Organocatalytic Enantioselective Henry Reactions
Abstract
:1. Introduction
2. Enantioselective Henry Reaction with Aldehydes
3. Enantioselective Henry Reaction with Ketones
3.1. Henry Reaction with α-Ketoesters
3.2. Henry Reaction with α-Ketophosphonates
3.3. Henry Reaction with Fluoromethylketones
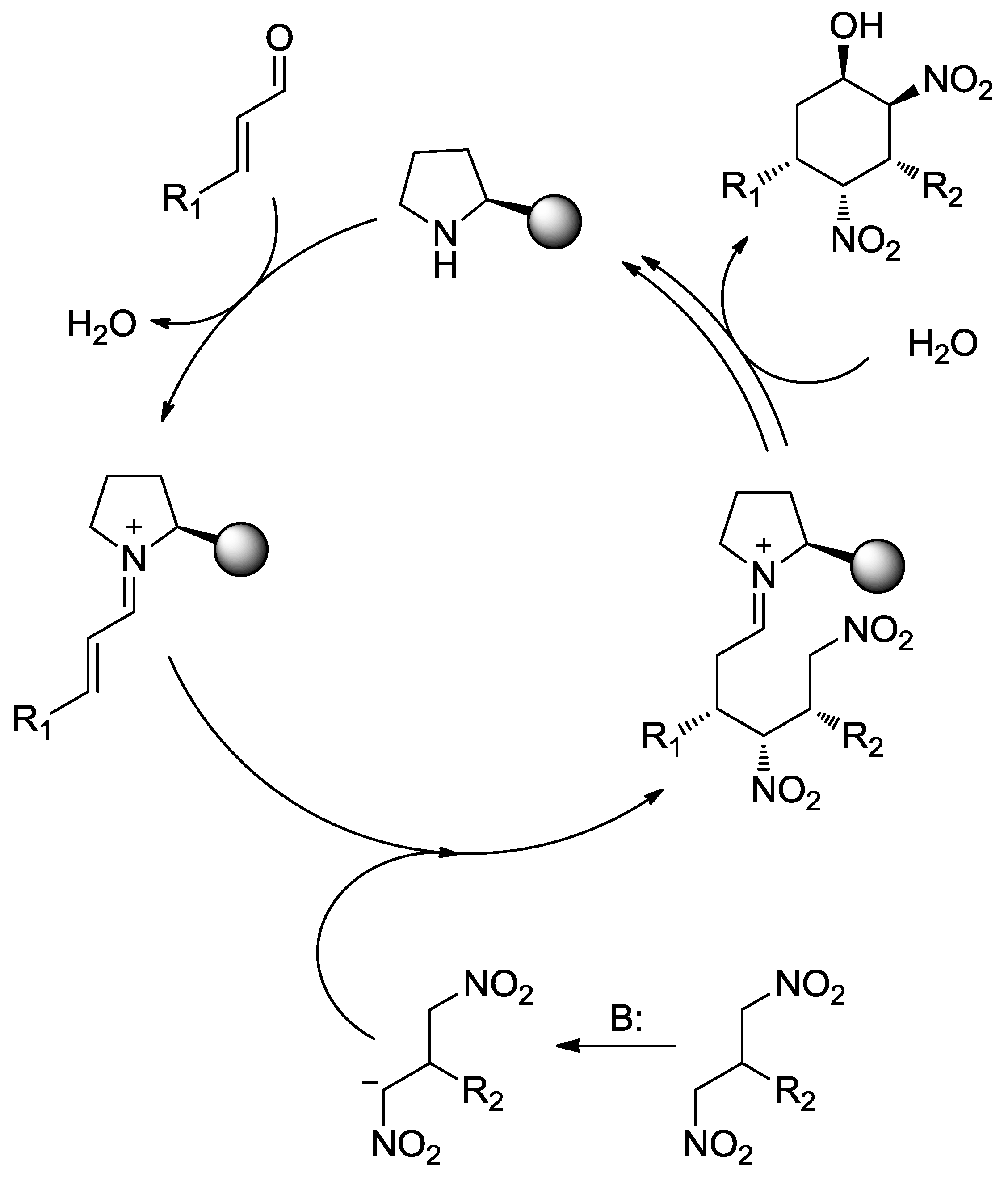
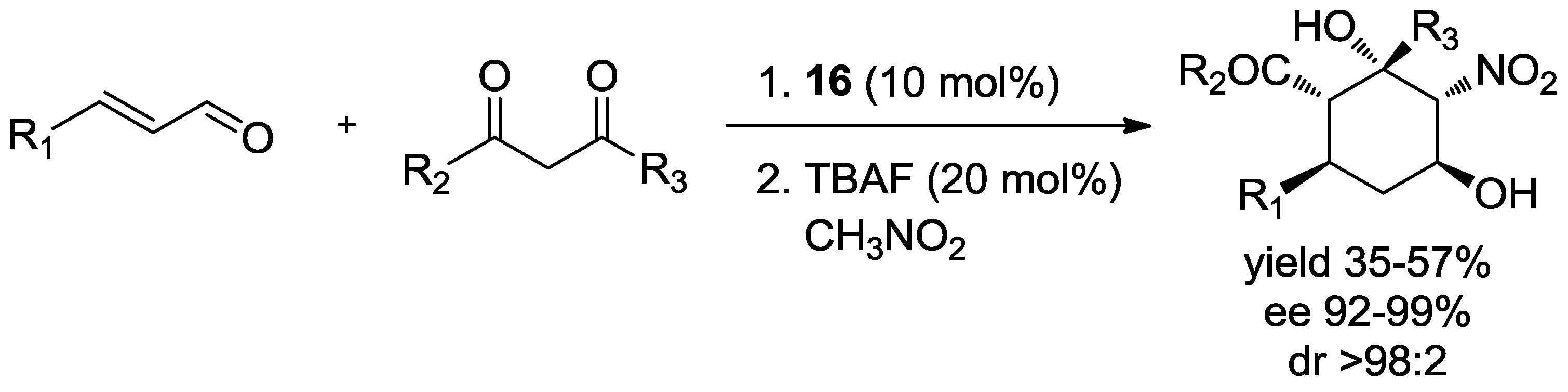
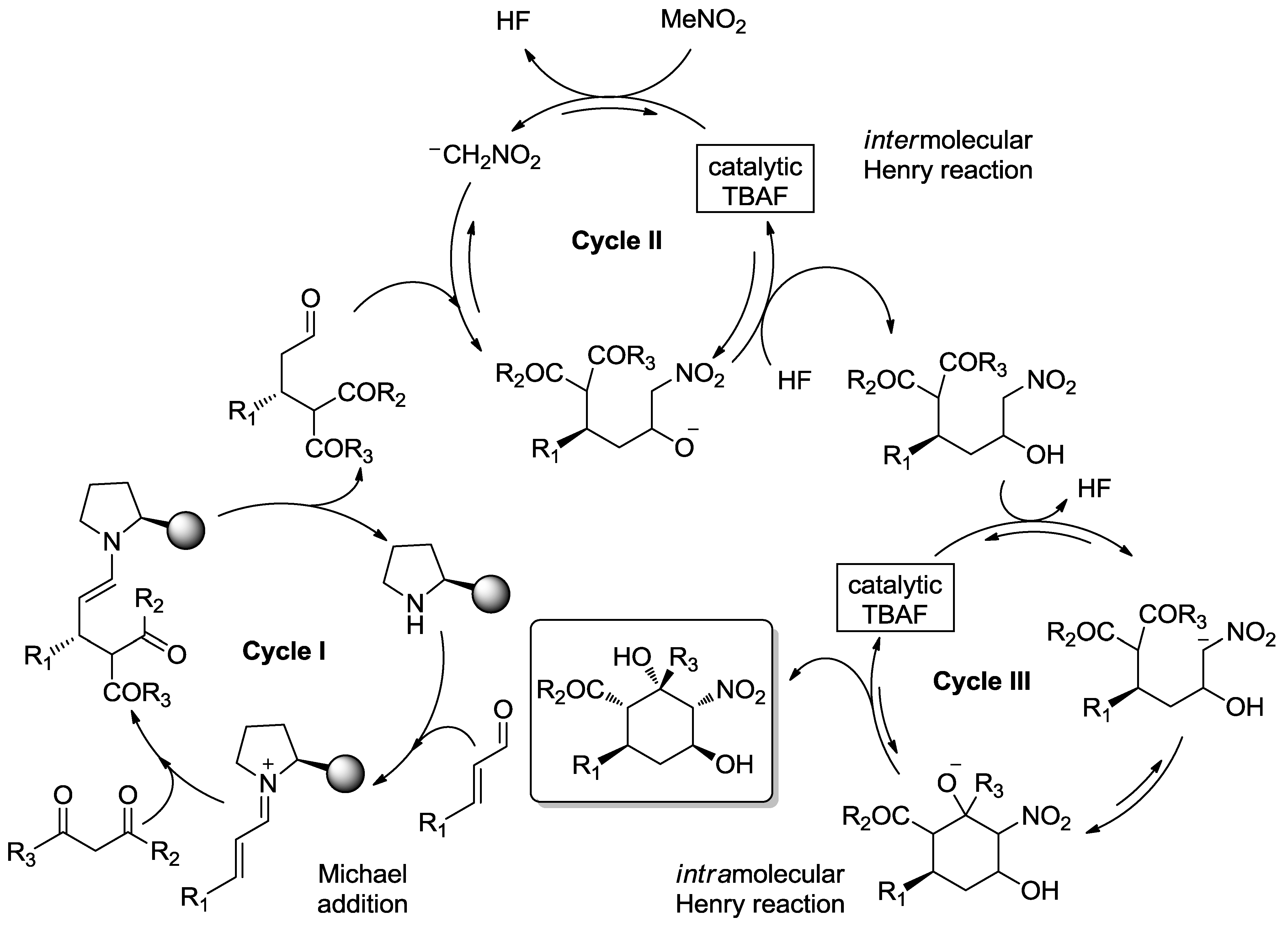
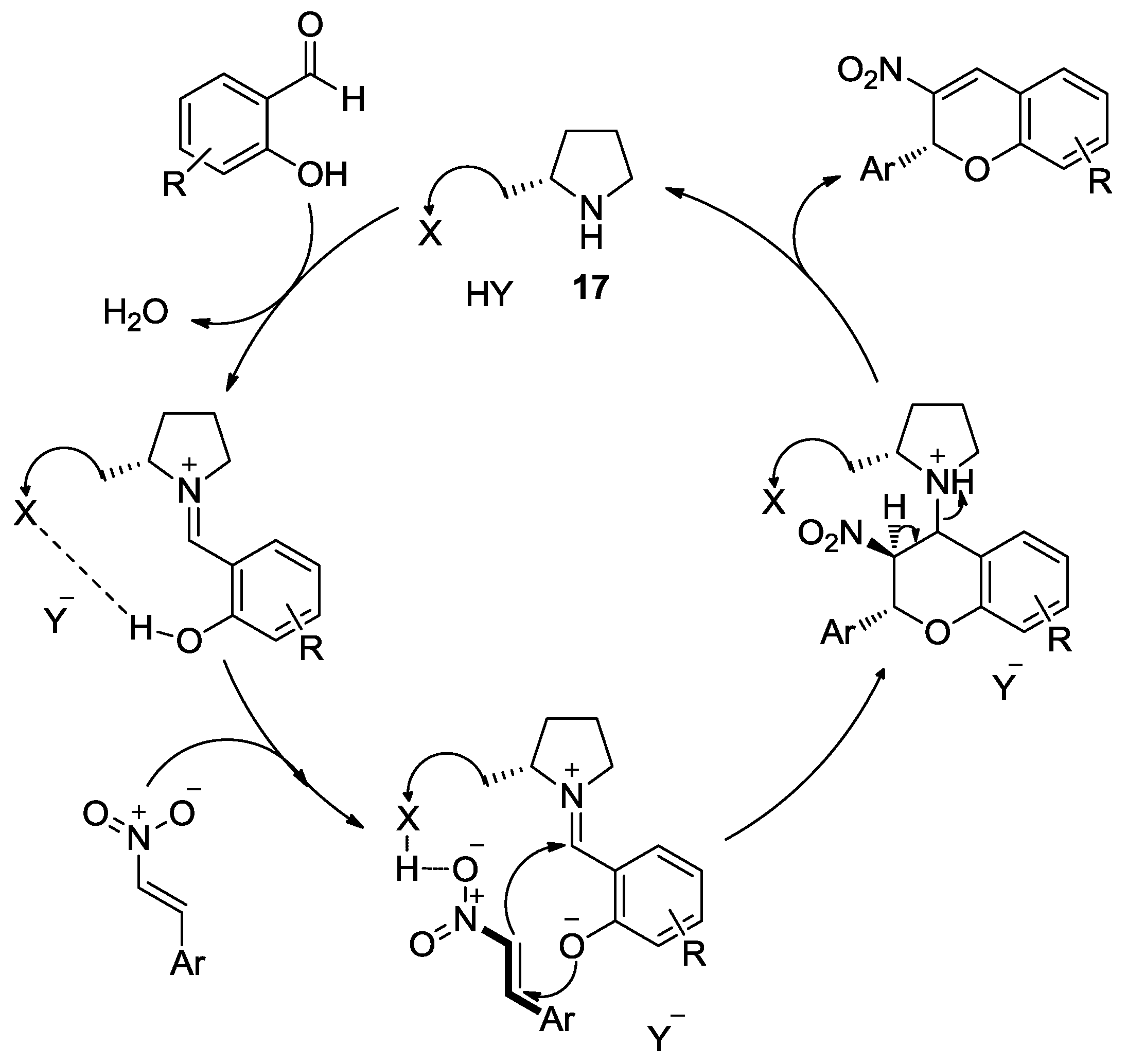
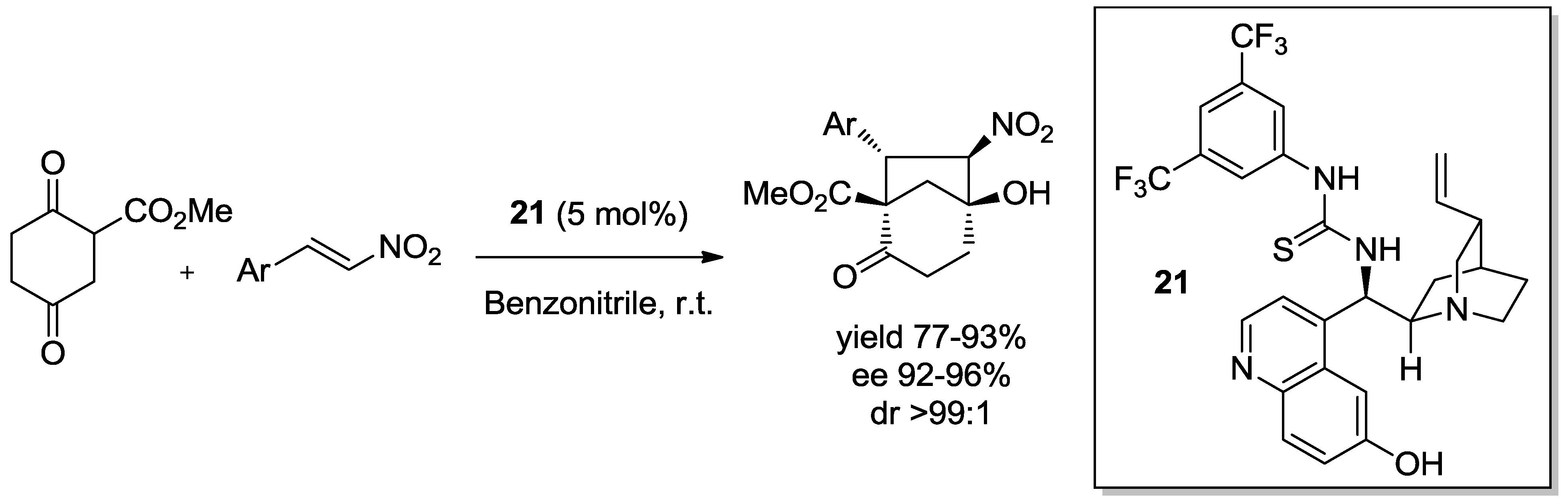
4. Enantioselective Domino Michael/Henry Reactions
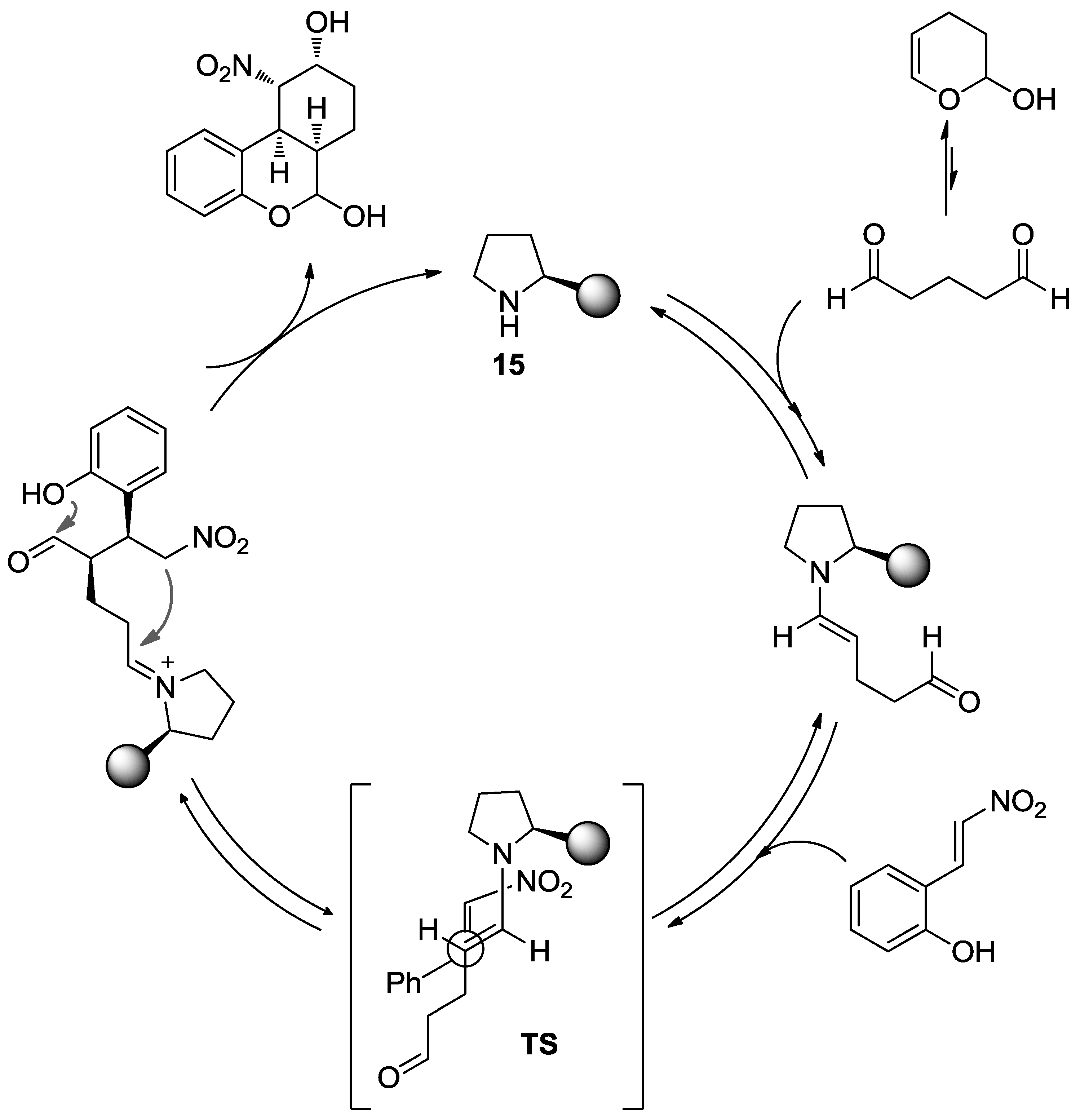
4.1. Aminocatalysis
4.2. Cinchona Alkaloids
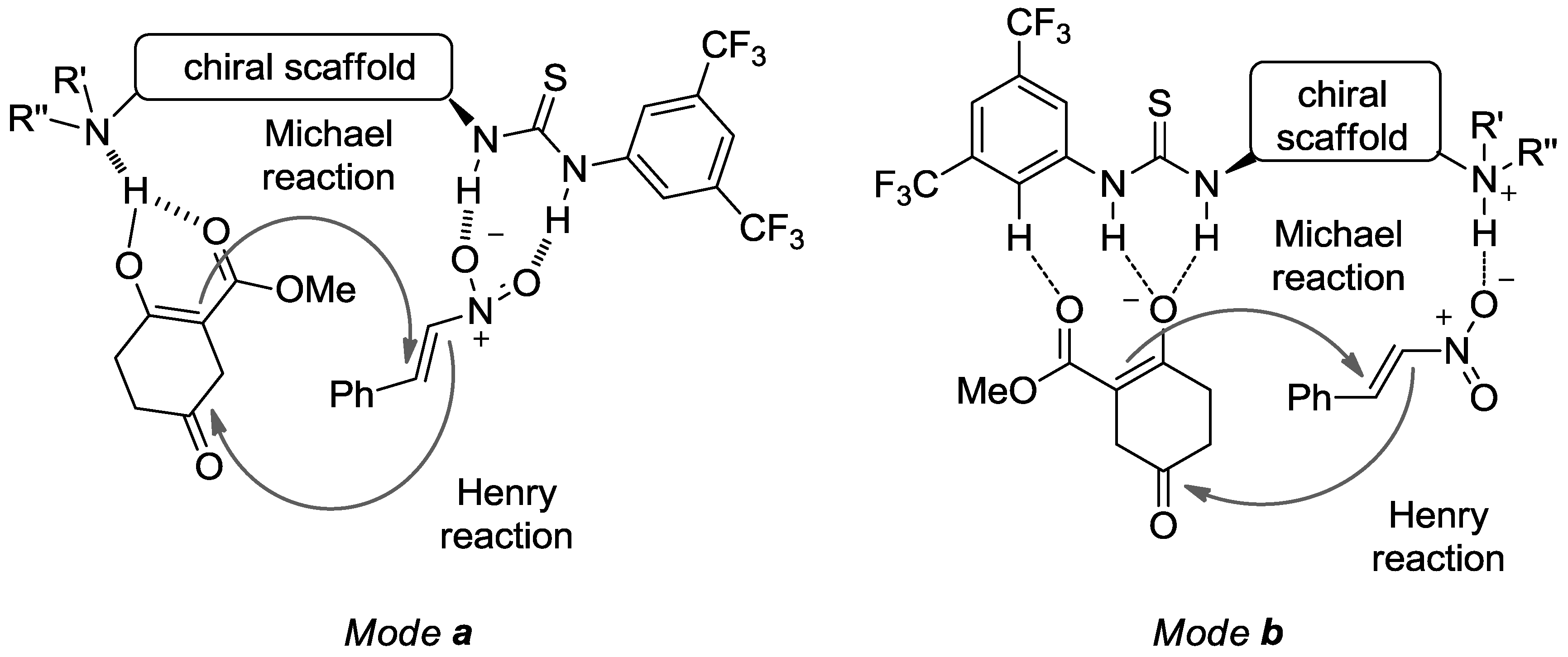
4.3. Thiourea Catalysts
5. Conclusions
Acknowledgements
References
- Luzzio, F.A. The Henry Reaction: Recent Examples. Tetrahedron 2001, 57, 915–945. [Google Scholar] [CrossRef]
- Henry, L. Nitro-alcohols. Compt. Rend. Hebd. Séances Acad. Sci. 1895, 120, 1265–1268. [Google Scholar]
- The Nitro Group in Organic Synthesis; Ono, N. (Ed.) Wiley-VCH: New York, NY, USA, 2001. [Google Scholar]
- Bergmeier, S.C. The Synthesis of Vicinal Amino Alcohols. Tetrahedron 2000, 56, 2561–2576. [Google Scholar] [CrossRef]
- Berkessel, A.; Gröger, H. Asymmetric Organocatalysis; Wiley-VHC: Weinheim, Germany, 2004. [Google Scholar]
- Enantioselective Organocatalysis; Dalko, P.I. (Ed.) Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Organocatalysis; Reetz, M.T.; List, B.; Jaroch, S.; Weinmann, H. (Eds.) Springer-Verlag: Berlin, Germany, 2008. [Google Scholar]
- Wong, O.A.; Shi, Y. Chiral Ketones and Iminium Catalysts for Olefin Epoxidation. Top. Curr. Chem. 2010, 291, 201–232. [Google Scholar]
- Guizzetti, S.; Benaglia, M. Trichlorosilane-Mediated Stereoselective Reduction of C=N Bonds. Eur. J. Org. Chem. 2010, 2010, 5529–5541. [Google Scholar] [CrossRef]
- Marqués-López, E.; Merino, P.; Tejero, T.; Herrera, R.P. Catalytic Enantioselective aza-Henry Reactions. Eur. J. Org. Chem. 2009, 15, 2401–2420. [Google Scholar] [CrossRef]
