3.1. Djurleite-Bearing Synthetic Gold Ore
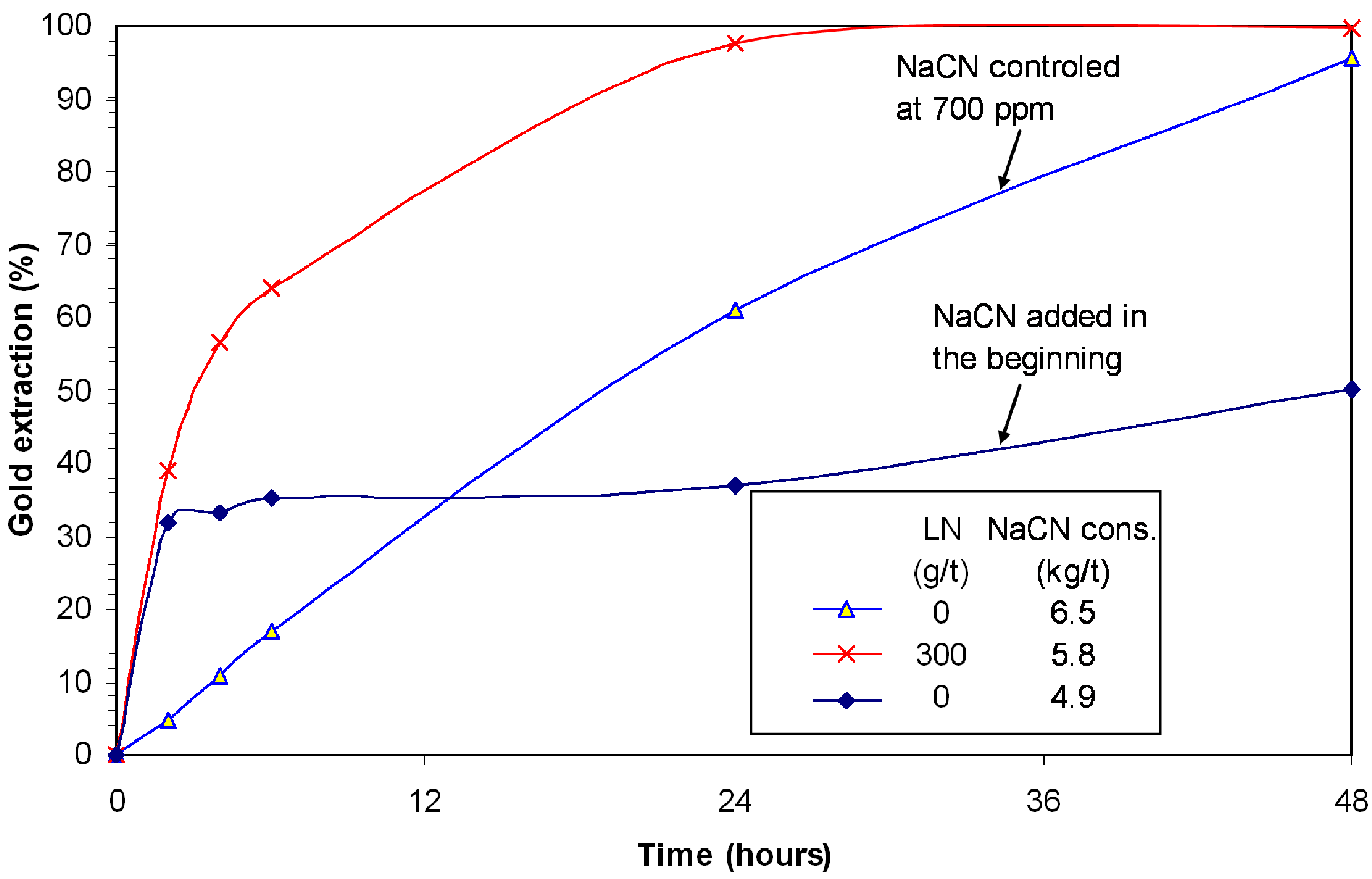
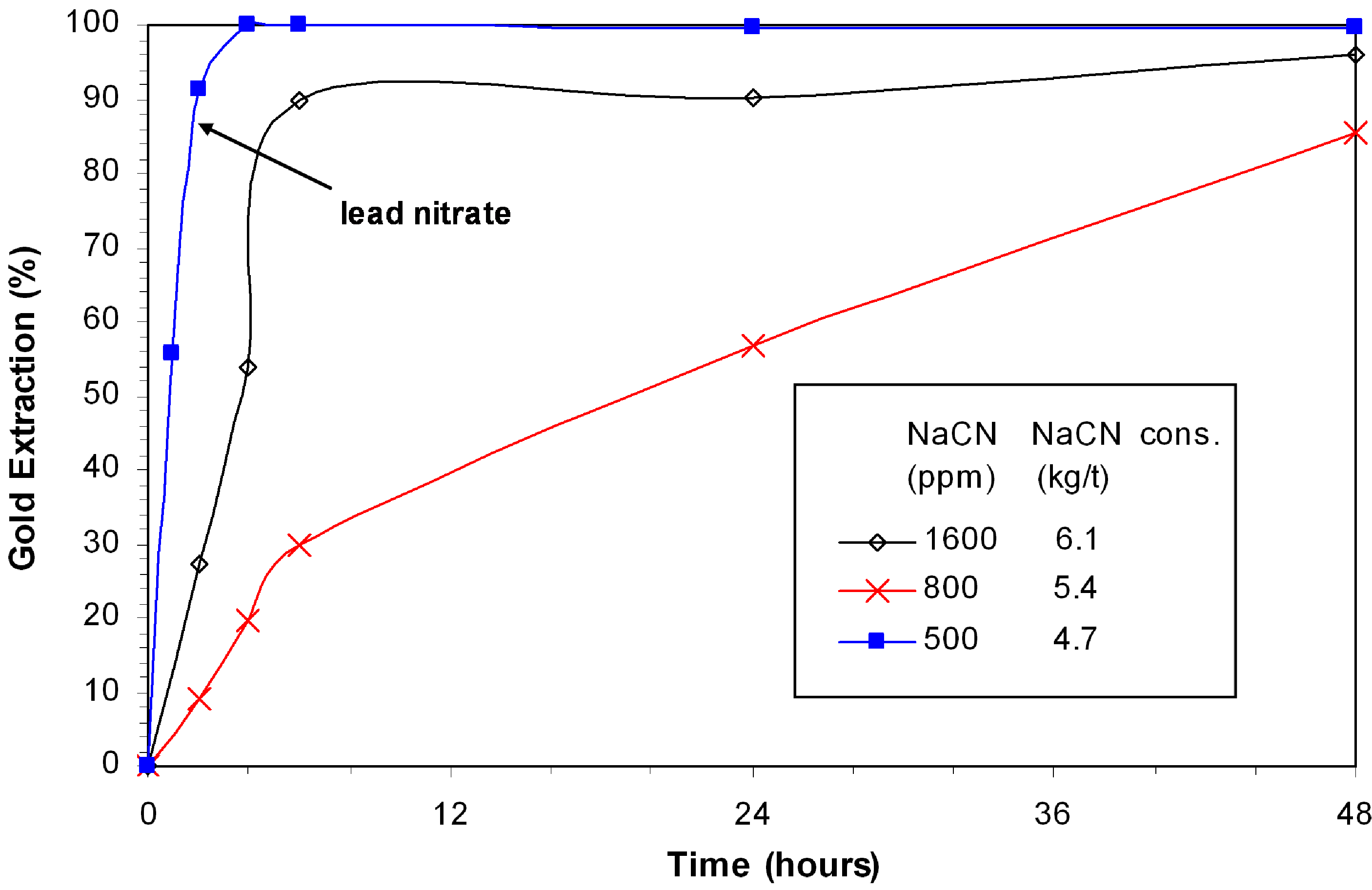
Figure 1 illustrates the gold leaching kinetics in the presence of djurleite. With 6.1 kg/t NaCN added at the start of the experiment, the leaching curve reaches a plateau after two hours due to passivation of gold grains. The gold extraction slowly increases to a maximum of 51% in 48 h. The leaching curve has a totally different profile with the control of free cyanide at 700 ppm NaCN (linear for the first 24 h). Under these conditions, the curve is almost linear. This profile is also an indication of passivation by sulphide, but the passivation is less severe. The overall gold extraction reached 95.8% in 48 h. The addition of lead nitrate alleviated the passivation and enhanced the gold leaching kinetics. Over 95% of gold was leached within 24 h and the extraction was complete (99.7%) in 48 h. The cyanide consumption was reduced from 6.5 to 5.8 kg/t due to lead nitrate addition.
Figure 1.
Effect of NaCN concentration and lead nitrate on gold extraction in presence of djurleite; 0.3% Cu, pH 11, 8 ppm DO.
Figure 1.
Effect of NaCN concentration and lead nitrate on gold extraction in presence of djurleite; 0.3% Cu, pH 11, 8 ppm DO.
