Water- and Boron-Rich Melt Inclusions in Quartz from the Malkhan Pegmatite, Transbaikalia, Russia
Abstract
:1. Introduction
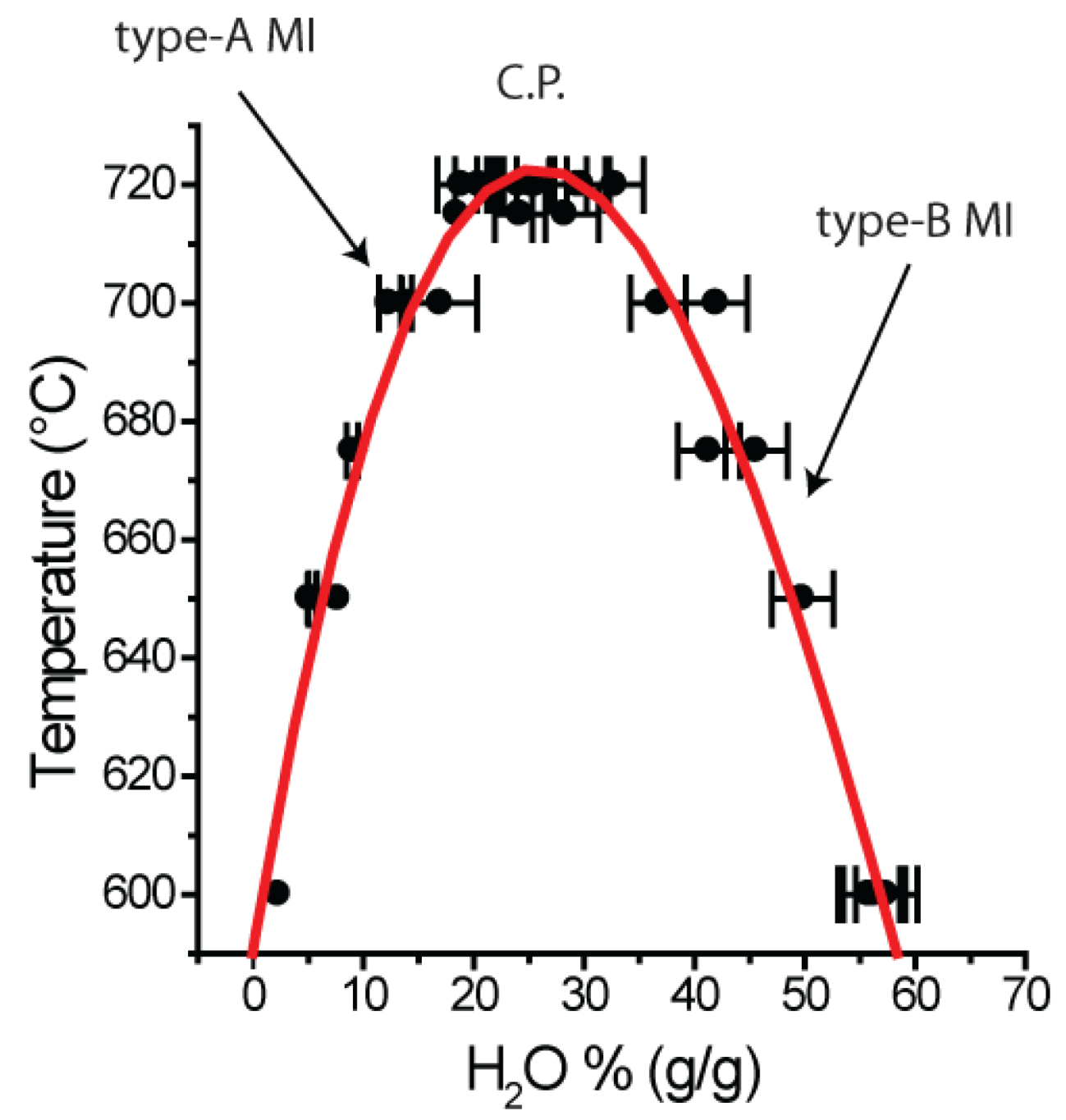
- Type-A MI: <20% (g/g) H2O
- Near-critical MI: ~26% (g/g) H2O
- Type-B MI: >30 and <60% (g/g) H2O
- Type-C MI: high B and H2O concentrations (~50% H3BO3) as well as small amounts of silicates.
2. Geological Setting
3. Samples and Experimental and Analytical Methods
3.1. Samples
| Pegmatite vein | Sample | Position in the pegmatites |
|---|---|---|
| Mokhovaya | MX-483 | Quartz-oligoclase zone (with only vague graphic structure) |
| Mokhovaya | MX-1, MX-2, MX-4 | Miarolitic cavities* |
| Oreshnaya | MX-73-74 | Graphic granite |
| Oktyabr’skaya | MX-707 | Quartz-oligoclase zone (with only vague graphic structure) |
| Sosedka | MX-467 | Graphic granite |
| Zapadnaya | MX-27 | Graphic granite |
| Granite | ||
| Oreshnyi Massif | MX-49 | Biotite-muscovite granite |
3.2. Cold-Seal Pressure Vessel Homogenization Experiments
3.3. Microprobe Analysis of the Melt Inclusion Glass
3.4. Micro-Raman Spectroscopy and Water Determination in Melt Inclusion Glass
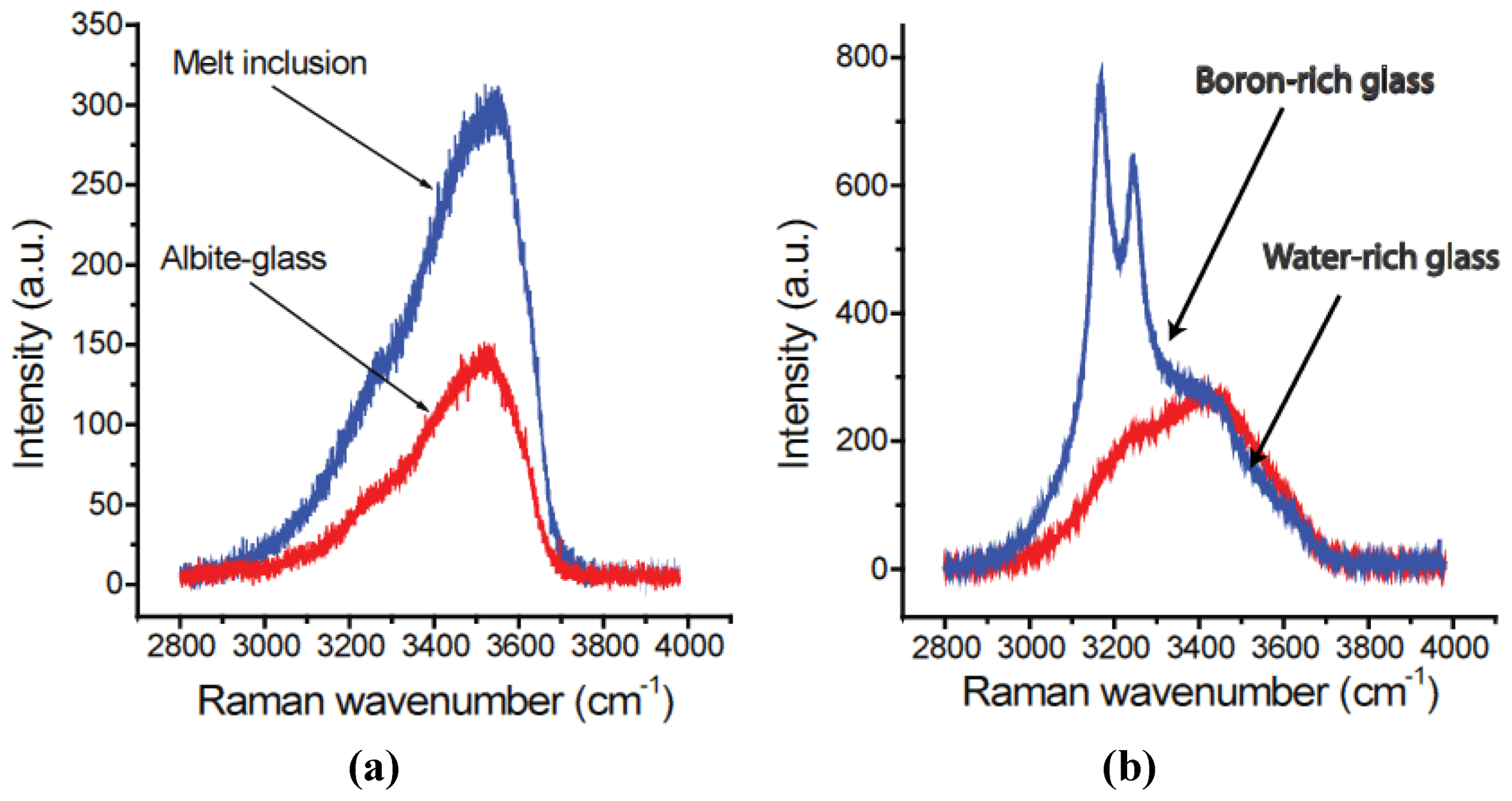
3.5. Interpreting Melt Inclusion Analyses
4. Results
4.1. Inclusions in Quartz from the Oreshnyi Massif Biotite-muscovite Granite
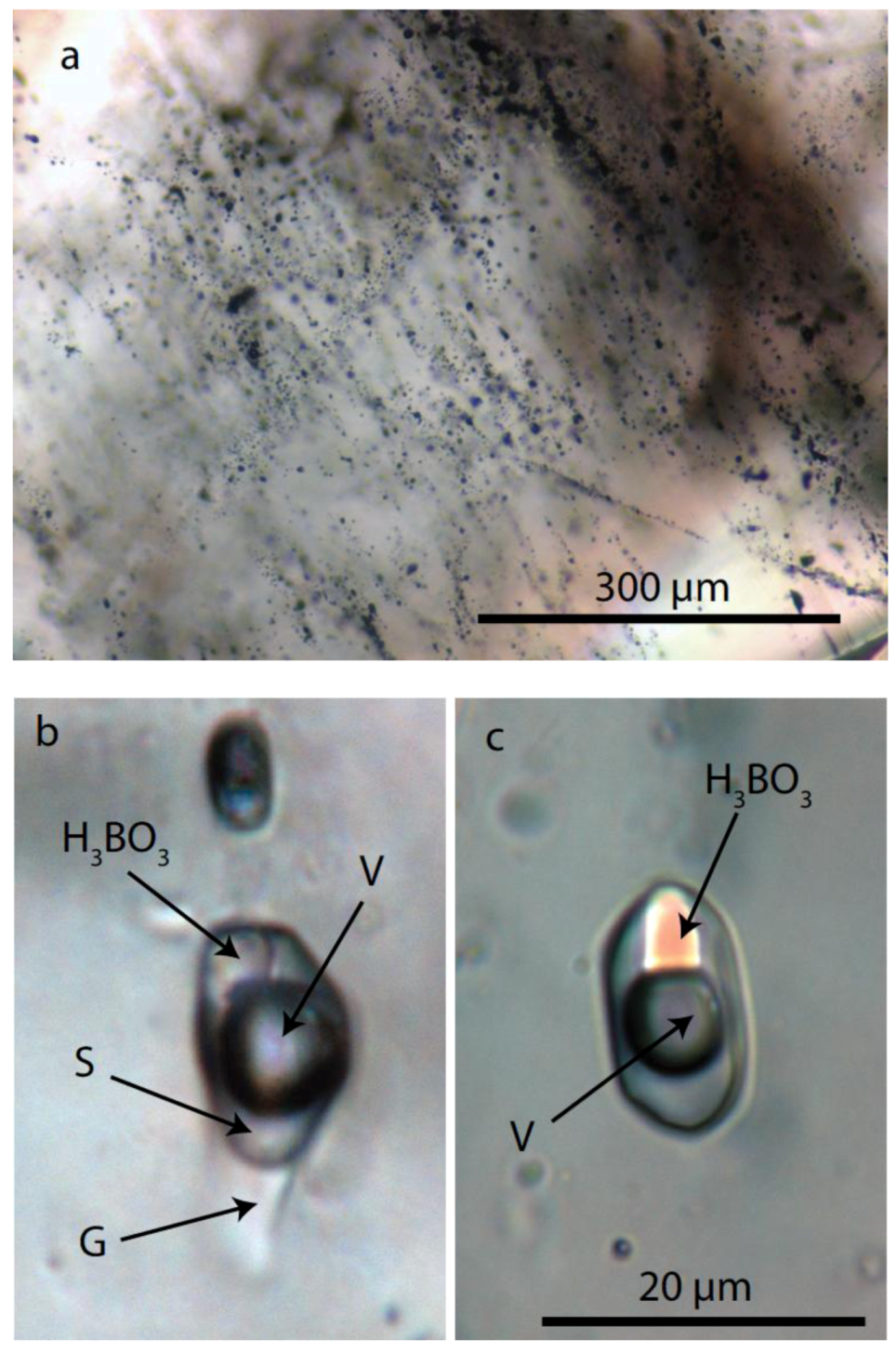
4.2. Inclusion in Pegmatite Quartz
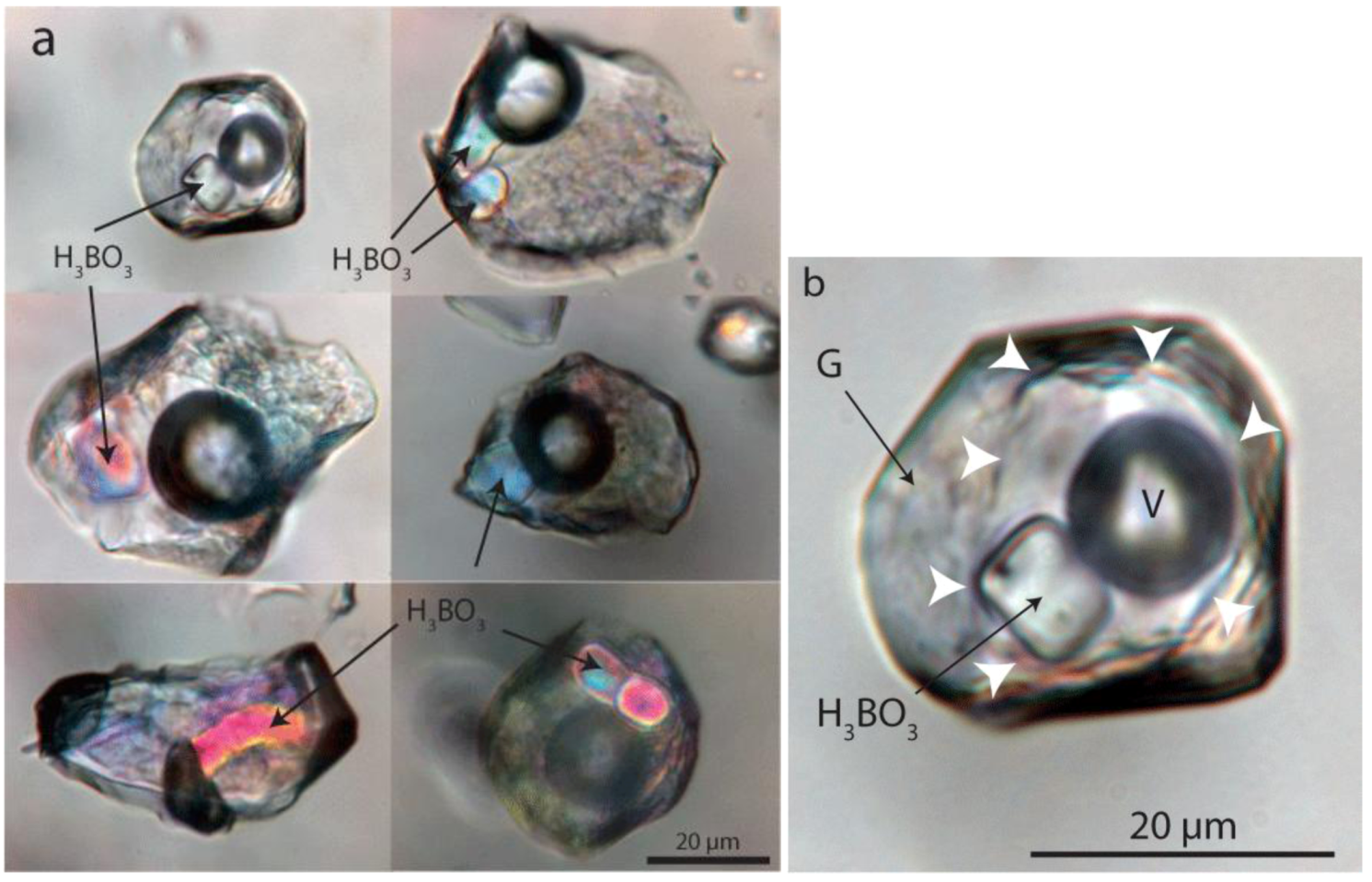
4.2.1. Melt Inclusion Data
| Pegmatite | T (°C) | MI-type | Glass% (vol/vol) | SD | H2O% (g/g) | SD | n | B2O3% (g/g) | SD | n |
|---|---|---|---|---|---|---|---|---|---|---|
| Mokhovaya | 650 | A | 5.1 | 0.4 | 38 | |||||
| 600 | B | 18.6 | 55.8 | 2.8 | 8 | 8.2 | 0.1 | 3 | ||
| 700 | A | 17.0 | 3.3 | 10 | ||||||
| 700 | B | 35.8 | 36.8 | 2.6 | 2 | |||||
| 720 | B | 62.9 | 2 | 20.5 | 2.2 | 11 | ||||