- Masson, G.; Housseman, C.; Zhu, J. The Enantioselective Morita-Baylis-Hillman Reaction and its aza Counterpart. Angew. Chem. Int. Ed. Engl. 2007, 46, 4614–4628. [Google Scholar] [CrossRef]
- Pihko, P.M.; Majander, I.; Erkkilä, A. Enamine Catalysis. Top. Curr. Chem. 2010, 291, 29–75. [Google Scholar]
- Merino, P.; Marqués-López, E.; Herrera, R.P. Catalytic Enantioselective Hydrophosphonylation of Aldehydes and Imines. Adv. Synth. Catal. 2008, 350, 1195–1208. [Google Scholar] [CrossRef]
- Merino, P.; Marqués-López, E.; Tejero, T.; Herrera, R.P. Organocatalyzed Strecker Reactions. Tetrahedron 2009, 65, 1219–1234. [Google Scholar] [CrossRef]
- Marqués-López, E.; Diez-Martinez, A.; Merino, P.; Herrera, R.P. The Role of the Indole in Important Organocatalytic Enantioselective Friedel-Crafts Alkylation Reactions. Curr. Org. Chem. 2009, 13, 1585–1609. [Google Scholar] [CrossRef]
- Terrasson, V.; de Figueiredo, R.M.; Campagne, J.M. Organocatalyzed Asymmetric Friedel-Crafts Reactions. Eur. J. Org. Chem. 2010, 2010, 2635–2655. [Google Scholar] [CrossRef]
- Almaşi, D.; Alonso, D.A.; Nájera, C. Organocatalytic Asymmetric Conjugate Additions. Tetrahedron: Asymmetry 2007, 18, 299–365. [Google Scholar] [CrossRef]
- Vicario, J.L.; Badía, D.; Carrillo, L. Organocatalytic Enantioselective Michael and Hetero-Michael Reactions. Synthesis 2007, 14, 2065–2092. [Google Scholar] [CrossRef]
- Tsogoeva, S.B. Recent Advances in Asymmetric Organocatalytic 1,4-Conjugate Additions. Eur. J. Org. Chem. 2007, 1701–1716. [Google Scholar] [CrossRef]
- Catalytic Asymmetric Conjugate Reactions; Córdova, A. (Ed.) Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Roca-López, D.; Sadaba, D.; Delso, I.; Herrera, R.P.; Tejero, T.; Merino, P. Asymmetric Organocatalytic Synthesis of γ-Nitrocarbonyl Compounds through Michael and Domino Reactions. Tetrahedron: Asymmetry 2010, 21, 2561–2601. [Google Scholar]