The dissolution of djurleite in a cyanide solution (using chalcocite as a proxy for djurleite) may be expressed as follow:
An XPS analysis of the gold surface identified various compounds (Cu/Ag oxides, Cu(I)cyanide and sulphide). By adding lead nitrate, the amount of Cu(I)cyanide was reduced. In addition, the sulphide disappeared and lead oxide and hydroxide precipitated. These findings are in line with previous experimental results identifying lead compounds at the surface of gold [
10]. The formation of these compounds reduced the passivation of gold.
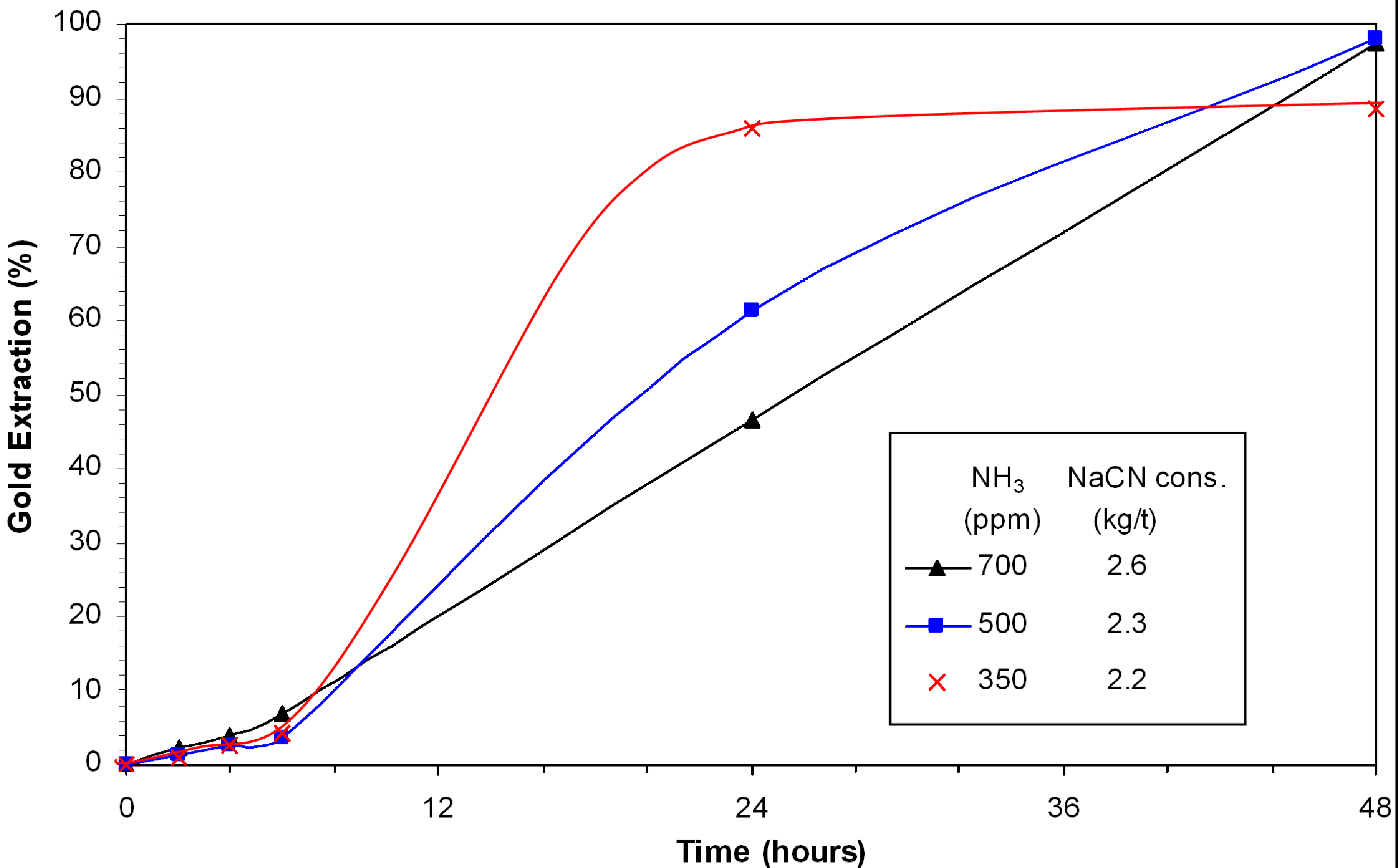
The effect of ammonia was investigated using 350 ppm, 500 ppm and 700 ppm ammonia with 400 ppm NaCN. The leaching rate in the final six hours remained low under all conditions (
Figure 2). The fastest leaching rate was achieved with 350 ppm ammonia. However, the passivation of gold occurred after 24 h to level the extraction of gold at 88.5%. High concentration of ammonia is detrimental for gold extraction after 24 h. With 500 ppm and 700 ppm NH
3, the gold extraction approached completion (>99%) within 48 hours. The associated cyanide consumptions were 2.3 and 2.6 kg/t NaCN. The copper concentration did not vary much and remained in the range of 115 ppm. The results demonstrate that ammonia concentration is critical for producing a high overall gold extraction.
