3.1. Bulk Mineralogy and Geochemistry
Calcite was observed in samples from experiments containing marble-hornfels and exoskarn waste rock (
Table 2). The calcite content of the marble-hornfels, which generally occurs at the margins of the deposit, was 67 wt % for FC-1-3A and 53 wt % for Pile 1. Calcite contents for experimental samples containing exoskarn were 9.1 wt % (FC-3-2A) and 3.0 wt % (Pile 3). Calcite was not detected by powder XRD analysis of intrusive rock samples (FC-2-2B, FC-2-3A and Pile 2); however, this acid-neutralizing phase is present in intrusive rocks from the Antamina deposit. The pH of drainage emanating from experimental piles and field cells containing marble-hornfels or exoskarn ranged from 7.3 to 7.9 between July 2008 and June 2009 (
Table 1). Calcite was detected as a primary component of the mineral assemblage in these samples, and the observed pH values are indicative of carbonate-mineral dissolution.
Table 2.
Results of mineralogical investigation using X-ray diffraction (XRD) and scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS). Minerals detected by XRD are reported in wt % determined during Rietveld refinement.
Table 2.
Results of mineralogical investigation using X-ray diffraction (XRD) and scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDS). Minerals detected by XRD are reported in wt % determined during Rietveld refinement.
| Lithologic Unit | Experimental ID | Mineral Abundance (wt %) |
|---|
| Cal | Py | Ccp | Sp | Mo | Gn | Apy | Po |
|---|
| Marble-Hornfels | Pile 1 | 53 | 0.8 | | 0.7 | 0.3 | | | |
| FC-1-3A | 67 | 1.4 | | | * | 0.2 | | |
| Intrusive | Pile 2 | | 0.3 | 0.9 | | <0.1 | | | |
| FC-2-2B | | 0.5 | 0.8 | | | | * | |
| FC-2-3A | | 1.7 | 2.6 | | 0.2 | | | * |
| Exoskarn | Pile 3 | 3.0 | 13.0 | 3.7 | 1.1 | | | | |
| FC-3-2A | 9.1 | 8.6 | 0.9 | 5.1 | | | | |
Sulfide minerals were detected in waste rock from all experiments; however, samples of exoskarn consistently exhibited the greatest sulfide-mineral content. Pyrite was the most abundant sulfide mineral in the exoskarn (Pile 3, FC-3-2A) and marble-hornfels (Pile 1, FC-1-3A) rock types, while chalcopyrite was the most abundant sulfide mineral in the intrusive (Pile 2, FC-2-2B, FC-2-3A) waste rock. In addition to chalcopyrite, samples from experiments that contained intrusive waste rock also contained pyrite (0.3 to 1.7 wt %) and molybdenite (≤0.2 wt %). Subsequent examination of these samples by SEM-EDS confirmed the presence of arsenopyrite (FC-2-2B) and pyrrhotite (FC-2-3A).