- Merino, P.; Marqués-López, E.; Tejero, T.; Herrera, R.P. Enantioselective Organocatalytic Diels-Alder Reactions. Synthesis 2010, 1, 1–26. [Google Scholar] [CrossRef]
- Verkade, J.M.M.; van Hemert, L.J.C.; Quaedflieg, P.J.L.M.; Rutjes, F.P.J.T. Organocatalysed Asymmetric Mannich Reactions. Chem. Soc. Rev. 2008, 37, 29–41. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, R.M.; Christmann, M. Organocatalytic Synthesis of Drugs and Bioactive Natural Products. Eur. J. Org. Chem. 2007, 2007, 2575–2600. [Google Scholar] [CrossRef]
- Marqués-López, E.; Herrera, R.P.; Cristmann, M. Asymmetric Organocatalysis in Total Synthesis—a Trial by Fire. Nat. Prod. Rep. 2010, 27, 1138–1167. [Google Scholar] [CrossRef] [PubMed]
- Boruwa, J.; Gogoi, N.; Saikia, P.P.; Barua, N.C. Catalytic Asymmetric Henry Reaction. Tetrahedron: Asymmetry 2006, 17, 3315–3326. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; Laso, A. Recent Advances in the Catalytic Asymmetric Nitroaldol (Henry) Reaction. Eur. J. Org. Chem. 2007, 2007, 2561–2574. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C.; Sánchez-Agulló, P. Enantiomerically Pure Guanidine-Catalysed Asymmetric Nitroaldol Reaction. Tetrahedron: Asymmetry 1994, 5, 1393–1402. [Google Scholar] [CrossRef]
- Allingham, M.T.; Howard-Jones, A.; Murphy, P.J.; Thomas, D.A.; Caulkett, P.W.R. Synthesis and Applications of C2-Symmetric Guanidine Bases. Tetrahedron Lett. 2003, 44, 8677–8680. [Google Scholar] [CrossRef]
- Ma, D.; Pan, Q.; Han, F. Diastereoselective Henry Reactions of N,N-Dibenzyl α-Amino Aldehydes with Nitromethane Catalyzed by Enantiopure Guanidines. Tetrahedron Lett. 2002, 43, 9401–9403. [Google Scholar] [CrossRef]
- Sohtome, Y.; Hashimoto, Y.; Nawasaga, K. Guanidine-Thiourea Bifunctional Organocatalyst for the Asymmetric Henry (Nitroaldol) Reaction. Adv. Synth. Catal. 2005, 347, 1643–1648. [Google Scholar] [CrossRef]
- Sohtome, Y.; Takemura, N.; Toshitsugu, I.; Hashimoto, Y.; Nawasaga, K. Diastereoselective Henry Reaction Catalyzed by Guanidine-Thiourea Bifunctional Organocatalyst. Synlett 2006, 1, 144–146. [Google Scholar] [CrossRef]