Figure 2.
Effect of NaCN:NH3 ratio on gold leaching in presence of djurleite; 0.3% Cu, 400 ppm NaCN, pH 11.5–11.8, 8 ppm DO.
Figure 2.
Effect of NaCN:NH3 ratio on gold leaching in presence of djurleite; 0.3% Cu, 400 ppm NaCN, pH 11.5–11.8, 8 ppm DO.
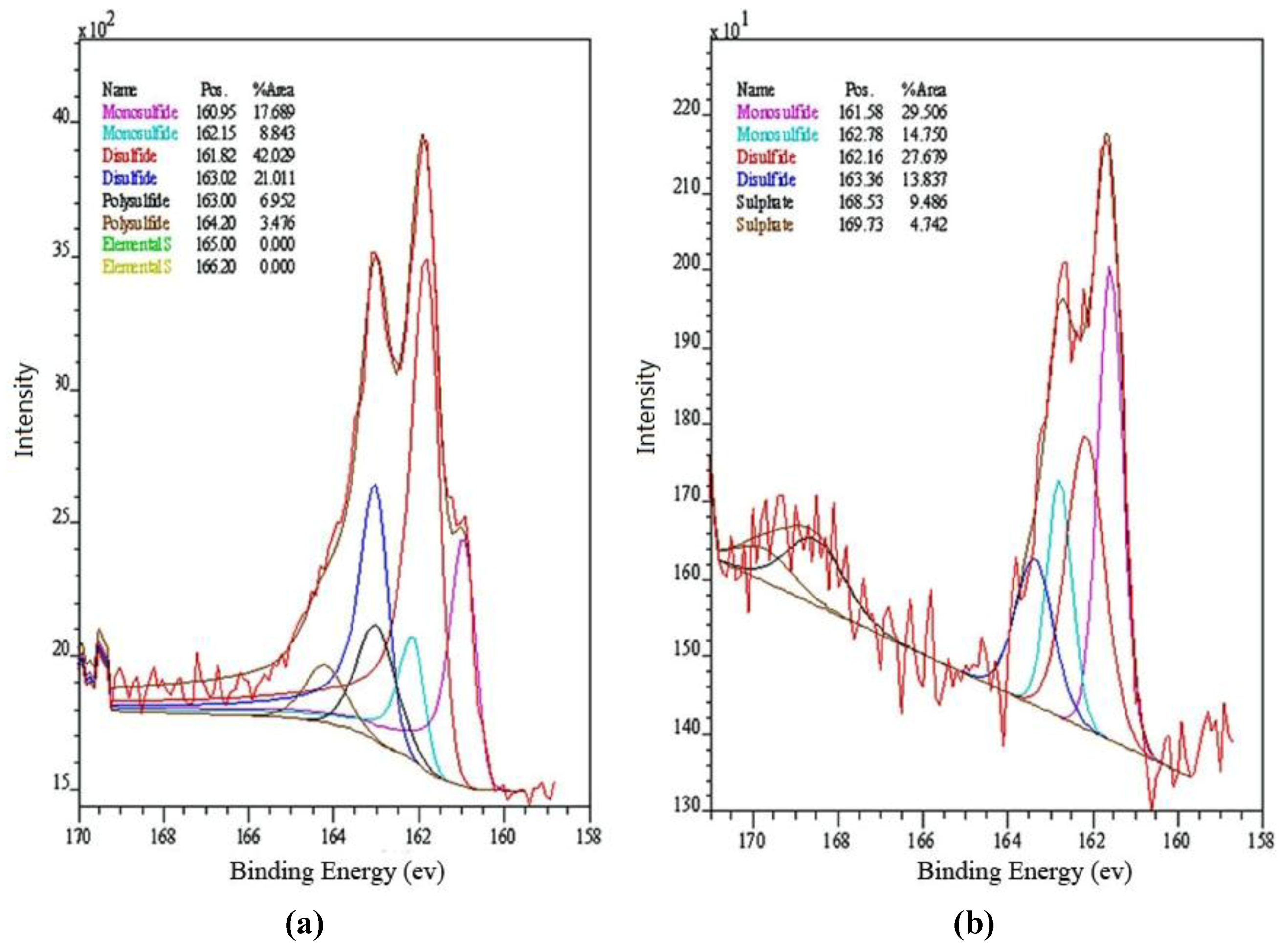
XPS analysis of leached djurleite samples indicated a significant amount of CuS and trace amount of Cu(II) on the mineral surface for the experiment using 350 ppm ammonia while, on the djurleite surface leached with 700 ppm ammonia, CuS was not significant, instead Cu(II) was found possibly as Cu(OH)
2. The formation of CuS, in the 350 ppm ammonia experiment, indicates the formation of a metal deficient sulphide at the mineral surface and it appears that it has a chemical composition that is similar to covellite, (Cu
3)
+(S
2)
2−(S
−) (
Figure 3, [
11,
12,
13]). Some Cu(I)sulphide, two N species (possibly ammonia and cyanide or their complexes) and polymerized sulphur were also found on the gold surfaces of the two leaching systems. Because of the similarities in the XPS spectra, it was not possible to identify the nature of the compounds that modified the leaching kinetics.