| 720 | critical | 100 | 25.3 | 3.1 | 7 | |||||
| 720 | B | 45.2 | 29.8 | 2.5 | 11 | 6.9 | 0.1 | 53 | ||
| Oreschnaya | 650 | A | 5.4 | 0.4 | 3 | 1.6 | 3 | |||
| 650 | B | 23 | 3.6 | 49.8 | 2.8 | 8 | ||||
| 675 | A | 9.2 | 0.5 | 5 | 2.4 | 0.9 | 14 | |||
| 675 | B | 30.6 | 4.2 | 41.3 | 2.8 | 7 | 8.4 | 1 | ||
| 700 | A | 12.3 | 0.9 | 7 | 4.4 | 0.6 | 7 | |||
| 700 | B | 29.9 | 42.0 | 2.8 | 10 | 8.7 | 2 | |||
| 720 | A | 22.1 | 1.8 | 39 | 6.8 | 0.6 | 9 | |||
| 720 | B | 45.7 | 0.13 | 29.4 | 2.5 | 27 | 7.1 | 1 | ||
| Oktyabr'skaya | 600 | B | 17.6 | 57.4 | 2.8 | 1 | 8.1 | 1 | ||
| 675 | B | 26.5 | 1 | 45.6 | 2.8 | 1 | 8.4 | 1 | ||
| 715 | A | 18.5 | 1 | 6.7 | 1 | |||||
| 715 | B | 47.6 | 1.7 | 28.3 | 3.0 | 22 | 7.8 | 0.6 | 2 | |
| 720 | A | 25.6 | 1 | 6.0 | 1 | |||||
| Sosedka | 600 | A | 2.3 | 1 | 0.8 | 0.3 | 5 | |||
| 715 | B | 55 | 24.2 | 2.3 | 1 | 7.2 | 1 | |||
| 720 | A | 24.1 | 2.5 | 15 | 6.9 | 0.7 | 15 | |||
| 720 | B | 67.1 | 8 | 18.9 | 2.2 | 1 | 7.1 | 1 | ||
| Zapadnaya | 600 | B | 18.3 | 0.5 | 56.3 | 2.8 | 3 | 8.3 | 1 | |
| 650 | A | 7.7 | 2 | 2.7 | 2 | |||||
| 675 | A | 9.0 | 0.5 | 9 | 2.5 | 1.1 | 5 | |||
| 700 | A | 13.9 | 0.5 | 34 | 4.6 | 0.3 | 5 | |||
| 720 | A | 21.6 | 0.4 | 9 | 5.8 | 0.5 | 7 | |||
| 720 | B | 40.8 | 32.8 | 2.6 | 2 | 6.5 | 5 | |||
| Sum of n | 296 | 146 |
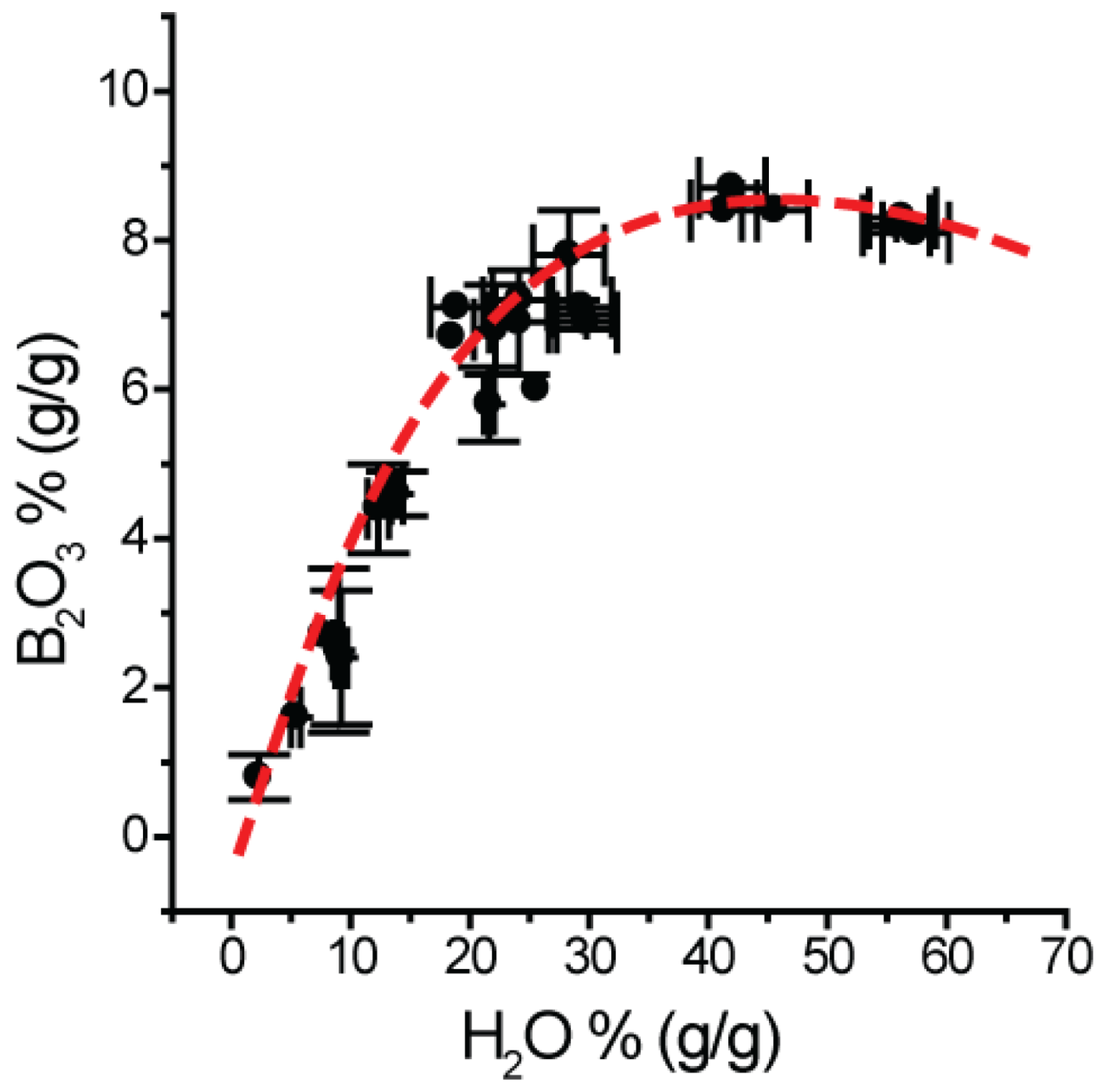

| Type-A MI Zapadnaya | Type-B MI Zapadnaya(2) | Type-A MI Sosedka | Type-A MI Sosedka | Type-B MI Sosedka(2)(3) | Type-A MI Oreschnaya | Type-A MI Oreschnaya | |
|---|---|---|---|---|---|---|---|
| SiO2 | 72.5 | 67.8 | 73.7 | 76.7 ± 2.9 | 67.2 | 68.7 ± 1.5 | 67.2 |
| TiO2 | 0.03 | 0.03 | 0.01 | 0.03 | d.l. | 0.03 | 0.01 |
| Al2O3 | 12.7 | 10.0 | 11.9 | 12.4 ± 1.3 | 10.8 | 11.2 ± 0.7 | 10.6 |