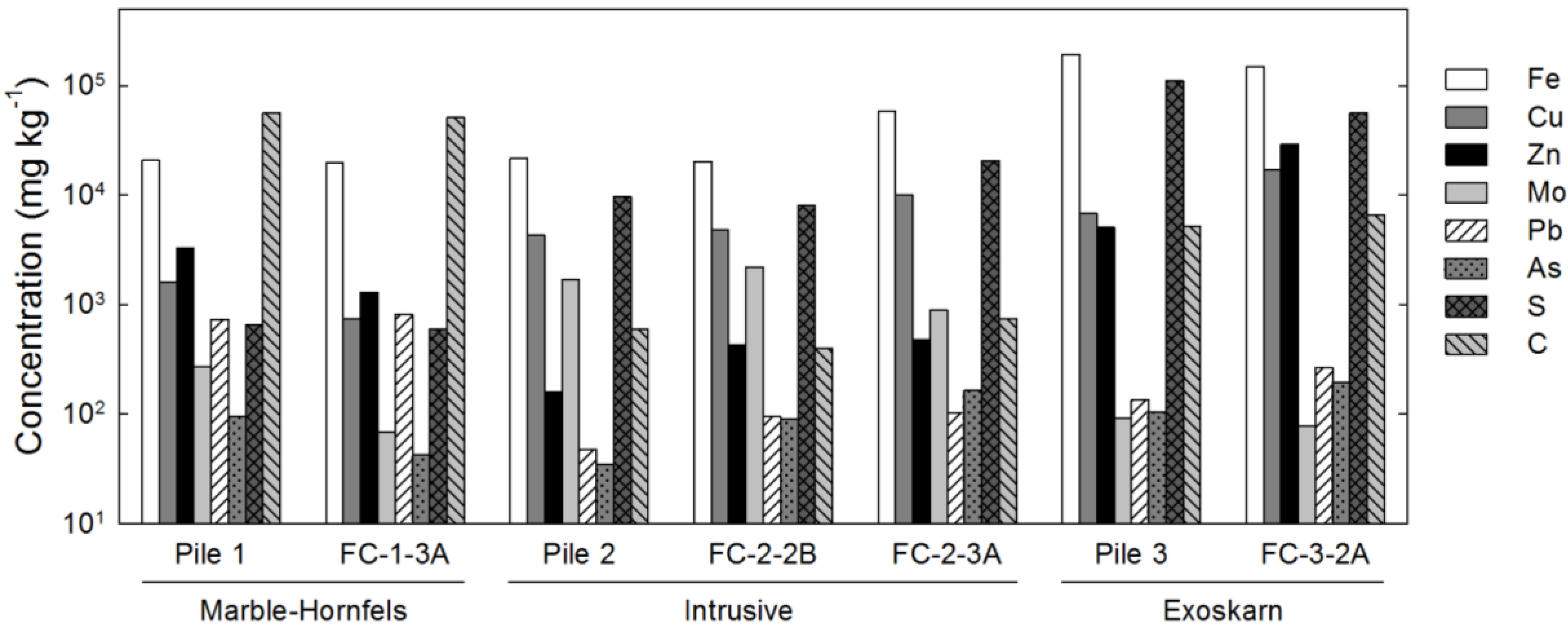
The bulk geochemistry of the samples largely reflected the mineral assemblage of the waste rock (
Figure 1). Concentrations of Fe, Cu and Zn generally corresponded to the relative abundance of pyrite, chalcopyrite and sphalerite, respectively. Low concentrations of Mo and Pb were detected in samples where molybdenite and galena were not detected by XRD, suggesting these phases were present in low amounts. Furthermore, trace concentrations of As were observed in waste rock samples, which is indicative of the presence of arsenopyrite or other As-bearing minerals at low abundance in these waste rock types. Total-S contents generally corresponded to total sulfide-mineral abundance, whereas elevated total-C concentrations were observed for samples where calcite was identified by XRD. Low total-C concentrations also were detected in samples of intrusive waste rock (Pile 2, FC-2-2B, FC-2-3A) where calcite could not be detected in XRD patterns.
Figure 1.
Whole-rock analyses by X-ray fluorescence (XRF) (Fe, Cu, Zn, Mo, Pb and As) or combustion-infrared detection (S and C) for waste rock samples collected in February 2009.
Figure 1.
Whole-rock analyses by X-ray fluorescence (XRF) (Fe, Cu, Zn, Mo, Pb and As) or combustion-infrared detection (S and C) for waste rock samples collected in February 2009.
3.2. Microbiology
Populations of neutrophilic thiosulfate oxidizers were clearly abundant in all piles; exceeding a million per gram, with populations ranging from 3.6 × 10
6 to 2.2 × 10
8 MPN∙g
−1 of washed sediment (
Figure 2). Growth of acidophiles was observed only for samples of intrusive waste rock collected from Pile 2 and FC-2-3A. Elevated populations of acidophilic iron oxidizers (6.5 × 10
6 MPN∙g
−1) and sulfur oxidizers (1.1 × 10
7 MPN∙g
−1) were associated with the field cell, while only acidophilic iron oxidizers (4.9 × 10
1 MPN∙g
−1) were successfully cultured from Pile 2. Cultured acidophiles were capable of oxidizing both Fe and S, which is indicative of
Acidithiobacillus ferrooxidans as opposed to other commonly-identified acidophiles (e.g.,
Acidithiobacillus thiooxidans,
Leptospirillum ferrooxidans).