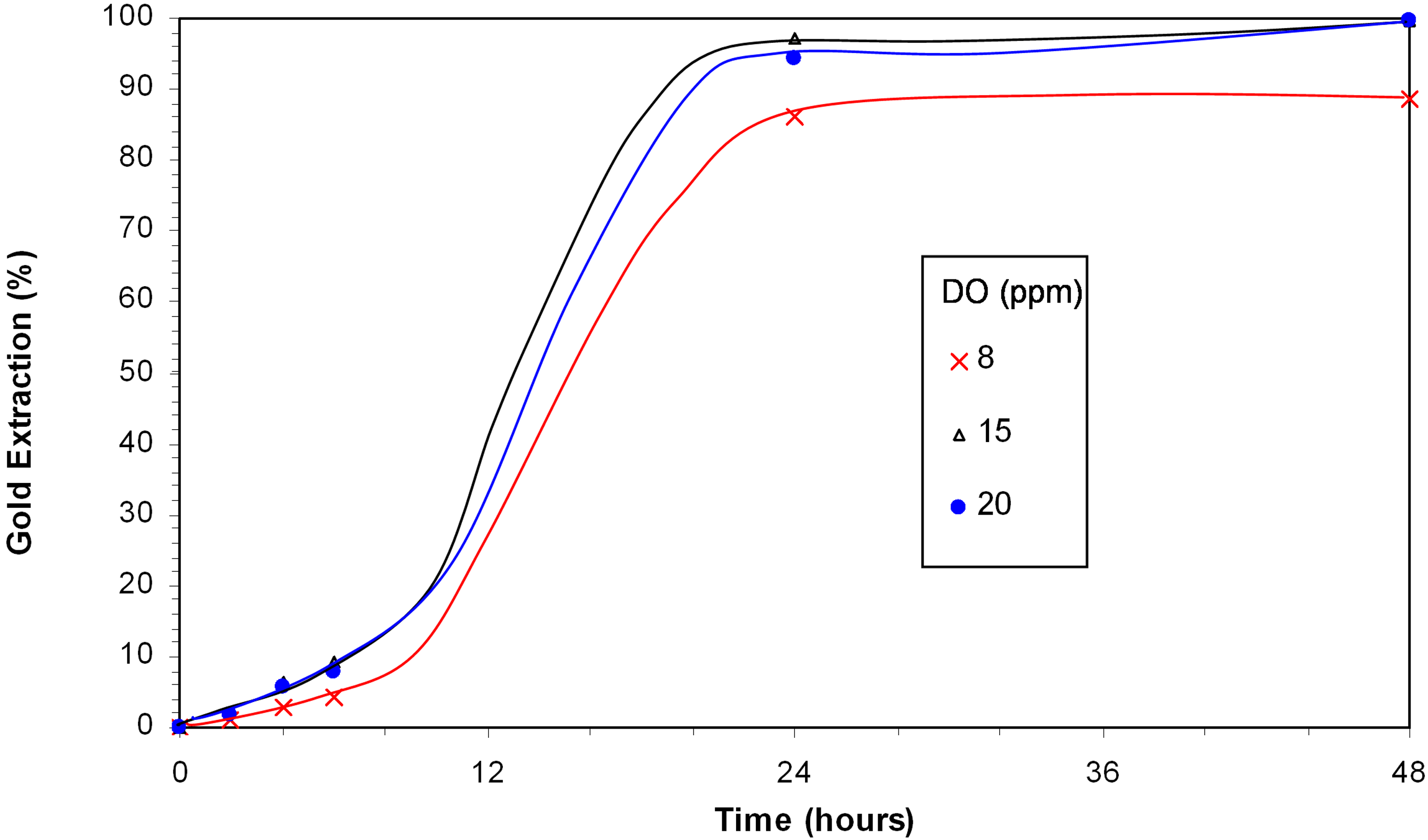
Oxygen oxidizes copper(I) to copper(II), which forms a copper amine in the presence of ammonia. Copper amine plays an important role in the oxidation of gold [
9]. Meanwhile, the oxygen level may affect mineral oxidation and precipitation of copper. Therefore, tests were carried out at DO levels of 8, 15 and 20 ppm to determine the effect of dissolved oxygen concentration.
Figure 4 shows that the gold extraction increased from 88.5% with a DO of 8 ppm to 99.7% when the DO increased to 15 ppm or above. The change in oxygen concentrations, however, has no significant effects on the solution compositions.
The effect of NaCN concentration was investigated by changing the concentration of NaCN from 200 to 350 ppm (
Figure 5). The consumption of cyanide was reduced from 2.4 kg/t at 350 ppm NaCN and 350 ppm ammonia to 1.2 kg/t at 200 ppm NaCN and 100 ppm ammonia. The gold leaching kinetics decreased with the decrease in cyanide concentration but the overall gold extraction still reached 99.7% in 48 h. The ammonia concentration has to be adjusted in tandem with the cyanide concentration to target the right ammonia/cyanide ratio. Surprisingly, the dissolved copper increased from about 130 ppm to about 390 ppm when the cyanide concentration was 250 ppm or lower. The increase of copper cyanide species is likely to be the result of the decrease in copper hydroxide formation. Only 1.2 kg/t NaCN was required to have a complete gold dissolution using the Hunt process, compared to 5.8 kg/t for cyanidation.
Figure 3.
The S2p spectra from djurleite powder reacted in (a) 350 ppm ammonia; and (b) 700 ppm ammonia.
Figure 3.
The S2p spectra from djurleite powder reacted in (a) 350 ppm ammonia; and (b) 700 ppm ammonia.
Figure 4.
Effect of dissolved oxygen concentration of gold leaching in presence of djurleite; 0.3% Cu, 400 ppm NaCN, 350 ppm NH3, pH from 11.5 to 11.8.
Figure 4.
Effect of dissolved oxygen concentration of gold leaching in presence of djurleite; 0.3% Cu, 400 ppm NaCN, 350 ppm NH3, pH from 11.5 to 11.8.
Figure 5.
Effect of NaCN concentration on gold extraction in presence of djurleite; 0.3% Cu, 15 ppm DO, pH >11.
Figure 5.
Effect of NaCN concentration on gold extraction in presence of djurleite; 0.3% Cu, 15 ppm DO, pH >11.