| B2O3 | 3.8 ± 0.5(1) | 8.0 | 3.0 | 2.8 ± 1.0 | 8.6 | 6.3 ± 0.3 | 7.1 ± 0.8(1) |
| MgO | d.l. | 0.01 | 0.03 | 0.02 | 0.03 | 0.01 | d.l. |
| CaO | 0.11 | 0.44 | 0.1 | 0.9 ± 0.3 | 0.09 | 0.08 | 0.05 |
| MnO | d.l. | 0.01 | 0.02 | 0.1 | 0.02 | 0.02 | 0.02 |
| FeO | 0.02 | d.l. | 0.04 | 0.04 | 0.04 | d.l. | 0.02 |
| Na2O | 3.0 | 5.5 | 2.0 | 2.8 ± 0.4 | 3.6 | 4.1 ± 0.2 | 4.4 |
| K2O | 3.1 | 3.5 | 3.0 | 2.6 ± 0.4 | 3.9 | 3.4 ± 0.5 | 4.1 |
| Rb2O | 0.22 | 0.12 | 0.82 | 0.2 ± 0.1 | 0.75 | 0.2 ± 0.03 | 0.1 |
| Cs2O | 3.0 | 3.2 | 4.0 | 0.75 ± 0.2 | 3.6 | 3.5 ± 0.2 | 3.6 |
| F | 1.6 | 1.5 | 1.3 | 0.55 ± 0.2 | 1.2 | 2.3 ± 0.7 | 2.9 |
| Cl | d.l. | d.l. | 0.02 | 0.02 | 0.02 | d.l. | 0.01 |
| P2O5 | 0.04 | d.l. | 0.04 | 0.02 | 0.04 | 0.02 | 0.02 |
| Sum | 100.12 | 100.11 | 99.98 | 99.93 | 99.89 | 99.88 | 100.13 |
| ASI | 1.31 | 0.67 | 1.38 | 1.31 ± 0.19 | 0.90 | 0.94 ± 0.08 | 0.81 |
| n | 1 | 1 | 1 | 18 | 2 | 5 | 1 |
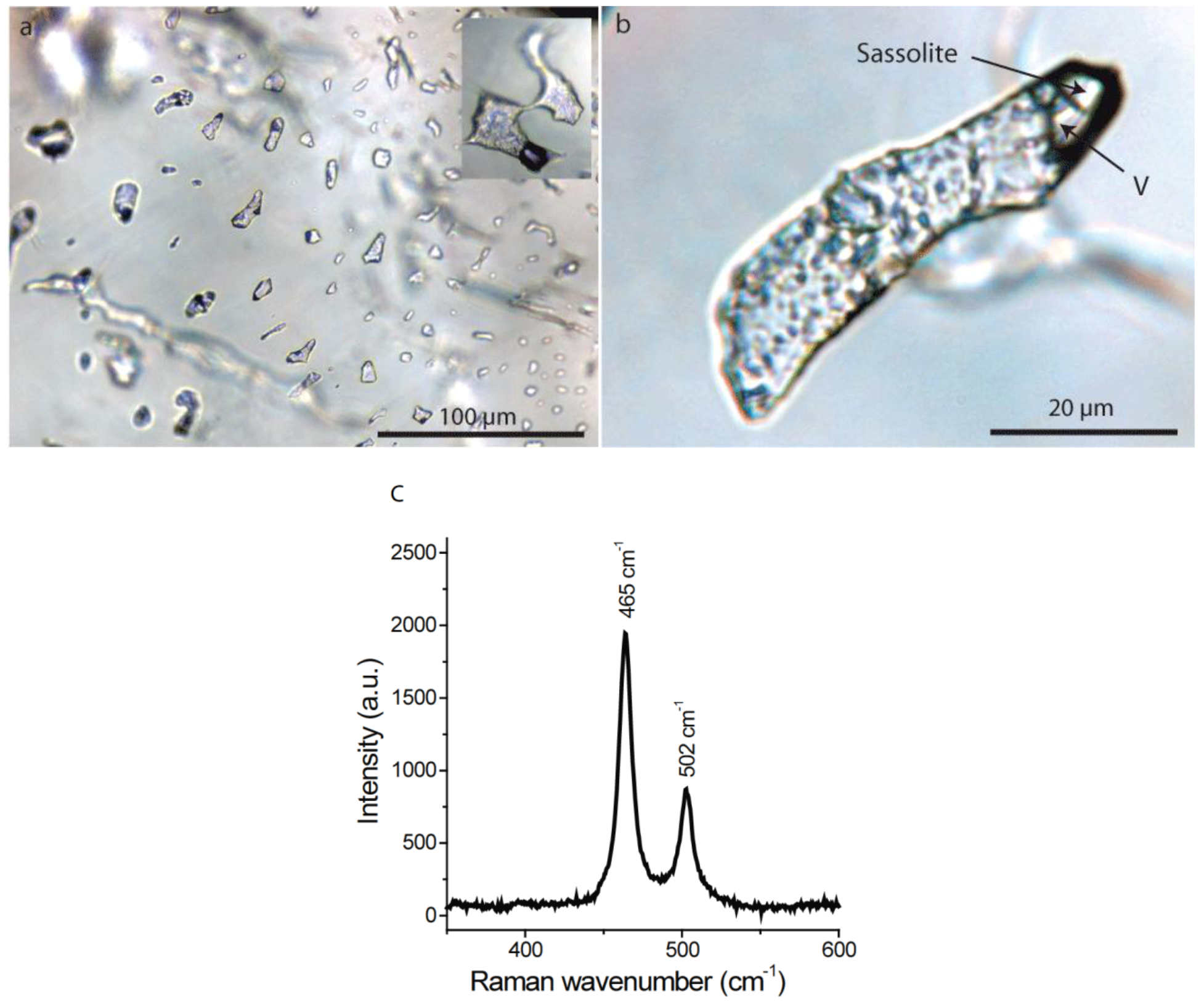
4.2.2. Fluid Inclusions
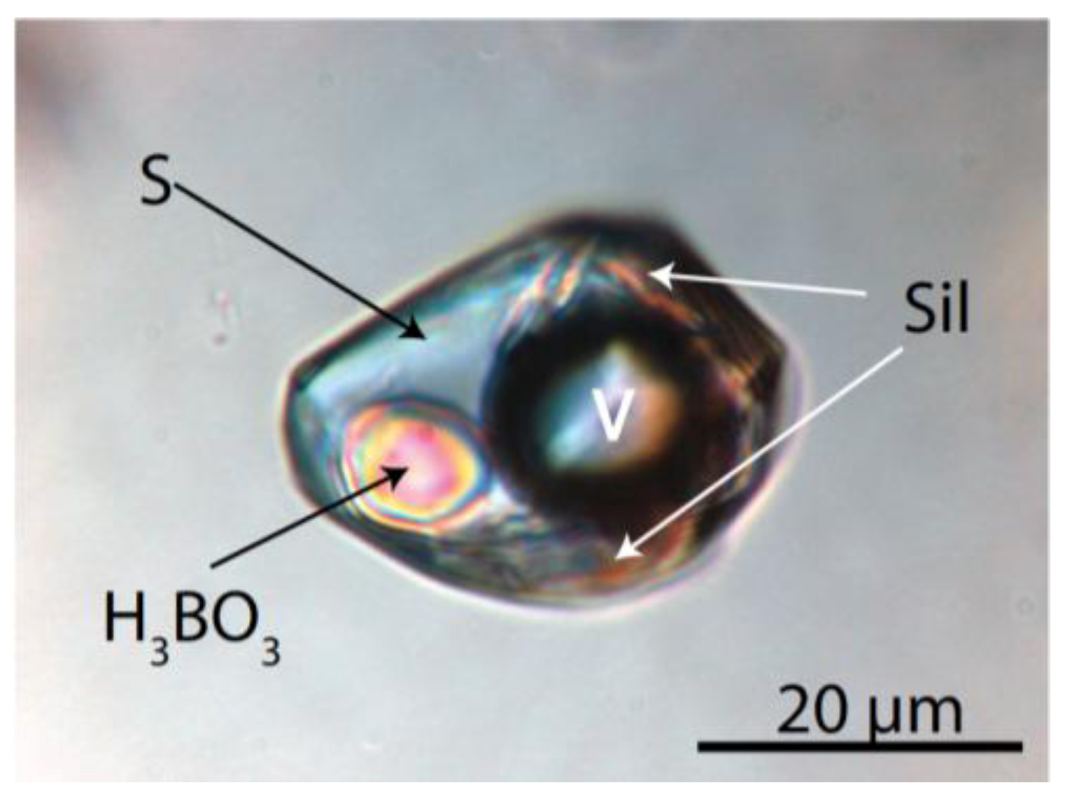
4.2.3. Gel Inclusions
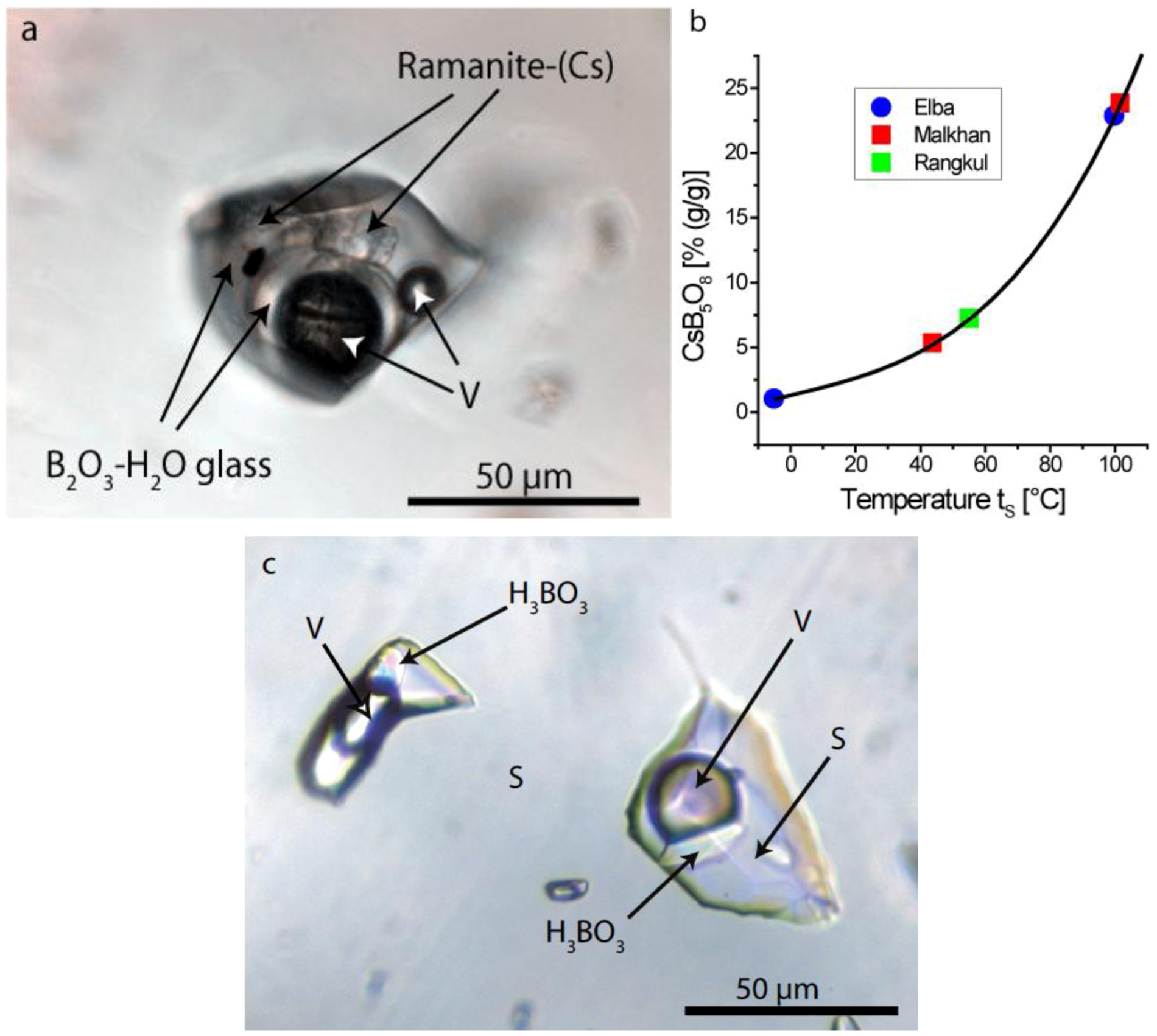
5. Discussion
5.1. The Significance of the Gel State in Pegmatite Evolution
5.2. Evolution of the Malkhan Pegmatite


Acknowledgements
References
- Peretyazhko, I.S.; Prokof’ev, V.Y.; Zagorskii, V.E.; Smirnov, S.Z. Role of boric acids in the formation of pegmatite and hydrothermal minerals: Petrologic consequences of sassolite (H3BO3) discovery in fluid inclusions. Petrology 2000, 8, 214–237. [Google Scholar]
- Smirnov, S.Z.; Peretyazhko, I.S.; Prokof’ev, V.Y.; Zagorskii, V.E.; Shebanin, A.P. The first finding of sassolite (H3BO3) in fluid inclusions in minerals. Russ. Geol. Geophys. 2000, 41, 193–205. [Google Scholar]
- Zagorsky, V.E.; Peretyazhko, I.S.; Shmakin, B.M. Miarolitic Pegmatites. In Granitic Pegmatites; Shmakin, B.M., Makagon, V.N., Eds.; Nauka: Novosibirsk, Russia, 1999; Volume 3, p. 488. [Google Scholar]