- Sohtome, Y.; Hashimoto, Y.; Nawasaga, K. Diastereoselective and Enantioselective Henry (Nitroaldol) Reaction Utilizing a Guanidine-Thiourea Bifuntional Organocatalyst. Eur. J. Org. Chem. 2006, 2006, 2894–2897. [Google Scholar] [CrossRef]
- Sohtome, Y.; Takemura, N.; Takada, K.; Takagi, R.; Toshitsugu, I.; Nawasaga, K. Organocatalytic Asymmetric Nitroaldol Reaction: Cooperative Effects of Guanidine and Thiourea Functional Groups. Chem. Asian J. 2007, 2, 1150–1160. [Google Scholar] [CrossRef]
- Ube, H.; Terada, M. Enantioselective Henry (Nitroaldol) Reaction Catalyzed by Axially Chiral Guanidines. Bioorg. Med. Chem. Lett. 2009, 19, 3895–3898. [Google Scholar] [CrossRef]
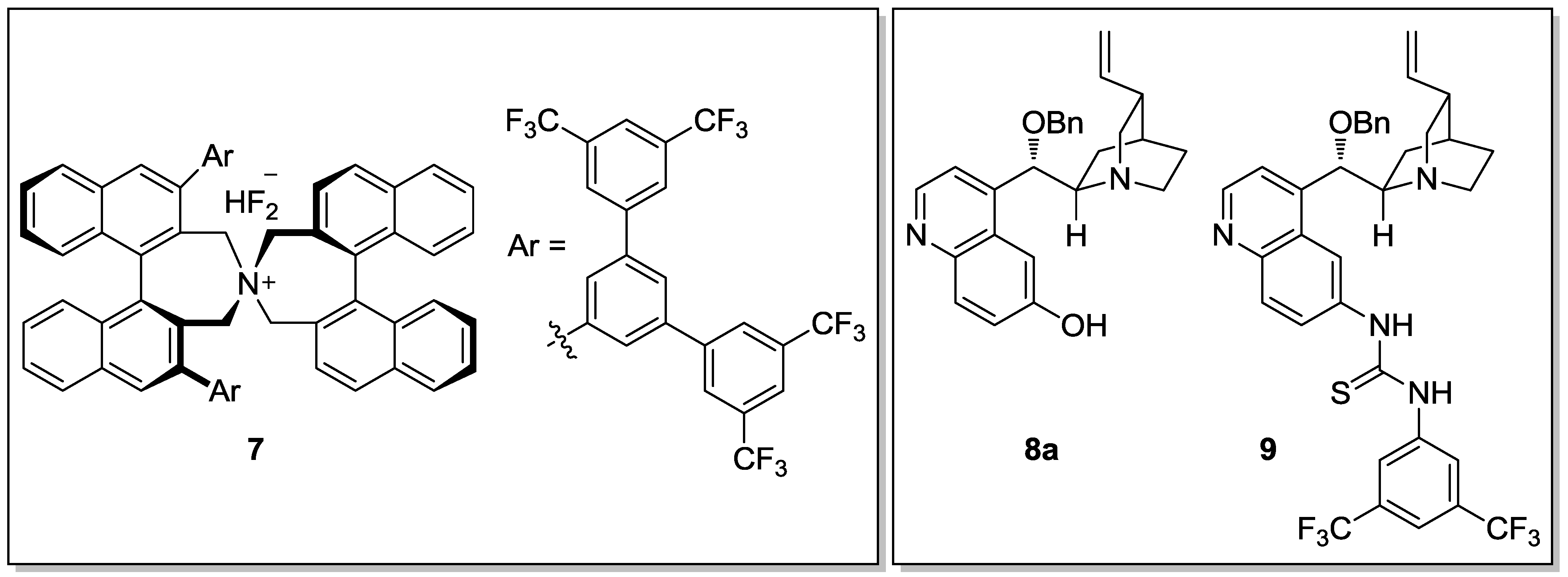
- Ooi, T.; Doda, K.; Maruoka, K. Designer Chiral Quaternary Ammonium Bifluorides as an Efficient Catalyst for Asymmetric Nitroaldol Reaction of Silyl Nitronates with Aromatic Aldehydes. J. Am. Chem. Soc. 2003, 125, 2054–2055. [Google Scholar] [CrossRef] [PubMed]
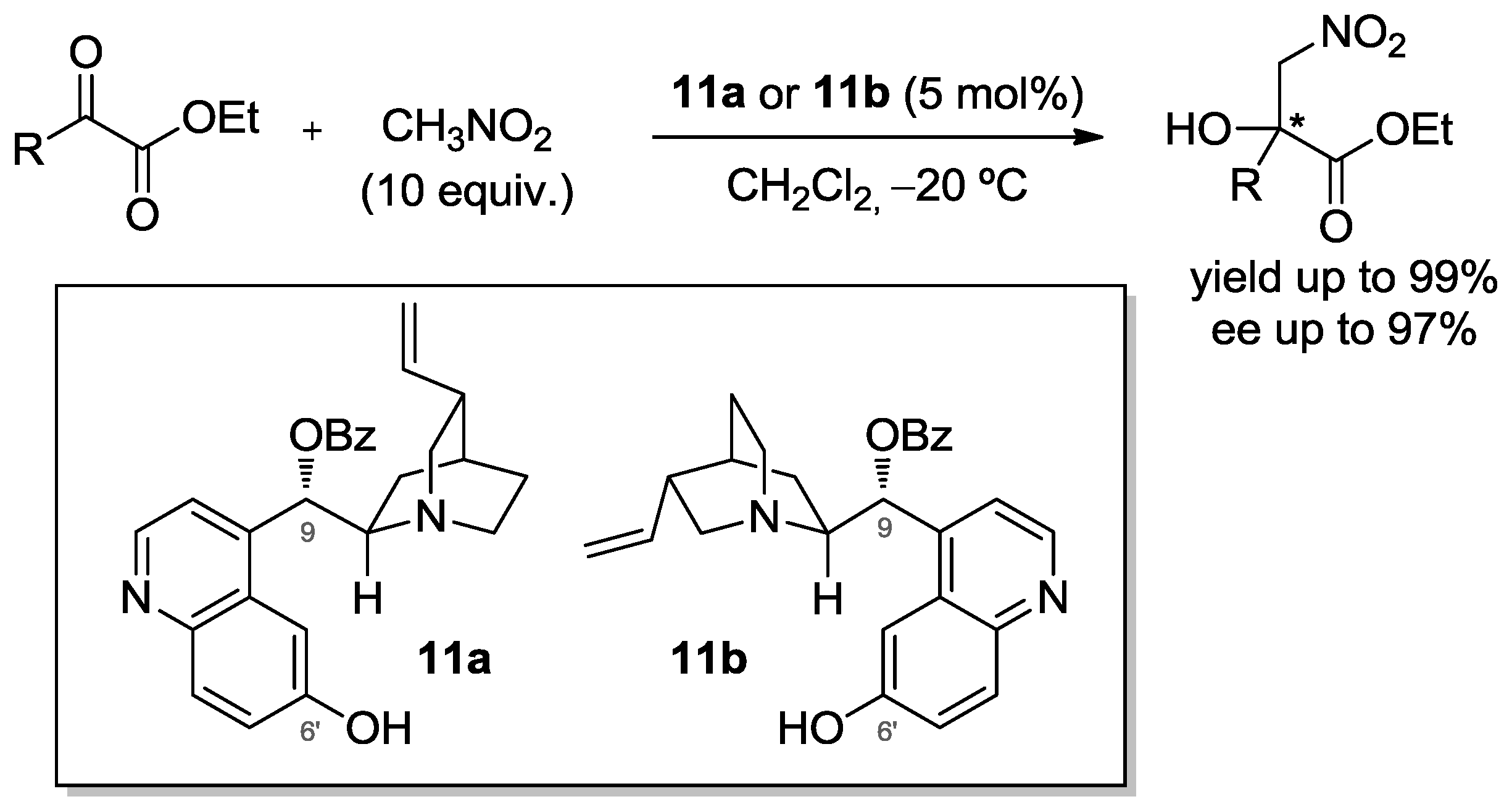
- Marcelli, T.; van der Haas, R.N.S.; van Maarseveen, J.H.; Hiemstra, H. Cinchona Derivatives as Bifunctional Organocatalysts for the Direct Asymmetric Nitroaldol (Henry) Reaction. Synlett 2005, 18, 2817–2819. [Google Scholar] [CrossRef]
- Marcelli, T.; van der Haas, R.N.S.; van Maarseveen, J.H.; Hiemstra, H. Asymmetric Organocatalytic Henry Reaction. Angew. Chem. Int. Ed. 2006, 45, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, J.; Shi, M. Development of Axially Chiral Bis(arylthiourea)-based Organocatalysts and their Application in the Enantioselective Henry Reaction. Tetrahedron: Asymmetry 2007, 18, 2773–2781. [Google Scholar] [CrossRef]
- Bella, M.; Gasperi, T. Organocatalytic Formation of Quaternary Stereocenters. Synthesis 2009, 10, 1583–1614. [Google Scholar] [CrossRef]