3.2. Bornite-Bearing Synthetic Gold Ore
The leaching kinetics profiles of experiments conducted at 800 ppm and 1600 ppm NaCN show that passivation of gold occurs in absence of lead nitrate as the gold extraction remains less than 99% (
Figure 6). Leaching with 800 ppm NaCN and 1600 ppm NaCN produced 85.4% and 96.1% respectively. The addition of 500 g/t lead nitrate totally eliminated gold passivation and a complete extraction of gold was obtained within 6 hours even with only 500 ppm NaCN. Obviously, the dissolution of bornite undergoes a different mechanism in the presence of lead nitrate. The decrease of free cyanide concentration in leaching reduced the cyanide consumption from 6.1 kg/t to 4.7 kg/t NaCN. The concentration of dissolved copper was reduced from 1370 ppm to 1074 ppm. The dissolution of bornite in a cyanide solution may be expressed as:
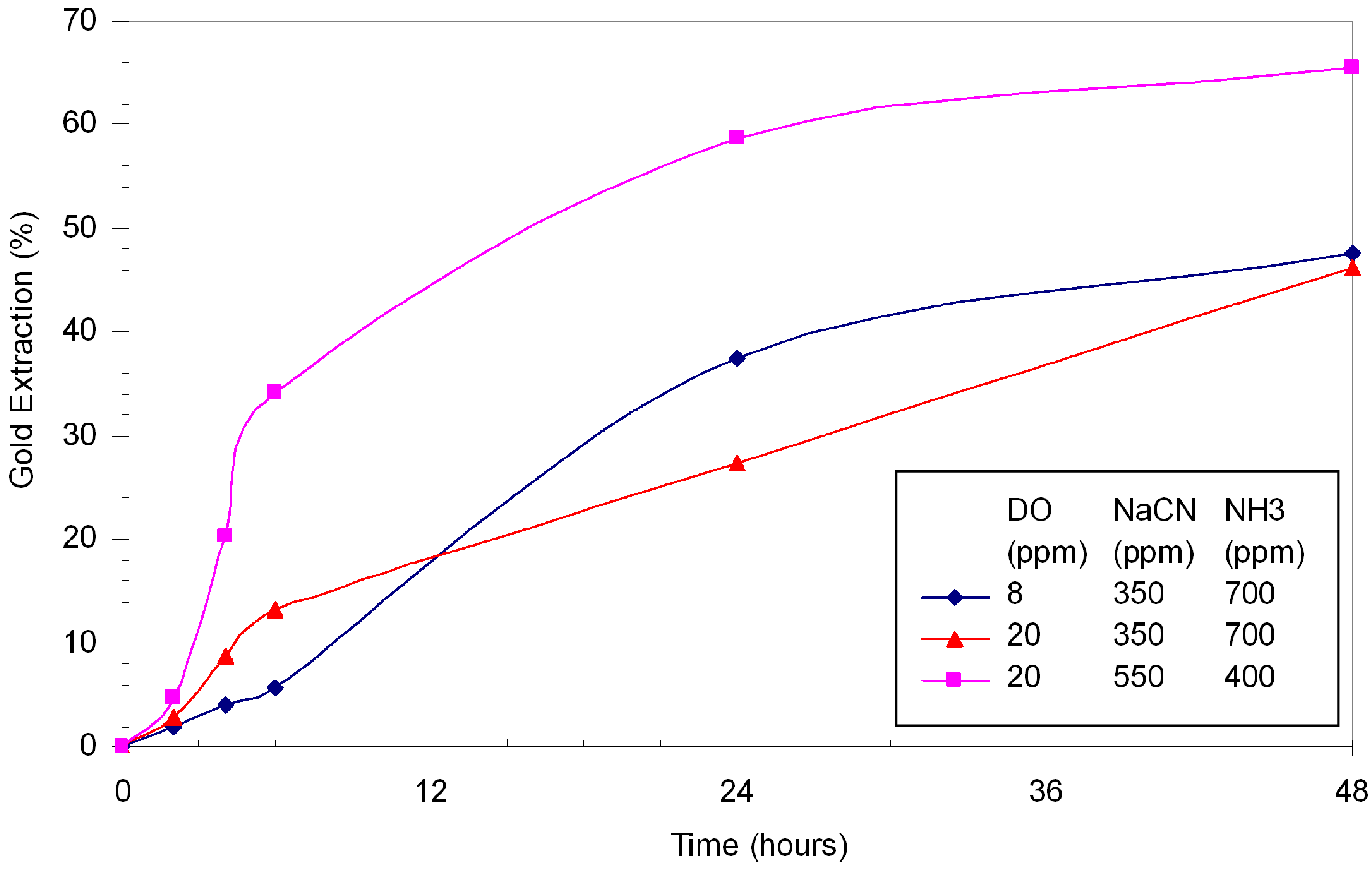
Three tests were conducted with different DO, NaCN and ammonia concentrations. In terms of gold extraction, these tests failed to increase gold extraction above 80% (
Figure 7). Increasing the DO level from 8 to 20 ppm did not introduce any significant enhancement of gold extraction at 350 ppm NaCN. However, the increase of NaCN from 350 ppm to 550 ppm brought the extraction up from 46.1% to 65.5% in 48 hours. The consumption of cyanide was similar to that in the straight cyanidation (2.0 kg/t at 350 ppm). At 550 ppm NaCN, the consumption increased to 3.2 kg/t. Gold dissolution at the initial stage of these Hunt tests was slower than straight cyanidation and it became faster after six hours. No significant reduction was observed on the dissolution of copper. In the straight cyanidation with 350 ppm NaCN, 560 ppm Cu was dissolved whereas, in these Hunt tests, the dissolved copper ranged from 439 to 818 ppm.
Figure 6.
Effect of NaCN concentration and lead nitrate addition on gold extraction in presence of bornite; 0.3% Cu, 8 ppm DO, pH >11.
Figure 6.
Effect of NaCN concentration and lead nitrate addition on gold extraction in presence of bornite; 0.3% Cu, 8 ppm DO, pH >11.
Figure 7.
Hunt leaching test under typical Hunt conditions; 0.3% Cu as bornite, pH >11.
Figure 7.
Hunt leaching test under typical Hunt conditions; 0.3% Cu as bornite, pH >11.
