef]
- Kutchko, B.; Kim, A. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Jegadeesan, G.; Al-Abed, S.; Pinto, P. Influence of trace metal distribution on its leachability from coal fly ash. Fuel 2008, 87, 1887–1893. [Google Scholar] [CrossRef]
- Yang, C. Leaching characteristics of metals in fly ash from coal-fired power plant by sequential extraction procedure. Michrochim. Acta 2009, 165, 91–96. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review of the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Reddy, K.; Attili, V. Solubility and fractionation of different metals in fly ash of the Powder River Basin coal. Water Air Soil Pollut. 2011, 220, 327–337. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching behavior of elements from coal combustion fly ash: An overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef] [Green Version]
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef]
- Huijgen, W.; Comans, R. Carbon Dioxide Sequestration by Mineral Carbonation: Literature Review; Report ECN-C-03-016 for Energy Research Centre of The Netherlands: Petten, The Netherlands, 2003. [Google Scholar]
- Oelkers, E.; Gislason, S.; Juerg, M. Mineral carbonation of CO2. Elements 2008, 4, 333–337. [Google Scholar] [CrossRef]
- Reddy, K.J.; Lindsay, W.L.; Boyle, F.W.; Redente, E.F. Solubility relationships and mineral transformations associated with recarbonation of retorted oil shales. J. Environ. Qual. 1986, 15, 129–133. [Google Scholar]
- Reddy, K.; Drever, J.; Hasfurther, V. Effects of a CO2 pressure process on the solubilities of major and trace elements in oil shale solid wastes. Environ. Sci. Technol. 1991, 25, 1466–1469. [Google Scholar] [CrossRef]
- Tawfic, T.; Reddy, K.; Gloss, S. Reaction of CO2 with clean coal technology ash to reduce trace element mobility. Wate Air Soil Pollut. 1994, 84, 385–398. [Google Scholar] [CrossRef]
- Wee, J. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Reddy, K. Coal Fly Ash Chemistry and Carbon Dioxide Infusion Process to Enhance Its Utilization. In Biogeochemistry of Trace Elements in Coal and Coal Combustion Byproducts; Sajwan, K., Alva, A., Keefer, R., Eds.; Springer: New York, NY, USA, 1999; pp. 133–143. [Google Scholar]
- Renforth, P.; Washbourne, C.; Taylder, J.; Manning, D. Silicate production and availability for mineral carbonation. Environ. Sci. Technol. 2011, 45, 2035–2041. [Google Scholar] [CrossRef]
- Attili, V. Capture and Mineralization of Carbon Dioxide from Coal Combustion Flue Gas Emissions. Ph.D Thesis, University of Wyoming, Laramie, WY, USA, 2009. [Google Scholar]
- Reddy, K.; Weber, H.; Bhattacharyya, P.; Argyle, M.; Taylor, D.; Christensen, M.; Foulke, T.; Fahlsing, P. Instantaneous Capture and Mineralization of Flue Gas Carbon Dioxide: Pilot Scale Study. Available online: http://precedings.nature.com/documents/5404/version/1 (accessed on 17 March 2014).
- Reddy, K.; John, S.; Weber, H.; Argyle, M.; Bhattacharya, P.; Taylor, D.; Christensen, M.; Foulke, T.; Fahlsing, P. Simultaneous capture and mineralization of anthropogenic carbon dioxide (CO2). Energy Procedia 2010, 4, 1574–1583. [Google Scholar]
- Bhattacharyya, P.; Reddy, K. Effect of flue gas treatment on the solubility and fractionation of different metals in fly ash of Powder River Basin coal. Water Air Soil Pollut. 2012, 223, 4169–4181. [Google Scholar] [CrossRef]
- Tessier, A. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Warren, C.; Dudas, M. Formation of secondary minerals in artificially weather fly ash. J. Environ. Qual. 1985, 14, 405–410. [Google Scholar] [CrossRef]
- Medina, A.; Gamero, P.; Querol, X.; Moreno, N.; DeLeon, B.; Almanza, M.; Vargas, G.; Izquierdo, M.; Font, O. Fly ash from a Mexican mineral coal I: Mineralogical and chemical characterization. J. Hazard. Mater. 2010, 181, 82–90. [Google Scholar] [CrossRef]
- Reardon, E.; Czank, C.; Warren, C.; Dayal, R.; Johnston, H. Determining controls on elemental concentrations in fly ash leachate. Waste Manag. Res. 1995, 13, 435–450. [Google Scholar]
- Jones, D. The Leaching of Major and Trace Elements from Coal Fly Ash. In Environmental Aspects of Trace Elements in Coal; Swaine, D., Goodzari, F., Eds.; Springer: Berlin, Germany, 1995; Volume 2, pp. 221–262. [Google Scholar]
- Dudas, M.; Warren, C. Submicroscopic model of fly ash particles. Geoderma 1987, 40, 101–114. [Google Scholar] [CrossRef]
- Summers, C.; Dahlin, D.; Ochs, T. The Effect of SO2 on Mineral Carbonation in Batch Tests; DOE/ARC-2004-022; Office of Fossil Energy (FE), U.S. Department of Energy: Washington, DC, USA, 2004.