- Riant, O.; Hannodouche, J. Asymmetric Catalysis for the Construction of Quaternary Carbon Centres: Nucleophilic Addition on Ketones and Ketimines. Org. Biomol. Chem. 2007, 5, 873–888. [Google Scholar] [CrossRef] [PubMed]
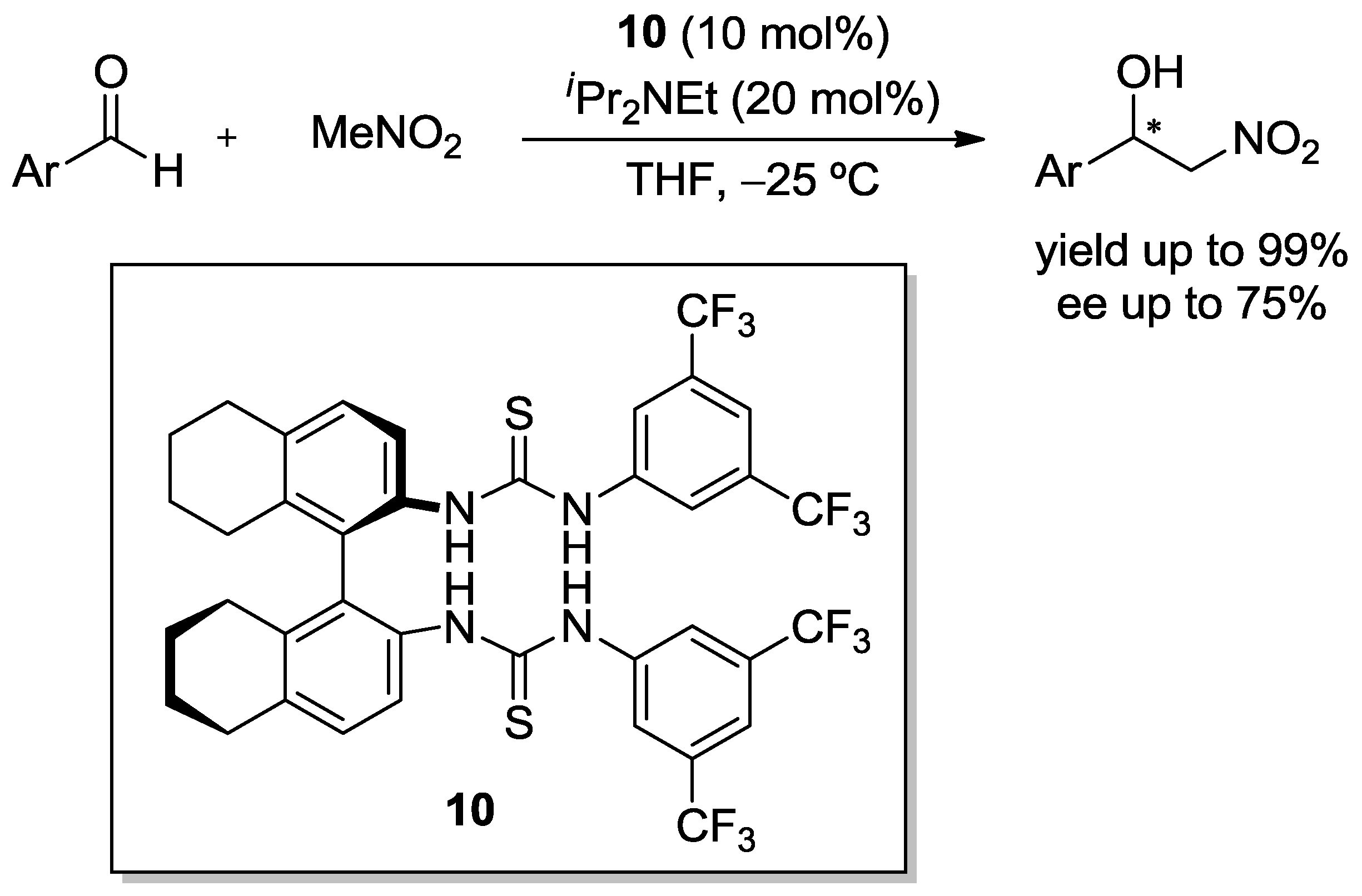
- Li, H.; Wang, B.; Deng, L. Enantioselective Nitroaldol Reaction of α-Ketoesters Catalyzed by Cinchona Alkaloids. J. Am. Chem. Soc. 2006, 128, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Takemura, N.; Cho, K.; Sohtome, Y.; Nawasaga, K. Asymmetric Organocatalytic Nitroaldol Reaction of α-Ketoesters: Stereoselective Construction of Chiral Tertiary Alcohols at Subzero Temperature. Tetrahedron Lett. 2008, 49, 1623–1626. [Google Scholar] [CrossRef]
- Mandal, T.; Samanta, S.; Zhao, C. Organocatalytic Highly Enantioselective Nitroaldol Reaction of α-Ketophosphonates and Nitromethane. Org. Lett. 2007, 9, 943–945. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Zhu, Y.; Shang, D.; Gao, B.; Liu, X.; Feng, X.; Su, Z.; Hu, C. A Secondary Amine Amide Organocatalyst for the Asymmetric Nitroaldol Reaction of α-Ketophosphonates. Chem. Eur. J. 2008, 14, 10896–10899. [Google Scholar] [CrossRef]
- Bandini, M.; Sinisi, R.; Umani-Ronchi, A. Enantioselective Organocatalyzed Henry Reaction with Fluoromethyl Ketones. Chem. Commun. 2008, 36, 4360–4362. [Google Scholar] [CrossRef] [PubMed]
- Guillena, G.; Ramón, D.J.; Yus, M. Organocatalytic Enantioselective Multicomponent Reactions (OEMCRs). Tetrahedron: Asymmetry 2007, 18, 693–700. [Google Scholar] [CrossRef]
- Bonne, D.; Coquerel, Y.; Constantieux, T.; Rodriguez, J. 1,3-Dicarbonyl Compounds in Stereoselective Domino and Multicomponent Reactions. Tetrahedron: Asymmetry 2010, 21, 1085–1109. [Google Scholar] [CrossRef]
- Enders, D.; Grondal, C.; Hüttl, M.R.M. Asymmetric Organocatalytic Domino Reactions. Angew. Chem. Int. Ed. 2007, 46, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, W. Organocatalysis: Asymmetric Cascade Reactions Catalysed by Chiral Secondary Amines. Org. Biomol. Chem. 2008, 6, 2037–2046. [Google Scholar] [CrossRef]