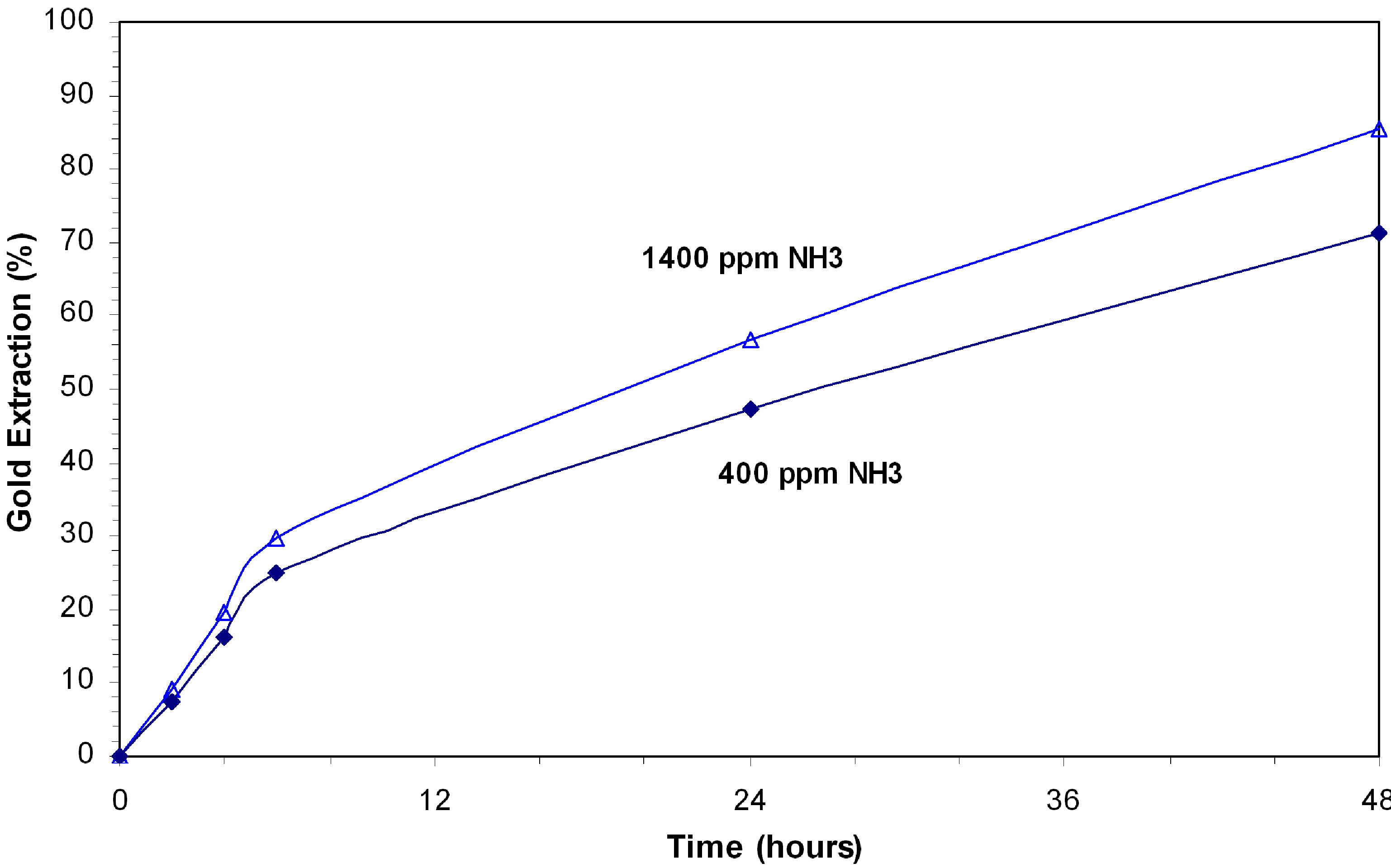
Tests were conducted at 400 ppm and 1400 ppm ammonia while maintaining the free cyanide at 850 ppm NaCN.
Figure 8 shows that gold extraction increased from 71% to 88% with cyanide consumption and 7.1 and 7.6 kg/t NaCN. The results indicate that the Hunt leaching has limited efficiency for leaching gold in presence of bornite. This approach had limited effect on retarding the dissolution of copper and consequently the consumption of cyanide. The bornite consumes more cyanide than djurleite. However, due to the deficiency of Fe in bornite, the FeOOH formed is not enough to prevent further dissolution and oxidation of bornite. The high copper concentration indicates that the precipitation of copper is low. The volume of oxygen required to maintain the dissolved oxygen concentration is significantly larger for bornite than for djurleite. This is possibly due to the highly reductive nature of bornite (Equations 1 and 2).
Figure 8.
Hunt leaching tests at high cyanide concentration; 0.3% Cu as bornite, 850 ppm NaCN, pH 11.7–11.9, 8 ppm DO.
Figure 8.
Hunt leaching tests at high cyanide concentration; 0.3% Cu as bornite, 850 ppm NaCN, pH 11.7–11.9, 8 ppm DO.
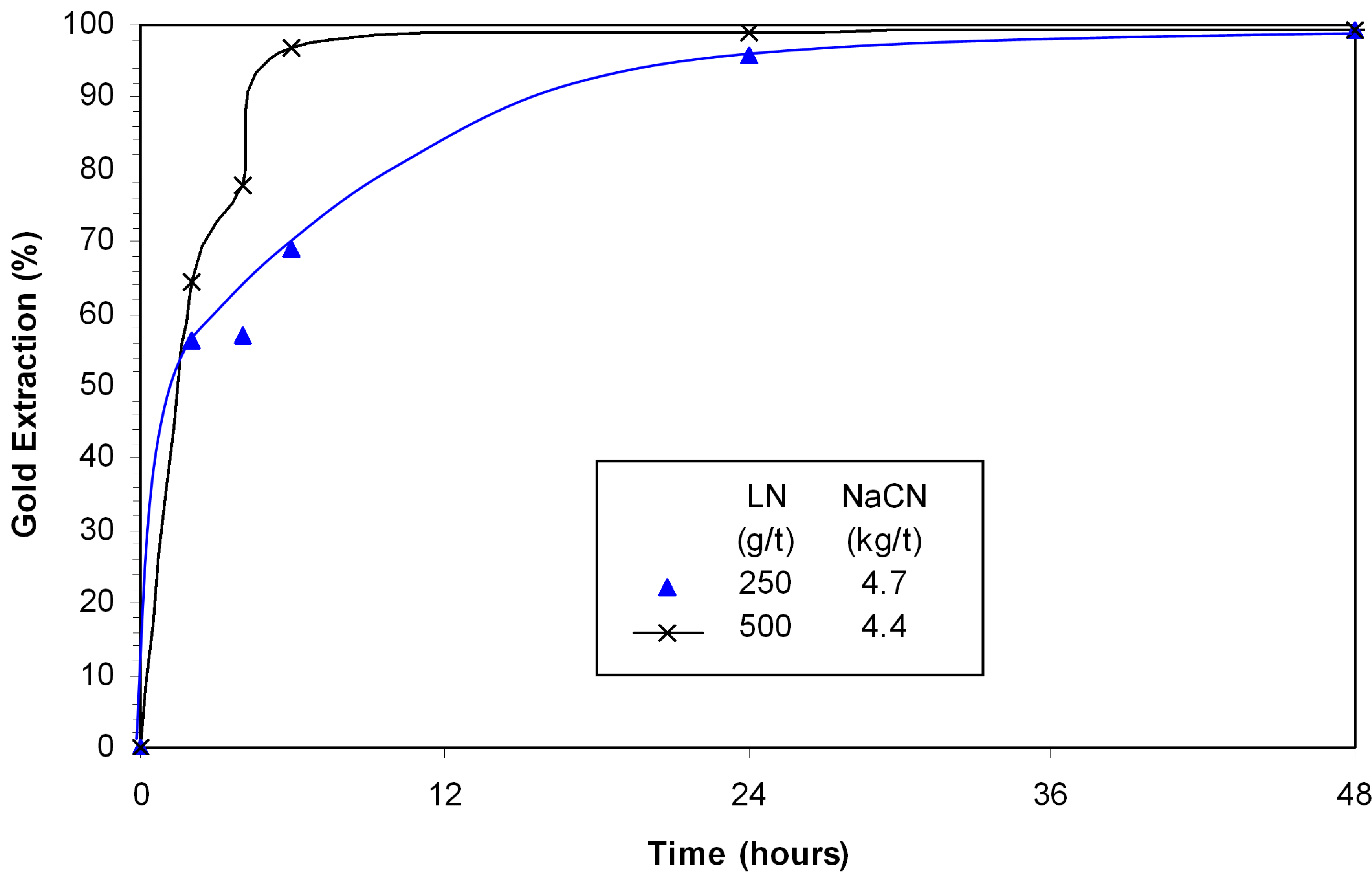
It was possible to improve the efficiency of ammonia-cyanide leaching by including a 4-hour pre-leach at pH 11 with the addition of 250 g/t or 500 g/t lead nitrate (
Figure 9) prior to gold leaching. The concentration of cyanide and ammonia were controlled at 550 ppm. The cyanide consumption was reduced to 4.4 kg/t NaCN. It is partly associated to a reduction of thiocyanate formed (~50% reduction).