Total bacterial populations estimated from direct counts of live and dead bacteria viability ranged from 1.0 × 10
7 to 3.4 × 10
8 bacteria g
−1 (
Figure 2). In five of the seven samples the bacteria were >75% viable, indicating that the microbial populations were likely active within the experimental waste rock piles and field cells. The two samples with highest populations as measured by both Live/Dead Baclight™ and MPN techniques (
Figure 2) also exhibited the lowest proportion of living bacteria (Pile 3, FC-3-2A). This difference may be indicative of geochemical stress resulting from biological activity in the exoskarn waste rock, which exhibited the greatest sulfide-mineral and metal contents, and an average annual pH of 7.4. The total count value can be less than the cultured count when you consider that bacteria occurring “underneath” minerals are not counted; therefore, the microscopic counts are underestimates of the total number of bacteria.
Figure 2.
Bacterial enumerations performed on waste rock samples collected in February 2009. Enumerations of total and live bacteria performed using Live/Dead Baclight™. Culture-based enumeration of Thiobacillus spp., e.g., A. thiooxidans and A. ferrooxidans, was performed using the most probable number (MPN) technique.
Figure 2.
Bacterial enumerations performed on waste rock samples collected in February 2009. Enumerations of total and live bacteria performed using Live/Dead Baclight™. Culture-based enumeration of Thiobacillus spp., e.g., A. thiooxidans and A. ferrooxidans, was performed using the most probable number (MPN) technique.
Fluorescent
in situ hybridization (FISH) imaging of samples revealed the presence of bacterial cells that hybridized with the genus-specific probes for
Acidithiobacillus sp. (Thio820) in four of the seven samples (Piles 2 and 3, FC-2-3A, and FC-3-2A). The highest proportion of cells stained with both Thio820 and DAPI were observed in samples from Pile 3. Three bacterial cell morphologies were observed; slightly curved rods, highly curved rods, and filamentous bacteria. The most abundant cell morphology was the slightly curved rod, which was commonly observed during FE-SEM-EDS and the Live/Dead Baclight™ microscopy. This bacterial morphology is characteristic of
Acidithiobacillus sp. and was observed in samples from Pile 2 and FC-2-3A. Less abundant were highly curved rod-shaped bacteria similar in morphology to
Leptospirillum spp. [
26] observed in sample FC-2-3A. Filamentous bacteria were observed in samples FC-2-2B, FC-3-2A, FC-1-3A, and Pile 3 during Live/Dead Baclight™ procedure. These filamentous bacteria are similar to
Thermothrix thiopara, which is capable of thiosulfate oxidation at circumneutral pH [
27]. Filamentous bacteria were not observed during FE-SEM imaging.
Although waste rock from Pile 1 and FC-1-3A (marble-hornfels) had been subjected to the longest period of oxidation, colonization by acidophiles was not apparent from MPN enumerations. Electron microscopy (FE-SEM) revealed a fine-grained secondary precipitate had accumulated within Pile 1; however, bacterial cells were not observed. Cumulative sulfate mass discharges were lowest for the marble-hornfels waste rock when comparing results among corresponding experiments. Although colonization by acidophiles was not observed, the presence of Zn and Cu in drainage from FC-1-3A is indicative of ferric leaching of sphalerite and chalcopyrite (Reactions (1)–(3)). Limited discharge of these metals from Pile 2 is attributed to secondary controls (
i.e., co-precipitation, sorption) on their mobility within the experimental waste rock piles. Despite the higher pyrite content of the exoskarn waste rock, microbial communities in samples from Pile 3 and FC-3-2A also were dominated by neutrophilic
Thiobacillus spp. at similar populations to those observed for the marble-hornfels waste rock. Calcite comprised a smaller component of the exoskarn mineral assemblage compared to that of marble-hornfels; however, this waste rock type exhibited the highest pyrite content (
Table 2). The presence of bacteria associated with a thin filamentous film at the grain margin suggests involvement in sulfide-mineral oxidation. However, limited biofilm development and a general lack of secondary Fe(III) precipitates suggests that growth on
A. ferrooxidans or other acidophilic bacteria were pH limited. Drainage from the experiments containing exoskarn also exhibited circumneutral average pH values of 7.5 (Pile 3) and 7.3 (FC-3-2A). Nonetheless, cumulative mass discharges for sulfate and zinc were the highest compared among experimental piles or field cells. The relatively rapid colonization of intrusive waste rock type by acidophiles, combined with a general lack of acidophiles in marble-hornfels and exoskarn waste rock, indicate that carbonate-mineral content and bulk pH controlled the colonization of sulfide-minerals by acidophilic bacteria.