- Alba, A.N.; Companyó, X.; Viciano, M.; Rios, R. Organocatalytic Domino Reactions. Curr. Org. Chem. 2009, 13, 1432–1474. [Google Scholar] [CrossRef]
- Hayashi, Y.; Okano, T.; Aratake, S.; Hazelard, D. Diphenylprolinol Silyl Ether as Catalyst in an Enantioselective, Catalytic, Tandem Michael/Henry Reaction for the Control of Four Stereocenters. Angew. Chem. Int. Ed. 2007, 46, 4922–4925. [Google Scholar] [CrossRef]
- Reyes, E.; Jiang, A.; Milelli, A.; Elsner, P.; Hazell, R.T.; Jørgensen, K.A. How to Make Five Contiguous Stereocenters in One Reaction: Asymmetric Organocatalytic Synthesis of Pentasubstituted Cyclohexanes. Angew. Chem. Int. Ed. 2007, 46, 9202–9205. [Google Scholar] [CrossRef]
- García Ruano, J.L.; Marcos, V.; Suanzes, J.A.; Marzo, L.; Alemán, J. One-Pot Synthesis of Pentasubstituted Cyclohexanes by a Michael Addition Followed by a Tandem Inter-Intra Double Henry Reaction. Chem. Eur. J. 2009, 15, 6576–6580. [Google Scholar] [CrossRef]
- Xu, D.Q.; Wang, Y.F.; Luo, S.P.; Zhang, S.; Zhong, A.G.; Chen, H.; Xu, Z.Y. A Novel Enantioselective Catalytic Tandem oxa-Michael/Henry Reaction: One-pot Organocatalytic Asymmetric Synthesis of 3-Nitro-2H-chromenes. Adv. Synth. Catal. 2008, 350, 2610–2616. [Google Scholar] [CrossRef]
- Hong, B.C.; Kotame, P.; Liao, J.H. Enantioselective Organocatalytic Domino Michael-Acetalization-Henry Reactions of 2-Hydroxynitrostyrene and Aldehydes for the Synthesis of Tetrahydro-6H-benzo[c]chromenones. Org. Biomol. Chem. 2011, 9, 382–386. [Google Scholar] [CrossRef] [PubMed]
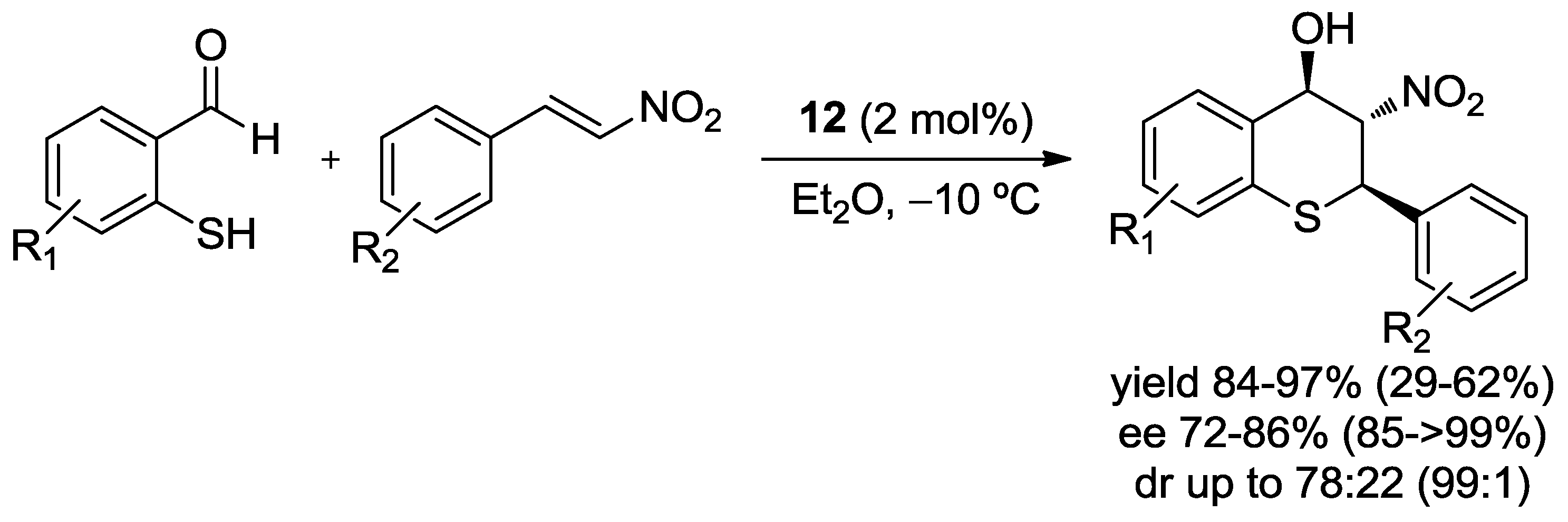
- Dodda, R.; Goldman, J.J.; Mandal, T.; Zhao, C.G.; Broker, G.A.; Tiekink, R.T. Synthesis of 2,3,4-Trisubstituted Thiochromanes Using an Organocatalytic Enantioselective Tandem Michael/Henry Reaction. Adv. Synth. Catal. 2008, 350, 537–541. [Google Scholar] [CrossRef] [PubMed]
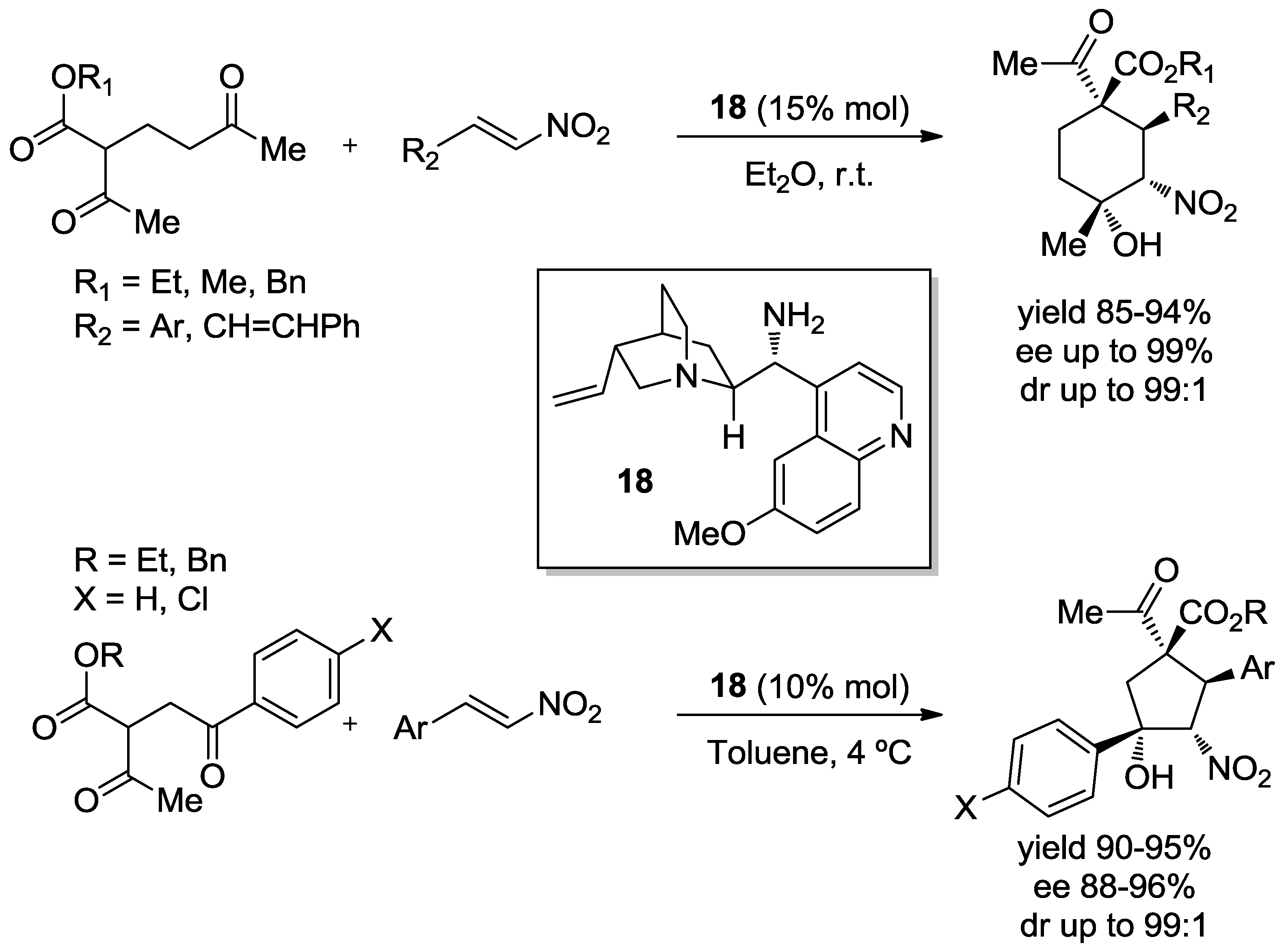