Figure 9.
Effect of lead nitrate on gold leaching in presence of bornite; 0.3% Cu, 550 ppm NaCN, 400 ppm NH3, pH 11.5–11.8.
Figure 9.
Effect of lead nitrate on gold leaching in presence of bornite; 0.3% Cu, 550 ppm NaCN, 400 ppm NH3, pH 11.5–11.8.
XPS study of the gold surface and bornite samples in the ammonia-cyanide leaching with or without lead nitrate showed several precipitated species. Gold was passivated by Cu(I) cyanide and possibly Cu(I) sulphide. With lead nitrate, the copper deposit on the surface was largely reduced. Instead, lead oxide and hydroxide appeared on the gold surface. Due to the decrease of copper precipitates, the gold dissolution is more active than that in the test without lead nitrate.
On the leached surface of bornite, the surface characteristics were found to be similar to djurleite. This change indicated that both copper and iron were dissolved. With lead nitrate, Cu(I) became Cu(I)sulphide and Cu(II)oxide and hydroxide at the surface of bornite. No Cu-cyanide species were found. The metal deficient sulphide surface produced thiosulphate and polysulphide. Analysis of the leach solution showed the presence of sulphate and cyanate were not significantly modified by adding lead nitrate (32 to 54 ppm for sulphate and 7 to 19 ppm for cyanate). The effect of lead was to reduce the concentration of sulphide and copper on the gold surface. Lead also reported on gold as an oxide or hydroxide. Such a change explains the improved gold extraction rate.
3.3. Chalcopyrite-Bearing Synthetic Gold Ore
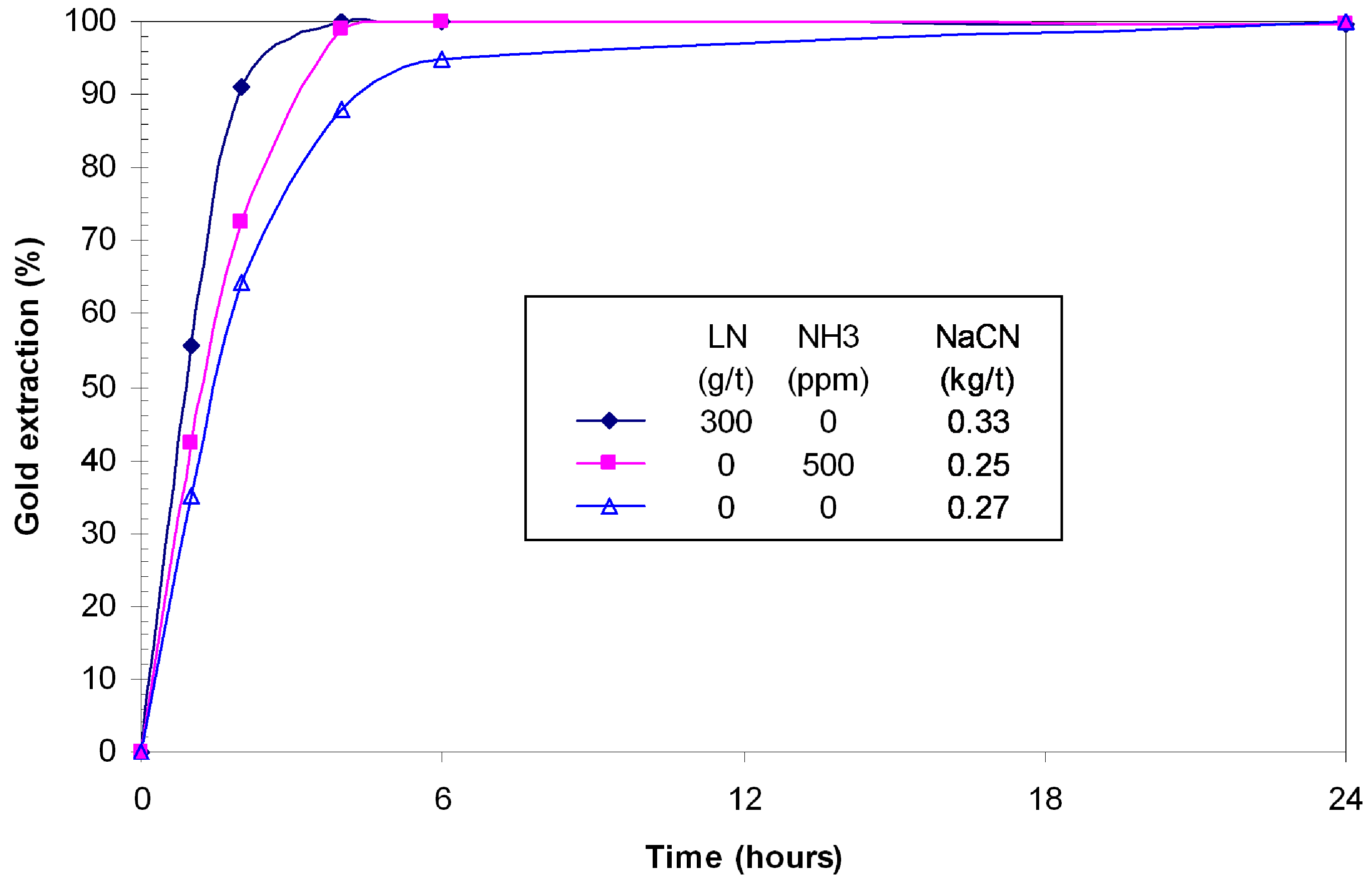
In the presence of chalcopyrite, the gold extraction in a cyanidation experiment with lead nitrate was 99.7% in four hours with 0.33 kg/t of cyanide consumed (
Figure 10). In absence of lead nitrate, the leaching kinetics were lower and the maximum extraction of gold was reached in 24 h. At the same concentration, the reactivity of this pure chalcopyrite is lower than the one contained in Mouska ore [
14]. However, it is comparable to the reactivity of chalcopyrite contained in Geant Dormant ore [
15]. The addition of 500 ppm ammonia improved gold leaching kinetics and overall extraction very close to the conventional cyanidation. The consumption of cyanide was slightly lower with ammonia (0.25 kg/t
vs. 0.33 kg/t NaCN).