- Zagorsky, V.Y.; Peretyazhko, I.S. The Malkhan gem tourmaline deposit in Transbaikalia, Russia. Miner. Obs. 2008, 13, 4–39. [Google Scholar]
- Badanina, E.V.; Thomas, R.; Syritso, L.F.; Veksler, I.V.; Trumbull, R.B. High boron concentration in a Li-F granitic melt. Dokl. Earth Sci. 2003, 390, 529–532. [Google Scholar]
- Badanina, E.V.; Thomas, R.; Syritso, L.F. Evolution of silicate melts during formation of the granite-pegmatite system of the Malkhany pegmatite field, Russia. Geochim. Cosmochim. Acta Suppl. 2005, 69, 240. [Google Scholar]
- Thomas, R.; Badanina, E.; Veksler, I. Extreme boron enrichment in granitic melt revealed by melt inclusions from Malkhan pegmatite, Russia. In Proceedings of the 8th Pan-American Current Research on Fluid Inclusions Meeting (PACROFI VIII), Halifax, Nova Scotia, Canada, 21-26 July 2002.
- Thomas, R.; Davidson, P. Water in granite and pegmatite-forming melts. Ore Geol. Rev. 2012, 46, 32–46. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P. Evidence of a water-rich silica gel state during the formation of a simple pegmatite. Mineral. Mag. 2012, 76. in press. [Google Scholar]
- Roedder, E. Fluid Inclusions. In Reviews in Mineralogy; Ribbe, P.H., Ed.; Mineralogical Society of America: Washington, DC, USA, 1984; Volume 12, p. 644. [Google Scholar]
- Zagorsky, V.E.; Peretyazhko, I.S. First 40Ar/39Ar age determination on the Malkhan granite-pegmatite system: Geodynamic implications. Dokl. Earth Sci. 2010, 430, 172–175. [Google Scholar] [CrossRef]
- Zagorsky, V.Y.; Peretyazhko, I.S. The Malkhan granite-pegmatite system. Dokl. Earth Sci. 2006, 406, 132–135. [Google Scholar] [CrossRef]