- Tan, B.; Chua, P.J.; Li, Y.; Zhong, G. Organocatalytic Asymmetric Tandem Michael/Henry Reactions: a Hihgly Stereoselective Synthesis of Multifunctionalized Cyclohexanes with Two Quaternary Stereocenters. Org. Lett. 2008, 10, 2437–2440. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Chua, P.J.; Zeng, X.; Lu, M.; Zhong, G.A. Highly Diastereo- and Enantioselective Synthesis of Multisubstituted Cyclopentanes with Four Chiral Carbons by the Organocatalytic Domino Michael/Henry Reaction. Org. Lett. 2008, 10, 3489–3492. [Google Scholar] [CrossRef] [PubMed]
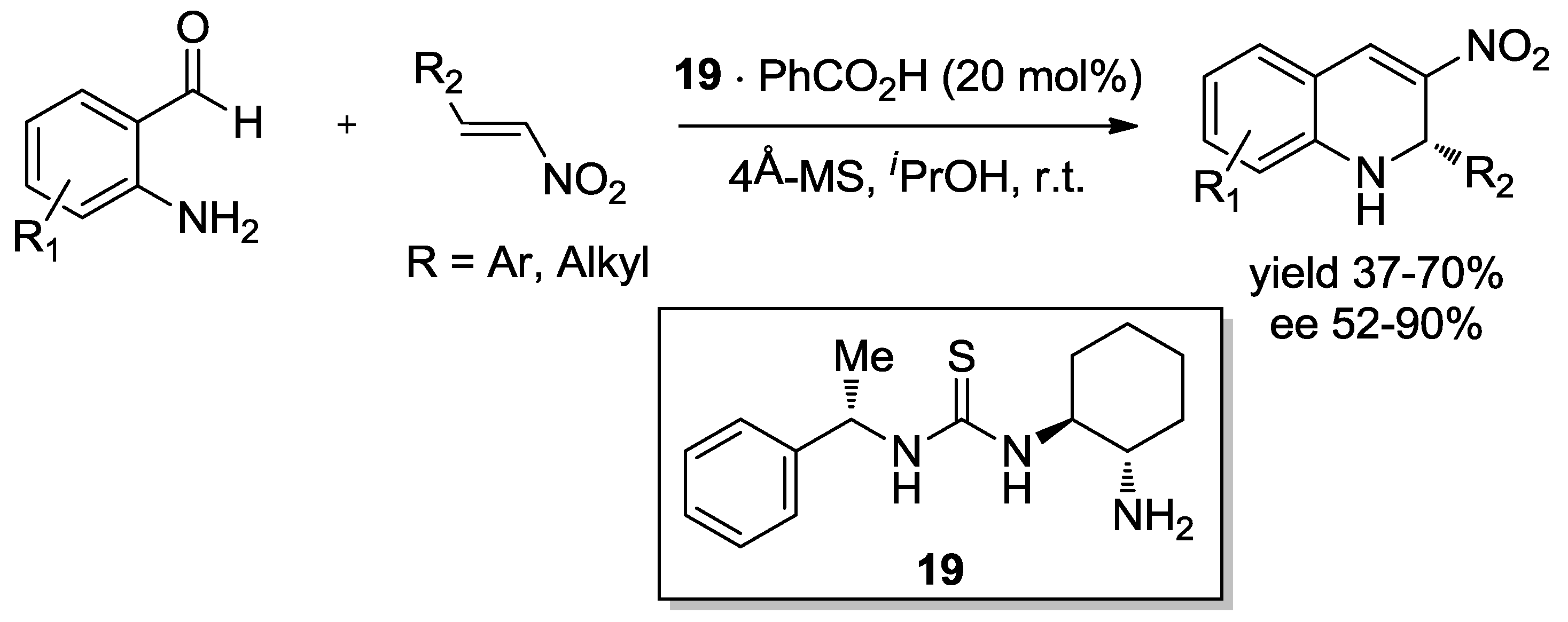
- Wang, Y.F.; Zhang, W.; Luo, S.P.; Li, B.L.; Xia, A.B.; Zhong, A.G.; Xu, D.Q. One-Pot Organocatalytic Asymmetric Synthesis of 3-Nitro-1,2-dihydroquinolines by a Dual-Activation Protocol. Chem. Asian J. 2009, 4, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Imashiro, R.; Hernández-Torres, G.; Barbas, C.F., III. Organocatalytic Asymmetric Assembly Reactions for the Syntheses of Carbohydrate Derivatives by Intermolecular Michael/Henry Reactions. Proc. Natl. Acad. Sci. USA 2010, 107, 20672–20677. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Lu, Y.; Zeng, X.; Chua, P.J.; Zhong, G. Facile Domino Access to Chiral Bicyclo[3.2.1]Octanes and Discovery of a New Catalytic Activation Mode. Org. Lett. 2010, 12, 2682–2685. [Google Scholar] [CrossRef] [PubMed]

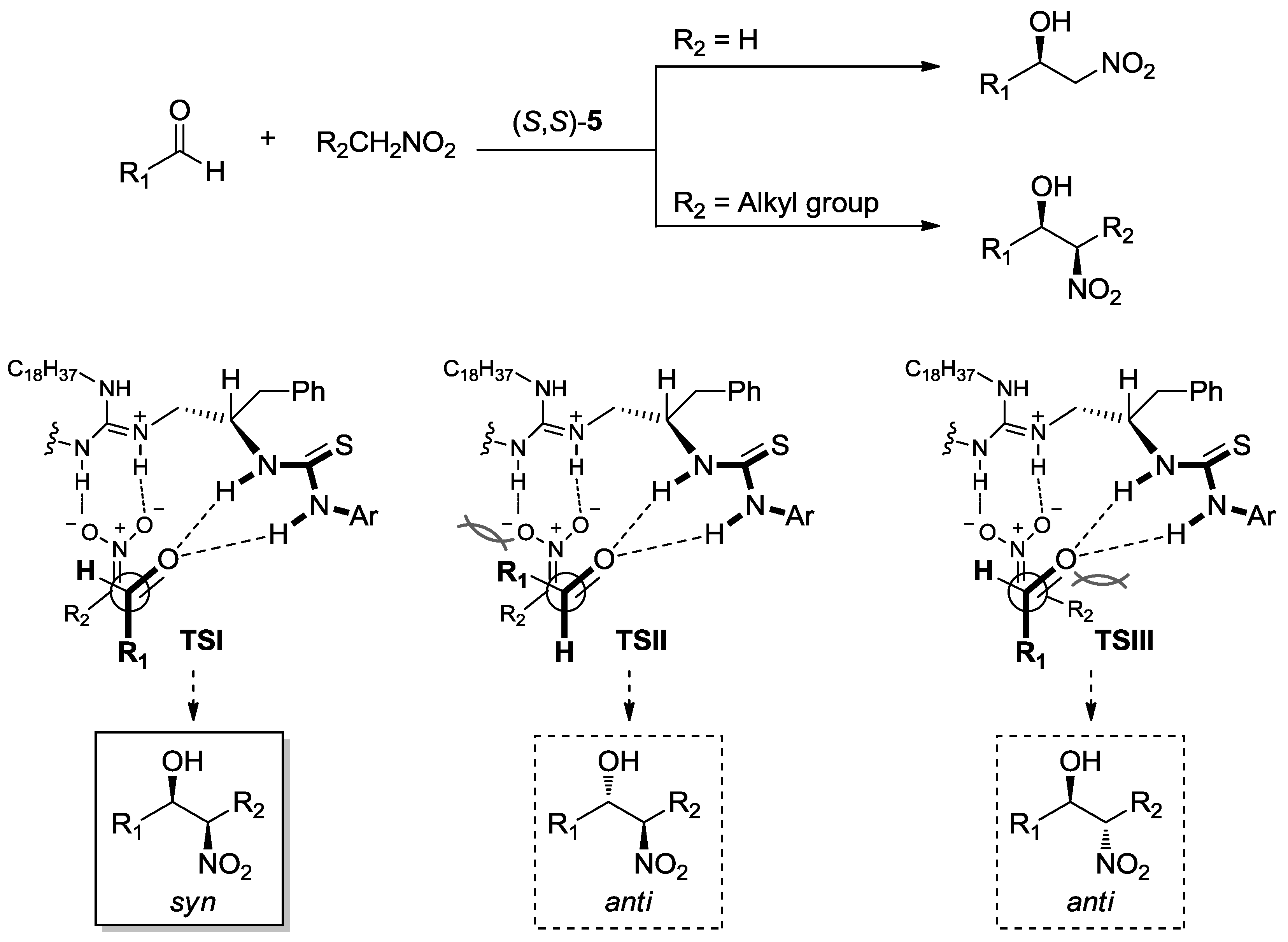
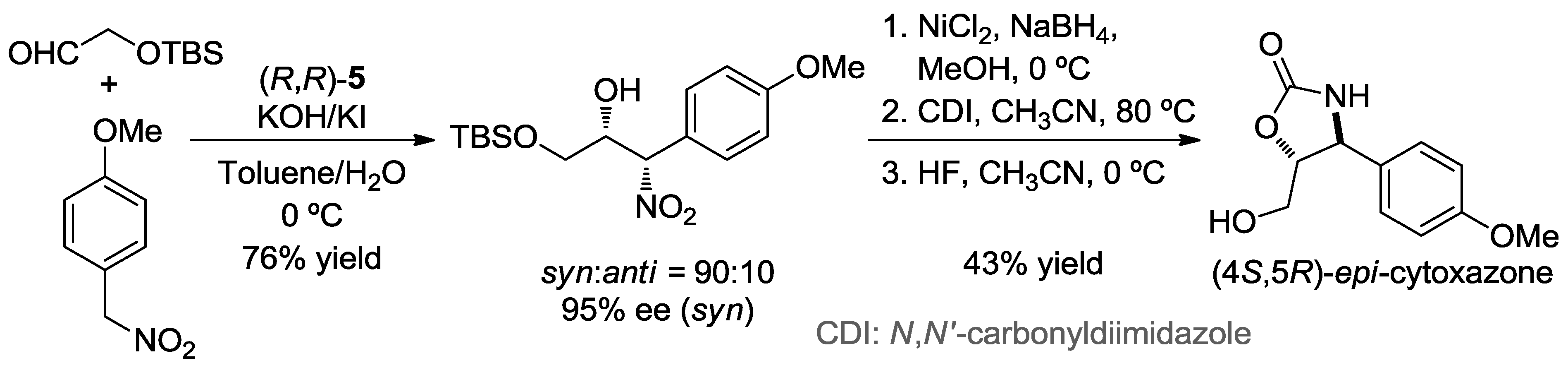
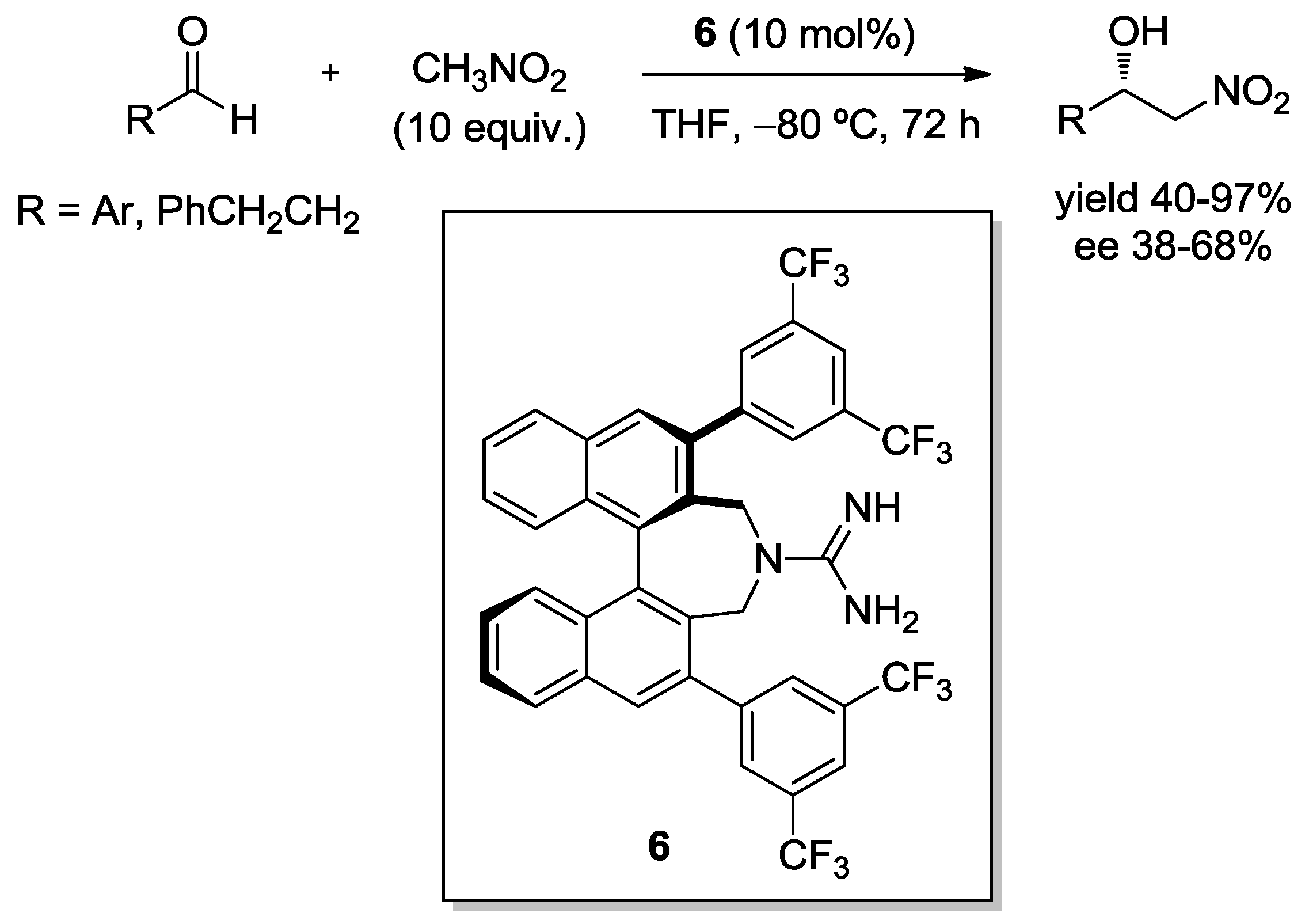
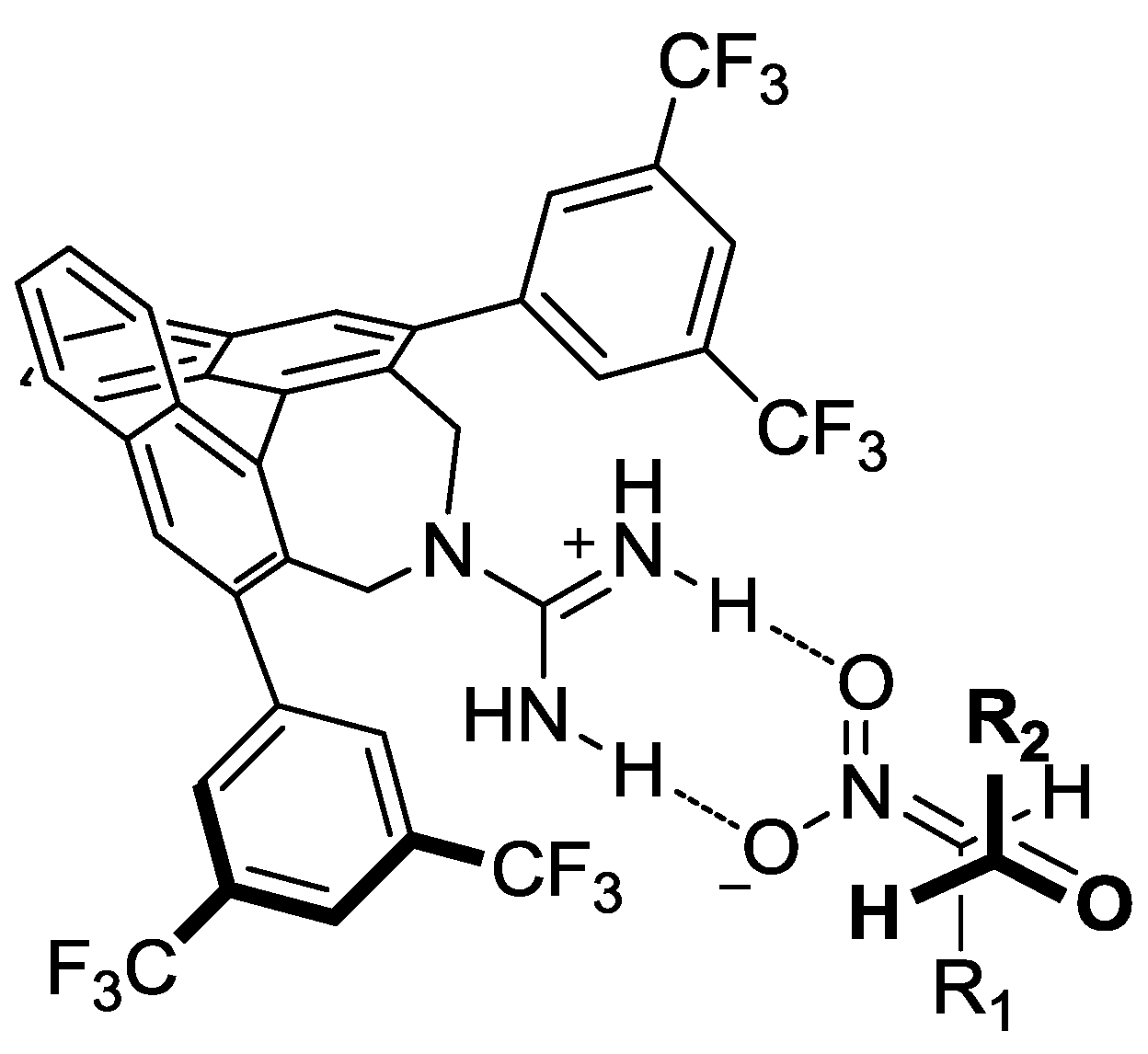
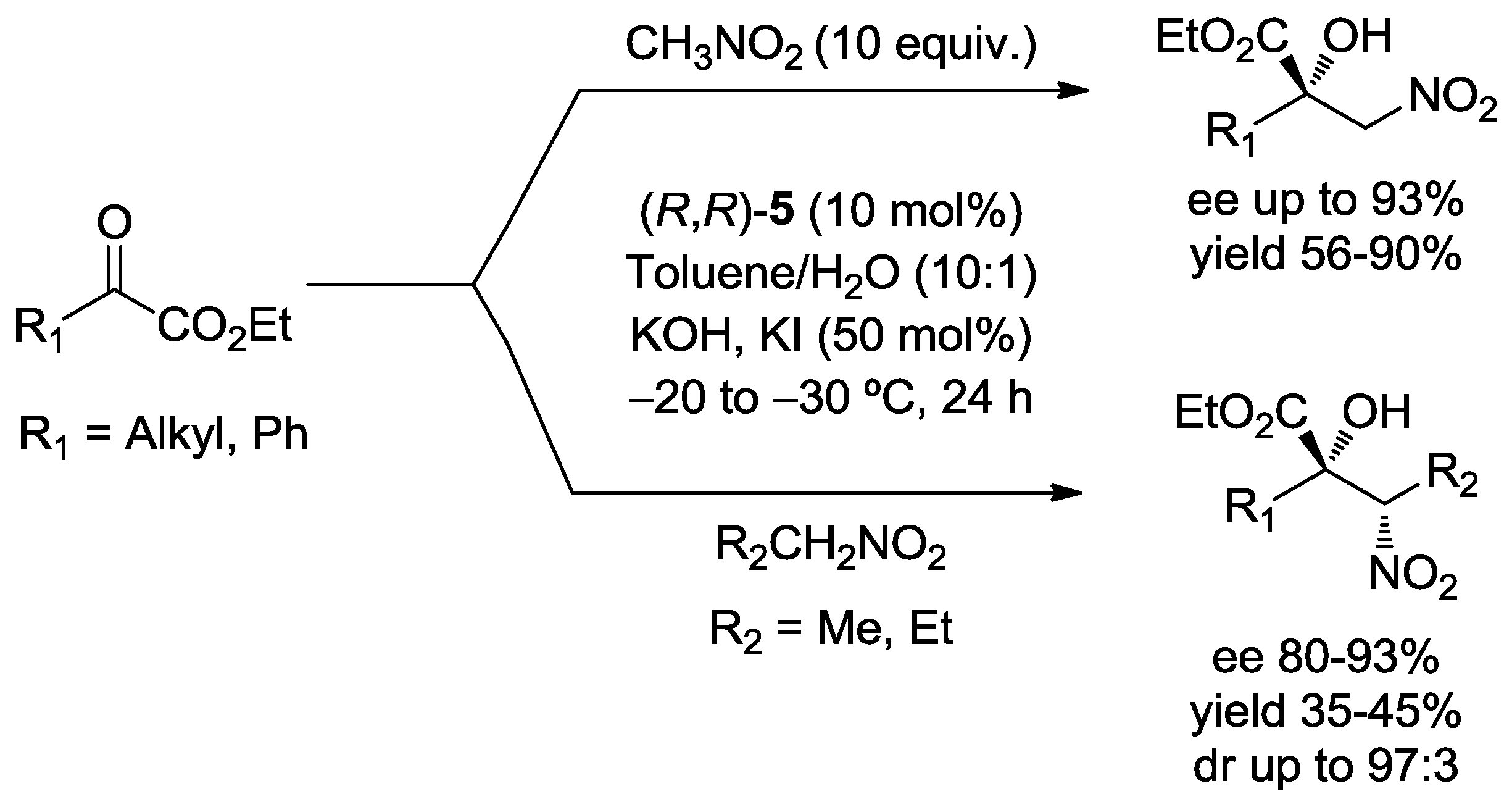


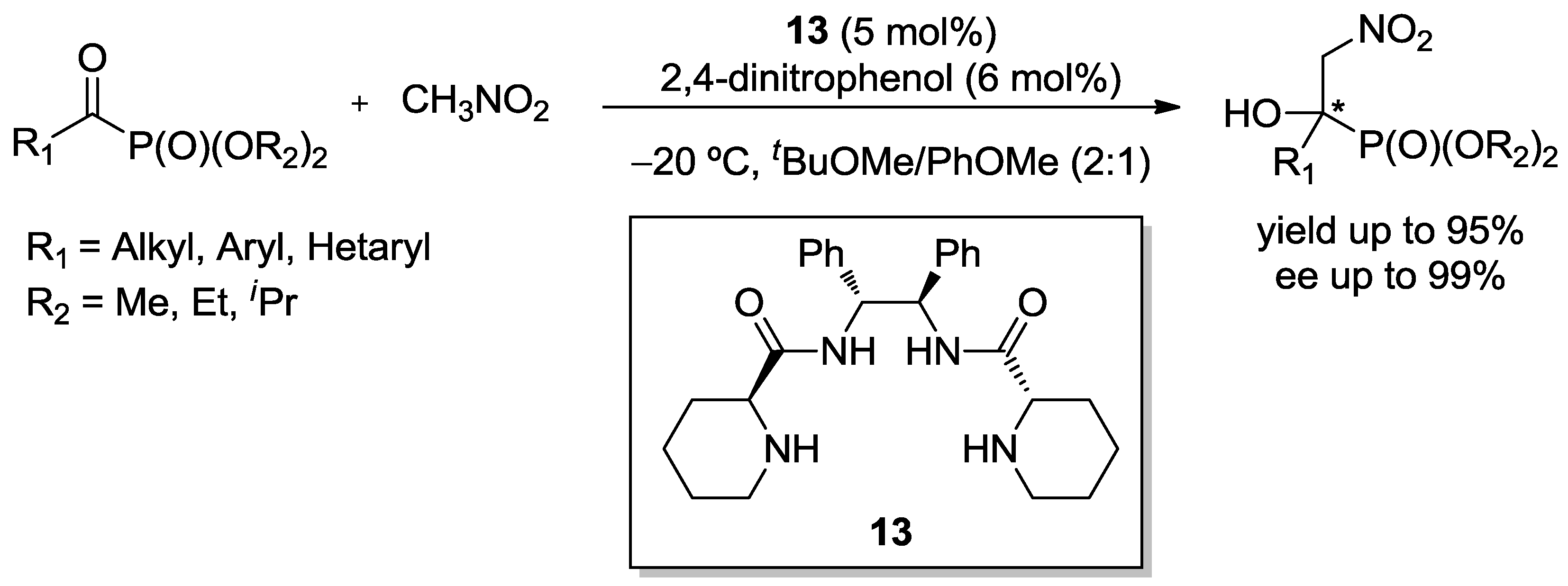
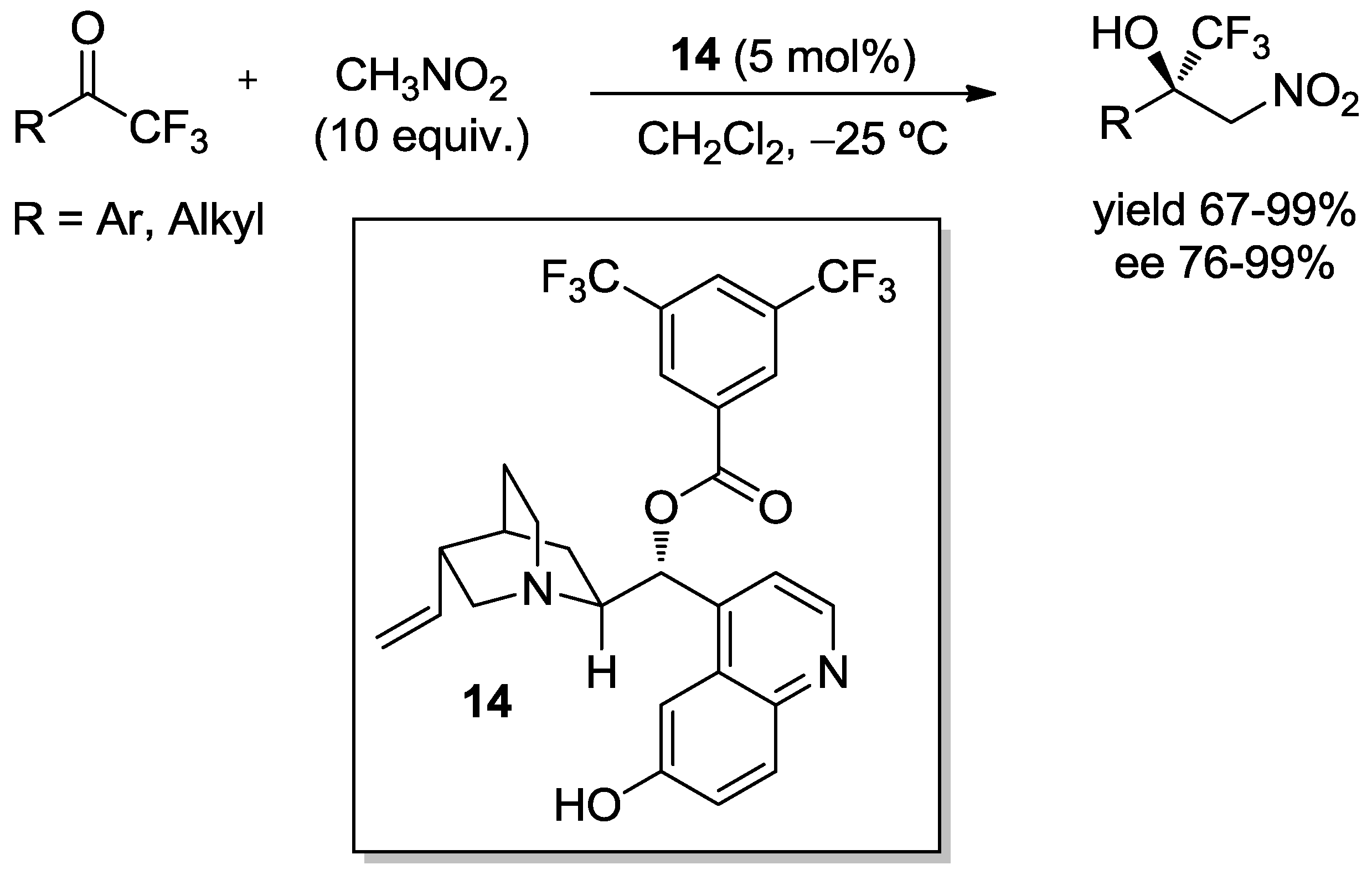
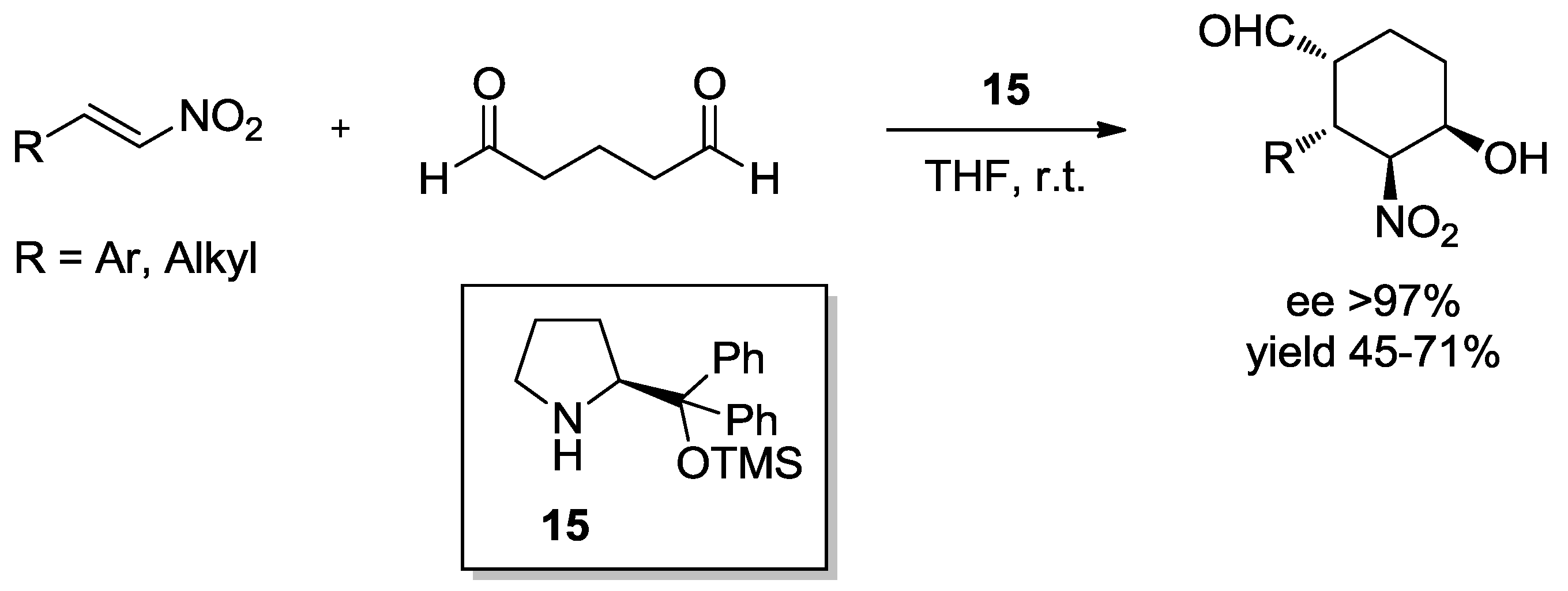
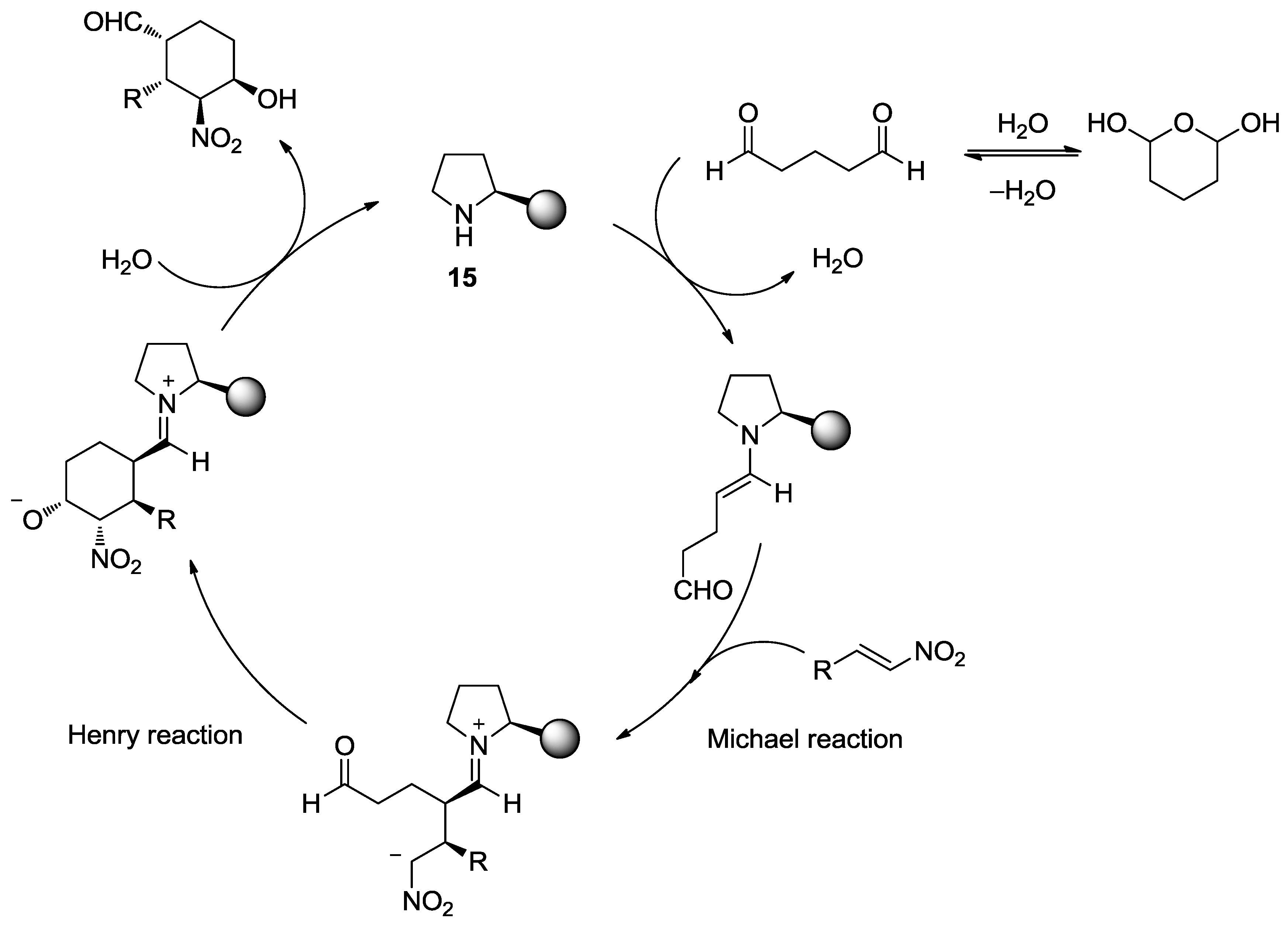



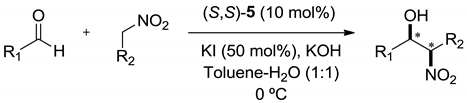
| R1 | R2 | KOH (mol%) | Time (h) | Yield (%) | syn:anti | ee (syn) (%) |
|---|---|---|---|---|---|---|
| CH3(CH2)2 | CH3(CH2)2NO2 | 5 | 48 | 63 | 90:10 | 85 |
| CH3(CH2)2 | TBSO(CH2)2NO2 | 3 | 48 | 51 | 97:3 | 87 |
| CH3(CH2)2 | TIPSO(CH2)2NO2 | 3 | 24 | 58 | 92:8 | 87 |