In addition to
Thiobacillus spp., microbial communities within samples from Pile 2 and FC-2-3A (intrusive) included viable populations of acidophilic iron oxidizers. The largest population of acidophilic iron oxidizers was found in FC-2-3A, which also was characterized by an elevated population of acidophilic sulfur oxidizers and an average annual drainage pH of 6.2. These bacteria appear to have colonized the sulfide mineral surface initially by direct attachment, cementation and formation of an acidic expansion front (Pile 2;
Figure 3), as proposed by Mielke
et al. [
18]. The extensive occurrence of microbially-colonized Fe(III) (oxy)hydroxysulfate, e.g., jarosite (KFe
3(OH)
6(SO
4)
2) and Fe(III) (oxy)hydroxide phases within FC-2-3A suggests that formation of these precipitates may support growth of acidophilic bacteria in NRD systems following the initial colonization stage (below). The occurrence of acidophiles in Pile 2 and FC-2-3A is attributed to the low calcite content of the intrusive waste rock, which may facilitate the rapid development of an acidic layer at sulfide-mineral surfaces. Cumulative mass discharges for sulfate were similar between intrusive and marble-hornfels waste rock for a given experiment (
i.e., experimental piles or field cells); however, precipitation of gypsum or other sulfate phases may complicate relative comparisons of sulfate discharge among waste rock types.
3.3. Mineralogy and X-ray Absorption Spectroscopy
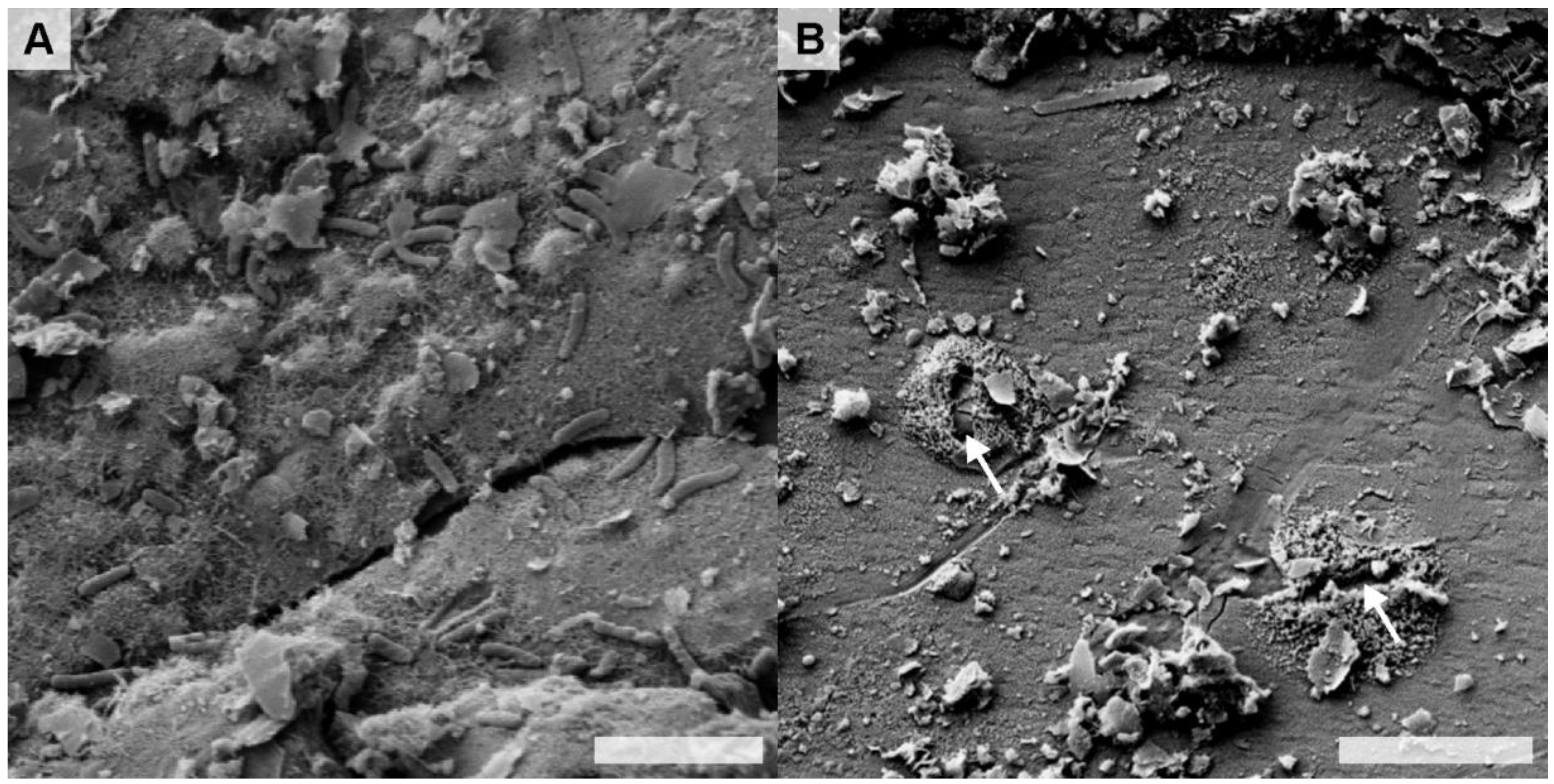