- Thomas, R.; Förster, H.-J.; Heinrich, W. The behaviour of boron in a peraluminous granite-pegmatite system and associated hydrothermal solutions: A melt and fluid inclusion study. Contrib. Mineral. Petrol. 2003, 144, 457–472. [Google Scholar] [CrossRef]
- Thomas, R. Estimation of the viscosity and the water content of silicate melts from meltinclusion data. Eur. J. Mineral. 1994, 6, 511–535. [Google Scholar]
- Massare, D.; Métrich, N.; Clocchiatti, R. High-temperature experiments on silicate melt inclusions in olivine at 1 atm: Inference on temperature of homogenization and H2O concentration. Chem. Geol. 2002, 183, 87–98. [Google Scholar] [CrossRef]
- Portnyagin, M.; Almeev, R.; Matveev, S.; Holtz, F. Experimental evidence for rapid water exchange between melt inclusions in olivine and host magma. Earth Planet. Sci. Lett. 2008, 272, 541–552. [Google Scholar] [CrossRef]
- Severs, M.J.; Azbej, T.; Thomas, J.B.; Mandeville, C.W.; Bodnar, R.J. Experimental determination of H2O loss from melt inclusions during laboratory heating: Evidence from Raman spectroscopy. Chem. Geol. 2007, 237, 358–371. [Google Scholar] [CrossRef]
- McGee, J.J.; Slack, J.F.; Herrington, J.R. Boron analysis by electron microprobe using MoB4C layered synthetic crystals. Am. Mineral. 1991, 76, 681–684. [Google Scholar]
- Thomas, R. Determination of water contents of granite melt inclusions by confocal laser Raman microprobe spectroscopy. Am. Mineral. 2000, 85, 868–872. [Google Scholar]
- Thomas, R.; Kamenetsky, V.S.; Davidson, P. Laser Raman spectroscopic measurements of water in unexposed glass inclusions. Am. Mineral. 2006, 91, 467–470. [Google Scholar] [CrossRef]
- Thomas, R.; Webster, J.D.; Heinrich, W. Melt inclusions in pegmatite quartz: Complete miscibility between silicate melts and hydrous fluids at low pressure. Contrib. Mineral. Petrol. 2000, 139, 394–401. [Google Scholar] [CrossRef]