- Schramke, J. Neutralization of alkaline coal fly ash leachates by CO2(g). Appl. Geochem. 1992, 7, 481–492. [Google Scholar] [CrossRef]
- Tsuchiai, H.; Ishizuka, T.; Ueno, T.; Hattori, H.; Kita, H. Highly active absorbent for SO2 removal prepared from coal fly ash. Ind. Eng. Chem. Res. 1995, 34, 1404–1411. [Google Scholar] [CrossRef]
- Serre, S.; Silcox, G. Adsorption of elemental mercury on the residual carbon in coal fly ash. Ind. Eng. Chem. Res. 2000, 39, 1723–1730. [Google Scholar] [CrossRef]
- Dunham, G.; DeWall, R.; Senior, C. Fixed-bed studies of the interactions between mercury and coal combustion fly ash. Fuel Process. Technol. 2003, 82, 197–213. [Google Scholar] [CrossRef]
- Niksa, S.; Naoki, F. Predicting extents of mercury oxidation in coal-derived flue gases. J. Air Waste Manag. Assoc. 2005, 55, 930–939. [Google Scholar] [CrossRef]
- Gale, T.; Lani, B.; Offen, G. Mechanisms governing the fate of mercury in coal-fired power systems. Fuel Process. Technol. 2008, 89, 139–151. [Google Scholar] [CrossRef]
- Goodzari, F.; Hower, J. Classification of carbon in Canadian fly ashes and their implications in the capture of mercury. Fuel 2008, 81, 1949–1957. [Google Scholar]
- Shah, P.; Strezov, V.; Prince, K.; Nelson, P. Speciation of As, Cr, Se, and Hg under coal fired power station conditions. Fuel 2008, 87, 1859–1869. [Google Scholar] [CrossRef]
- Hower, J.; Senior, C.; Suuberg, E.; Hurt, R.; Wilcox, J.; Olson, E. Mercury capture by native fly ash carbons in coal-fired power plants. Prog. Energy Combust. Sci. 2010, 36, 510–529. [Google Scholar] [CrossRef]
- Noel, J.; Biswas, P.; Giammar, D. Evaluation of a sequential extraction process used for determining mercury binding mechanisms to coal combustion byproducts. J. Air Waste Manag. Assoc. 2007, 57, 856–867. [Google Scholar] [CrossRef]
- Narukawa, T.; Takatsu, C.; Riley, K.; French, D. Investigation on chemical species of arsenic, selenium and antimony in fly ash from coal fuel thermal power stations. J. Environ. Monit. 2005, 7, 1342–1348. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, A.; VanGerven, T.; Vandecasteele, C. Leaching mechanism of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
- Rai, D.; Eary, L.; Zachara, J. Environmental chemistry of chromium. Sci. Total Environ. 1989, 86, 15–23. [Google Scholar]
- Meima, J.; Comans, N. The leaching of trace elements from municipal solid waste incinerator bottom ash at different stages of weathering. Appl. Geochem. 1999, 14, 159–171. [Google Scholar] [CrossRef]
- Meima, J.; van der Weijden, R.; Eighmy, T.; Comans, R. Carbonation processes in municipal solid waste incinerator bottom ash and their effect on the leaching of copper and molybdenum. Appl. Geochem. 2002, 17, 1503–1513. [Google Scholar] [CrossRef]
- Meima, J.; Comans, N. Application of surface complexation/precipitation modeling to contaminant leaching from weathered solid waste incinerator bottom ash. Environ. Sci. Technol. 1998, 32, 688–693. [Google Scholar] [CrossRef]
- Zachara, J.; Kittrick, J.; Harsh, J. The mechanism of Zn2+ adsorption on calcite. Geochem. Cosmochim. Acta 1988, 52, 2281–2291. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reynolds, B.; Reddy, K.J.; Argyle, M.D. Field Application of Accelerated Mineral Carbonation. Minerals 2014, 4, 191-207. https://doi.org/10.3390/min4020191
Reynolds B, Reddy KJ, Argyle MD. Field Application of Accelerated Mineral Carbonation. Minerals. 2014; 4(2):191-207. https://doi.org/10.3390/min4020191
Chicago/Turabian StyleReynolds, Brandon, K. J. Reddy, and Morris D. Argyle. 2014. "Field Application of Accelerated Mineral Carbonation" Minerals 4, no. 2: 191-207. https://doi.org/10.3390/min4020191