Weathered sulfide minerals from the three waste rock piles, and one field cell FC-2-3A that exhibited elevated populations of acidophilic bacteria, were selected for detailed examination by FE-SEM-EDS. Bacterial cells were generally sparse on samples from the field cells; however, surfaces of weathered pyrite grains from Pile 2 were colonized by bacteria (
Figure 3A). For Pile 2, dissolution pits surrounded by secondary minerals were observed in the absence of bacterial cells at the surface of a chalcopyrite grain (
Figure 3B). The occurrence of secondary precipitates surrounding the dissolution pit is indicative of bacterial attachment, which has been associated with localized precipitation of Fe(III) phases at cell margins and on EPS deposits [
18,
28,
29]. Despite exhibiting the highest MPN population of neutrophilic thiosulfate oxidizers, relatively few bacterial cells were observed at chalcopyrite or pyrite surfaces in samples collected from Pile 3 (data not shown).
Figure 3.
Field emission-scanning electron microscopy secondary electron (FE-SEM SE) micrographs of: (A) bacterially-colonized secondary Fe(III) (oxy)hydroxysulfate precipitates at the sulfide-mineral surface in FC-2-3A (EDS analysis indicated the presence of Fe, S and O—data not shown); and (B) relic direct attachment pits at the surface of a chalcophyrite grain in Pile 2. Scale bars equal 3 µm.
Figure 3.
Field emission-scanning electron microscopy secondary electron (FE-SEM SE) micrographs of: (A) bacterially-colonized secondary Fe(III) (oxy)hydroxysulfate precipitates at the sulfide-mineral surface in FC-2-3A (EDS analysis indicated the presence of Fe, S and O—data not shown); and (B) relic direct attachment pits at the surface of a chalcophyrite grain in Pile 2. Scale bars equal 3 µm.