- Sun, Q.; Qin, C. Raman OH stretching band of water as an internal standard to determine carbonate concentrations. Chem. Geol. 2011, 283, 274–278. [Google Scholar] [CrossRef]
- Oliver, B.G.; Davis, A.R. Vibrational spectroscopic studies of aqueous alkali metal bicarbonate and carbonate solutions. Can. J. Chem. 1973, 51, 698–702. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P. The application of Raman spectroscopy in the study of fluid and melt inclusions. Z. Dt. Ges. Geowiss. 2012, 163, 113–126. [Google Scholar]
- Thomas, R.; Webster, J.D.; Rhede, D.; Seifert, W.; Rickers, K.; Förster, H.-J.; Heinrich, W.; Davidson, P. The transition from peraluminous to peralkaline granitic melts: Evidence from melt inclusions and accessory minerals. Lithos 2006, 91, 137–149. [Google Scholar] [CrossRef]
- Thomas, R. Determination of the H3BO3 concentration in fluid and melt inclusions in granite pegmatites by laser Raman microprobe spectroscopy. Am. Mineral. 2002, 87, 56–68. [Google Scholar]
- Peretyazhko, I.S.; Smirnov, S.Z.; Thomas, V.G.; Zagorsky, V.Y. Gels and melt-like gels in high-temperature endogeneous mineral formation. In Metallogeny of the Pacific Northwest: Tectonics, Magmatism and Metallogeny of Active Continental Margin; Khanchuk, A.I., Ed.; Dalnauka: Vladivostok, Russia, 2004; pp. 306–309. [Google Scholar]
- Thomas, R.; Davidson, P.; Hahn, A. Ramanite-(Cs) and ramanite-(Rb): New cesium and rubidium pentaborate tetrahydrate minerals identified with Raman spectroscopy. Am. Mineral. 2008, 93, 1034–1042. [Google Scholar] [CrossRef]
- Gmelin, L. Gmelins Handbuch der Anorganischen Chemie System-No.25 Caesium; Springer: Berlin, Germany, 1938; p. 268. [Google Scholar]