The dissolution of chalcopyrite in a cyanide solution may be expresses as follow:
Figure 10.
Effect of lead nitrate and ammonia on gold extraction in presence of chalcopyrite; 0.3% Cu, 500 ppm NaCN, 8 ppm DO.
Figure 10.
Effect of lead nitrate and ammonia on gold extraction in presence of chalcopyrite; 0.3% Cu, 500 ppm NaCN, 8 ppm DO.
The XPS analysis indicated that the chalcopyrite powder was partially oxidized. The chalcopyrite surface contains Cu(I), an Fe(III) species, an Fe(II)–S species, and a small amount of Fe in chalcopyrite. The sulphur data shows sulphur in chalcopyrite, along with sulphur in disulphide, polysulphide, and sulphate species. The oxygen data shows that oxide and hydroxide species are present. Some pristine chalcopyrite is detected and this suggests that the oxidized material coating particles is either very thin, or it may also occur as islands or some combination of both. This may explain the lower reactivity of chalcopyrite with cyanide.
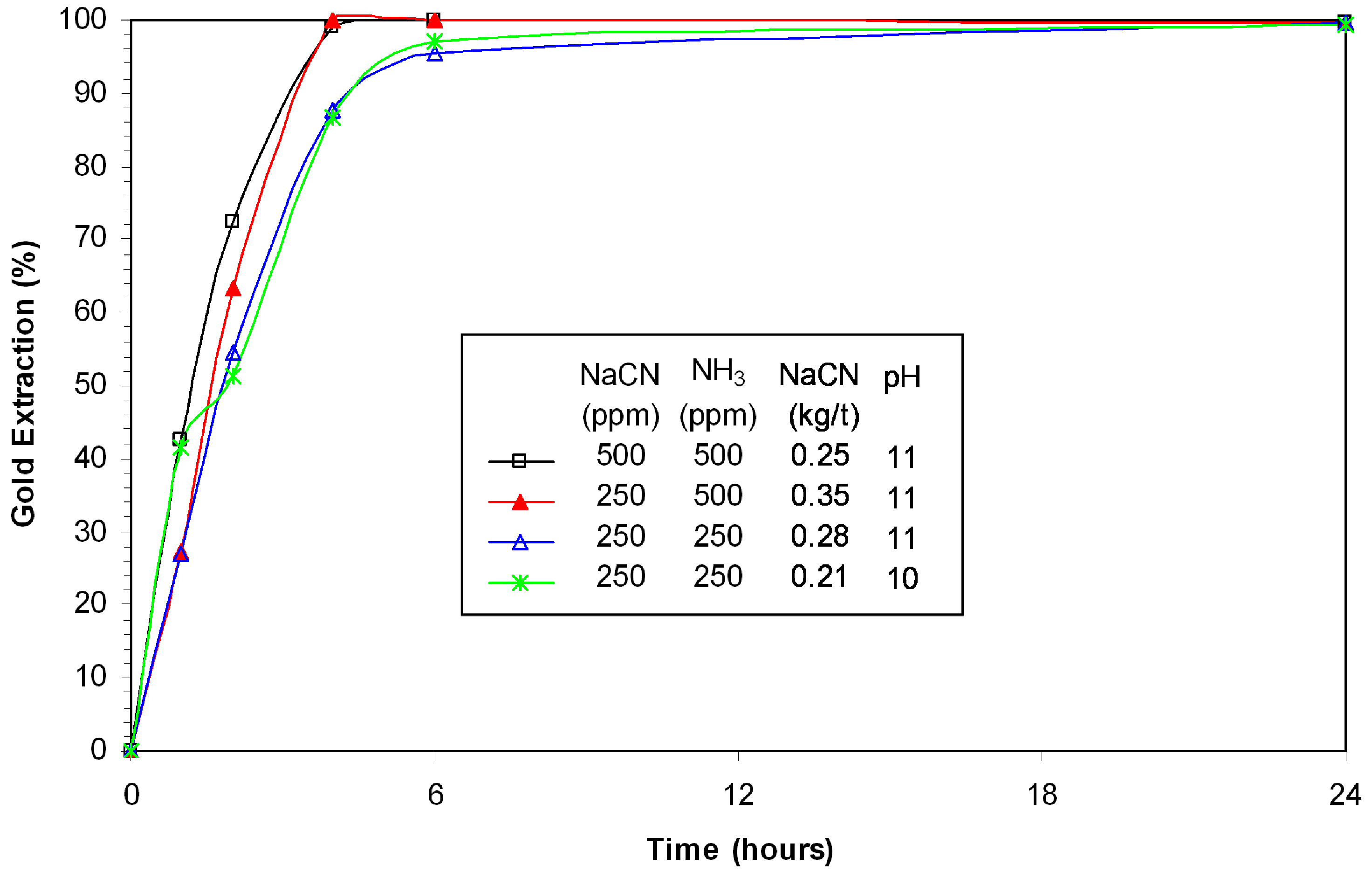
Figure 11 shows that using lower cyanide and ammonia did not compromise gold extraction, which remained >99%. The gold leaching kinetics slightly decreased with the reduction of reagents concentration. The lowest consumption of cyanide recorded is 0.21 kg/t NaCN. Comparison of actual leaching results with prior work on high purity chalcopyrite shows similarities in terms of low dissolution of copper [
16].
Figure 11.
Effect of cyanide and ammonia on gold extraction in presence of chalcopyrite; 0.3% Cu, 8 ppm DO.
Figure 11.
Effect of cyanide and ammonia on gold extraction in presence of chalcopyrite; 0.3% Cu, 8 ppm DO.