A macroscopic sulfide clast comprised of chalcopyrite, pyrrhotite, sphalerite and pyrite (based on EDS analyses), and characterized by the presence of extensive weathering products was selected from FC-2-3A for detailed analysis. Imaging of the weathered clast by FE-SEM revealed extensive secondary mineral-precipitation and biofilm formation at mineral surfaces. Bacterial cells were largely associated with porous precipitate localized at surfaces of weathered pyrite and pyrrhotite within the clast (
Figure 4,
Figure 5 and
Figure 6). The highest density of bacterial cells was observed within this porous layer rather than on the underlying mineral surfaces (
Figure 4). This secondary-precipitate layer exhibited the fibrous morphology characteristic of schwertmannite, and was commonly bound between the sulfide-mineral surface and a less porous, smooth precipitate crust (
Figure 4A). The mineralogical composition of this secondary Fe(III)-bearing coating could not be distinguished from primary phases in powder XRD analysis of liberated grains. However, LCF of bulk Fe K-edge EXAFS spectra (
Χ2 = 0.44) was achieved by fitting pyrite, schwertmannite [Fe
8O
8(OH)
6–4.5(SO
4)
1–1.75], lepidocrocite [γ-FeO(OH)] and K-jarosite [KFe
3(OH)
6(SO
4)
2] (
Figure 5). The fitted ratio of schwertmannite to lepidocrocite to K-jarosite was found to be 4.2:1.6:1, indicating that schwertmannite was the most abundant secondary Fe-bearing precipitate within the secondary mineral coating on this weathered sulfide grain. Pitting of the pyrrhotite surface was observed for a location where the schwertmannite had become detached, whereas pitting was limited to the adjacent pyrrhotite surface (
Figure 4D). This observation suggests that the Fe incorporated into schwertmannite is derived from dissolution of the underlying sulfide-mineral surface.
Figure 4.
FE-SEM SE micrographs of: (A) a exposed region of porous schwertmannite covered by a thin, non-porous crust; (B) bacteria within the porous schwertmannite layer observed at higher magnification; (C) bacterially-colonized porous schwertmannite beneath the thin, non-porous crust; and (D) an area of bacterially-colonized schwertmannite that separated from sulfide-mineral surface revealing extensive pitting. Scale bars equal 10 µm, 1 µm, 1 µm and 1 µm, respectively.
Figure 4.
FE-SEM SE micrographs of: (A) a exposed region of porous schwertmannite covered by a thin, non-porous crust; (B) bacteria within the porous schwertmannite layer observed at higher magnification; (C) bacterially-colonized porous schwertmannite beneath the thin, non-porous crust; and (D) an area of bacterially-colonized schwertmannite that separated from sulfide-mineral surface revealing extensive pitting. Scale bars equal 10 µm, 1 µm, 1 µm and 1 µm, respectively.
Figure 5.
FE-SEM SE micrograph of microbially-colonized, secondary Fe-bearing mineral coating on surface of a sulfide-mineral clast from intrusive waste rock (FC-2-3A). Scale bar equals 5 µm. Corresponding bulk Fe K-edge extended X-ray absorption fine structure (EXAFS) spectra (solid line) and linear combination fitting (LCF) fit (circles) of sulfide-mineral weathering products.
Figure 5.
FE-SEM SE micrograph of microbially-colonized, secondary Fe-bearing mineral coating on surface of a sulfide-mineral clast from intrusive waste rock (FC-2-3A). Scale bar equals 5 µm. Corresponding bulk Fe K-edge extended X-ray absorption fine structure (EXAFS) spectra (solid line) and linear combination fitting (LCF) fit (circles) of sulfide-mineral weathering products.