- Thomas, R.; Webster, J.D.; Davidson, P. Be-daughter minerals in fluid and melt inclusions: Implications for the enrichment of Be in granite-pegmatite systems. Contrib. Mineral. Petrol. 2011, 161, 483–495. [Google Scholar] [CrossRef]
- Thomas, R.; Davidson, P. Water and melt/melt immiscibility, the essential components in the formation of pegmatites; evidence from melt inclusions. Z. geol. Wiss. Berl. 2008, 36, 347–364. [Google Scholar]
- Götze, J.; Nasdala, L.; Kleeberg, R.; Wenzel, M. Occurence and distribution of “moganite” in agate/chalcedony: A combined micro-Raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol. 1998, 133, 96–105. [Google Scholar] [CrossRef]
- Peretyazhko, I.S.; Zagorskii, V.E.; Smirnov, S.Z.; Mikhailov, M.Y. Conditions of pocket formation in the Oktyabrskaya tourmaline-rich gem pegmatite (the Malkhan field, Central Transbaikalia, Russia). Chem. Geol. 2004, 210, 91–111. [Google Scholar] [CrossRef]
- Zagorskii, V.Y. Malkhan gem tourmaline deposit: Types and nature of miaroles. Dokl. Earth Sci. 2010, 431, 314–317. [Google Scholar] [CrossRef]
- McMillan, P.F.; Wolf, G.H. Vibrational spectroscopy of silicate liquids. Rev. Mineral. 1995, 32, 247–315. [Google Scholar]
- Ihinger, P.D.; Hervig, R.L.; McMillan, P.F. Analytical methods for volatiles in glasses. Rev. Mineral. 1994, 30, 67–121. [Google Scholar]
- Xue, X.; Kanzaki, M. Dissolution mechanisms of water in depolymerized silicate melts: Constraints from 1H and 29Si NMR spectroscopy and ab initio calculations. Geochim. Cosmochim. Acta 2004, 68, 5027–5057. [Google Scholar] [CrossRef]
- Mattson, J.; Wyss, H.M.; Fernandez-Nieves, A.; Miyazaki, K.; Hu, Z.; Reichmann, D.R.; Weitz, D.A. Soft colloids make strong glasses. Nature 2009, 462, 83–86. [Google Scholar]
- Thomas, R.; Davidson, P.; Schmidt, C. Extreme alkali bicarbonate- and carbonate-rich fluid inclusions in granite pegmatite from the Precambrian Rønne granite, Bornholm Island, Denmark. Contrib. Mineral. Petrol. 2011, 161, 315–329. [Google Scholar] [CrossRef]
- Smirnov, S.Z.; Thomas, V.G.; Kamenetsky, V.S.; Kozmenko, O.A.; Large, R.R. Hydrosilicate liquids in the system Na2O-SiO2-H2O with NaF, NaCl and Ta: Evaluation of their role in ore and mineral formation at high T and P. Petrologiya 2012, 20, 300–314. [Google Scholar]
- London, D.; Morgan, G.B., VI. The pegmatite puzzle. Elements 2012, 8, 263–268. [Google Scholar] [CrossRef]
- London, D. Pegmatites; Mineralogical Association of Canada: Ottawa, Canada, 2008. [Google Scholar]
- Thomas, R.; Davidson, P.; Beurlen, H. The competing models for the origin and internal evolution of granitic pegmatites in the light of melt and fluid inclusion research. Mineral. Petrol. 2012, 106, 55–73. [Google Scholar]
- McKenzie, D. The extraction of magma from the crust and mantle. Earth Planet. Sic. Lett. 1985, 74, 81–91. [Google Scholar] [CrossRef]
- Audétat, A.; Keppler, H. Viscosity of fluids in subduction zones. Science 2004, 303, 513–516. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thomas, R.; Davidson, P.; Badanina, E. Water- and Boron-Rich Melt Inclusions in Quartz from the Malkhan Pegmatite, Transbaikalia, Russia. Minerals 2012, 2, 435-458. https://doi.org/10.3390/min2040435
Thomas R, Davidson P, Badanina E. Water- and Boron-Rich Melt Inclusions in Quartz from the Malkhan Pegmatite, Transbaikalia, Russia. Minerals. 2012; 2(4):435-458. https://doi.org/10.3390/min2040435
Chicago/Turabian StyleThomas, Rainer, Paul Davidson, and Elena Badanina. 2012. "Water- and Boron-Rich Melt Inclusions in Quartz from the Malkhan Pegmatite, Transbaikalia, Russia" Minerals 2, no. 4: 435-458. https://doi.org/10.3390/min2040435