A transect measuring ~30 µm long was FIB milled through the schwertmannite-rich layer near an exposed pyrrhotite surface to facilitate FE-SEM-EDS examination across this layer (
Figure 6). Three distinct layers exhibiting different chemical composition and texture were observed in SE images showing this cross-section (
Figure 6D). The upper layer was composed of an Fe–S–O precipitate containing minor amounts of Si. The molar Fe:S ratio of this layer was 8:2.4, which is similar to the 8:(1–1.75) molar ratio that is characteristic of schwertmannite. Slight variation from the ideal molar ratio may result from the precipitation of sulfate phases during sample drying. A lighter-colored intermediate Fe–O–Si layer contained ~20% (molar) Si and trace concentrations of Al content. A distinct transition from the middle Fe–O–Si layer to a lighter colored Fe–O layer was observed, which was comprised solely of Fe and O. The porosity of the upper schwertmannite layer was estimated to be 8.2% based on two dimensional binary image analysis of the SE image. Approximately 50% of this porosity was associated with a large void located in the upper left quadrant of the image (
Figure 6D,E). The porosity of the upper schwertmannite layer, which was poorly preserved during FIB milling, is likely much greater at zones more distal to the primary sulfide-mineral surface.
Poorly ordered ferrihydrite [nominally Fe
2O
3∙½H
2O] and schwertmannite generally are the first Fe(III) phases to precipitate during oxidation of iron-sulfide minerals [
30,
31]. These minerals are metastable with respect to more crystalline and less soluble Fe(III) (oxy)hydroxides, and will transform to goethite [αFeOOH] at circumneutral pH with lepidocrocite [γFeOOH] commonly forming as an intermediate phase [
30,
32,
33,
34,
35]. Under low-pH conditions (
i.e., pH ≤ 3), these Fe(III) (oxy)hydroxides can transform to jarosite in the presence of monovalent cations [
30,
36,
37]. Due to the nano-crystalline nature schwertmannite, and the geochemical conditions under which this phase forms, schwertmannite has been commonly identified as the predominant Fe precipitate produced by acidophilic iron oxidizing bacteria [
36,
38,
39,
40,
41]. Dissolved Fe concentration in drainage from the experimental piles and field cells generally remained below the method detection limit (0.001 mg∙L
−1) due to the low solubility of Fe(III) in circumneutral pH waters. This result precluded geochemical equilibrium modeling of associated mineral saturation indices.
Figure 6.
FE-SEM SE micrographs of: (A) an overview of the surface of a bacterially-colonized, weathered pyrrhotite grain with areas selected for higher magnification imaging; (B) a bacterially-colonized layer of porous schwertmannite and adjacent pyrrhotite surface exhibiting no direct bacterial attachment; (C) a mineralised biofilm surrounded by porous schwertmannite with non-porous crust present at the top of the image; (D) three different layers of iron oxides exposed by the focused ion beam (FIB) cut; and (E) mineralized bacteria (arrows) within the micrometer-scale porous structure located approximately 5 µm below the outer margin of the schwertmannite layer. Scale bars equal 50 µm, 5 µm, 2 µm, 5 µm and 0.5 µm, respectively.
Figure 6.
FE-SEM SE micrographs of: (A) an overview of the surface of a bacterially-colonized, weathered pyrrhotite grain with areas selected for higher magnification imaging; (B) a bacterially-colonized layer of porous schwertmannite and adjacent pyrrhotite surface exhibiting no direct bacterial attachment; (C) a mineralised biofilm surrounded by porous schwertmannite with non-porous crust present at the top of the image; (D) three different layers of iron oxides exposed by the focused ion beam (FIB) cut; and (E) mineralized bacteria (arrows) within the micrometer-scale porous structure located approximately 5 µm below the outer margin of the schwertmannite layer. Scale bars equal 50 µm, 5 µm, 2 µm, 5 µm and 0.5 µm, respectively.
The biogeochemical conditions under which ferrihydrite or schwertmannite will precipitate remains somewhat uncertain [
42]. Thermodynamic-based predictions indicate that ferrihydrite precipitation is favored at circumneutral and alkaline pH, whereas schwertmannite precipitation is favored under circumneutral to acidic pH conditions [
33,
37,
38]. The average pH of drainage from FC-2-3A initially was mildly alkaline (pH > 8), but decreased to 6.2–6.5 after approximately 3 months. Under bulk circumneutral pH conditions observed in this field cell, the precipitation of schwertmannite may be thermodynamically favored in the presence of sulfate [
42]. Iron K-edge EXAFS spectra indicated that schwertmannite was the dominant Fe-bearing precipitate on the weathered sulfide clast from FC-2-3A (intrusive), while lesser amounts of K-jarosite and lepidocrocite were present. Bigham
et al. [
33] found that schwertmannite was the predominant Fe(III) phase in acid sulfate waters (
n = 28) at pH 2.8–4.5, whereas K-jarosite dominated at pH < 2.5 and mixtures of ferrihydrite and goethite occurred at pH > 6.5. The presence of both schwertmannite and K-jarosite on this weathered sulfide clast is indicative of the development of an acidic microenvironment at the sulfide mineral surface,
i.e., is an important habitat, in this intrusive waste rock (FC-2-3A), which at the time of sample collection was characterized by bulk NRD conditions.
3.4. Bacteria-Mineral Interactions
Examination of sulfides using FE-SEM revealed that bacteria in the FC-2-3A intrusive sample were commonly associated with schwertmannite at the weathered sulfide-mineral surface (
Figure 3C,
Figure 4,
Figure 5 and
Figure 6). These bacteria occurred within the porous structure of schwertmannite, which was often covered with a thin, non-porous crust (
Figure 5A,C). Bacteria were also found in porous schwertmannite on the fringe of the crusted region (
Figure 4C), and buried within schwertmannite between the crust and the sulfide mineral surface (
Figure 6D,E). The crust formed only in regions with thick schwertmannite layers, suggesting that changes in biogeochemical conditions at greater distance from the sulfide-mineral surface may not favor schwertmannite precipitation. The abundance of bacteria within the porous schwertmannite layer indicates that precipitation of this mineral phase is an important microbial habitat. This layer has potential to provide isolation from bulk geochemical conditions within the waste rock, thereby facilitating the development of an acidic microenvironment.
Intuitively, the porosity within the schwertmannite must be amenable to microbial growth. Sulfide surfaces covered with the non-porous lower two layers exposed in the FIB transect (
Figure 6D) demonstrate that sulfides may quickly become armored. However, the oxidation of sulfide minerals coated by porous schwertmannite may be subjected to ongoing oxidation due to the diffusive transport of oxidants through this layer. If sulfide mineral surfaces continue to be oxidized beneath the porous schwertmannite, acidic conditions could persist within the pore space of the schwertmannite. This mechanism was inferred where a schwertmannite layer became detached from the underlying pyrrhotite surface, exposing dissolution pitting uncharacteristic of bacterial attachment [
18,
19,
28,
43]. The occurrence of K-jarosite in FC-2-3A reflects the presence of acidic conditions at the sulfide-mineral surfaces, and indicates that Fe(III) contributes to sulfide-mineral oxidation in this NRD system.
The direct attachment model is often used to explain the colonization of acidophilic bacteria in circumneutral pH environments [
18,
44]. This model describes the development of acidic microenvironment at the bacteria-mineral interface, where increased Fe(III) solubility facilitates Fe redox cycling coupled with sulfide oxidation [
18]). Relic direct attachment sites (
i.e., pits) on the chalcopyrite surface in Pile 2 (
Figure 2B) were surrounded by a secondary Fe(III) precipitate, presumably reflecting direct attachment by acidophiles. The secondary Fe(III) precipitate associated with the attachment sites exhibited a tubular structure similar to schwertmannite, which is in distinct contrast to precipitates not associated with attachment sites. Acidophilic iron oxidizing bacteria can promote the precipitation of Fe(III) (oxy)hydroxide and Fe(III) (oxy)hydroxysulfate minerals on or near the cell surface [
29]). The close association of attachment sites and secondary Fe(III) precipitates, combined with limited pitting in surrounding areas of the chalcopyrite surface, suggest that acidophilic bacteria were facilitating sulfide-mineral oxidation via Fe(III) generation. Acidophilic iron oxidizing bacteria were not abundant, but viable populations were cultured from Pile 2 (intrusive), where these relic direct attachment sites were observed. The expansion of acidic microenvironments from initial attachment sites appears to have been facilitated through precipitation of schwertmannite and K-jarosite, which supported the larger populations of acidophilic bacteria observed in FC-2-3A.