| CH3(CH2)2 | PhCH2NO2 | 10 | 24 | 70 | 91:9 | 87 |
| c-C6H11 | CH3(CH2)2NO2 | 5 | 40 | 61 | 99:1 | 95 |
| c-C6H11 | TBSO(CH2)2NO2 | 7 | 48 | 63 | 99:1 | 90 |
| c-C6H11 | TIPSO(CH2)2NO2 | 6 | 48 | 60 | 99:1 | 90 |
| c-C6H11 | PhCH2NO2 | 7 | 48 | 67 | 99:1 | 95 |

| R | Yield (%) | anti:syn | ee (anti) (%) | ee (syn) (%) |
|---|---|---|---|---|
 | 72 | 79:21 | 78 | 87 |
 | 56 | 76:24 | 81 | 89 |
 | 75 | 94:6 | 69 | 74 |
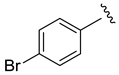 | 81 | 80:20 | 58 | 57 |
 | 66 | 87:13 | 56 | 10 |
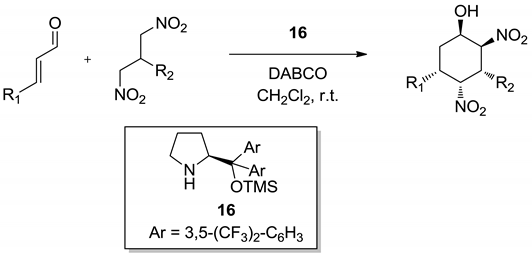
| R1 | R2 | Yield (%) | dr | ee (%) |
|---|---|---|---|---|
| Et |  | 45 | 4:2:1 | 90 |
| Me |  | 43 | 4:1:1 | 75 |
| n-Pr |  | 44 | 4:2:1 | 86 |
| i-Pr |  | 38 | 3:1:1 | 90 |
| n-Bu |  | 43 | 4:2:1 | 87 |
| CH2OTIPS |  | 56 | 3:1:0 | 94 |
| cis-C6H11 |  | 52 | 4:2:1 | 86 |
| C7H16 |  | 50 | 4:2:1 | 87 |
| Et | 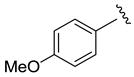 | 48 | 4:2:1 | 92 |
| i-Pr |  | 53 | 5:1:1 | 89 |
| i-Pr |  | 65 | 12:2:3 | 90 |
| i-Pr | 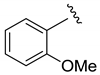 | 48 | 5:2:1 | 90 |
| i-Pr |  | 40 | 5:1:1.2 | 84 |
| i-Pr | 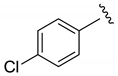 | 47 | 5:1:1 | 88 |
| i-Pr | 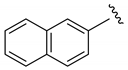 | 61 | 5:0:1 | 88 |
| Et | 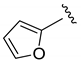 | 60 | 4:2:1 | 86 |
| i-Pr |  | 43 | 6:1:0 | 88 |
| Et | 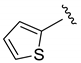 | 42 | 4:0:1 | 80 |
| i-Pr |  | 43 | 5:1:1 | 90 |
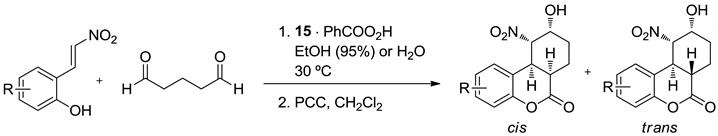
| R | in EtOH (95%) | in H2O | Yield (%) b | ee (%) c | ||
|---|---|---|---|---|---|---|
| t (h) | cis:trans a | t2 (h) | cis:trans a | |||
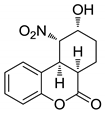 | 42 | 88:12 | 24 | 81:19 | 50 (76) | >99 |
 | 45 | 86:14 | 24 | 88:12 | 63 (77) | >99 |
 | 46 | 87:13 | 30 | 80:20 | 65 (81) | >99 |

| R | t1 (h) | t2 (h) | Yield (%) | dr | ee (%) | |
|---|---|---|---|---|---|---|
| A+B:C | A:B | |||||
 | 4 | 1 | 68 | >10:1 | 3:1 | 98 |
 | 4 | 0.5 | 62 | >10:1 | 4:1 | 98 |
 | 4 | 1.5 | 76 | >10:1 | 3:1 | 97 |
 | 5 | 0.5 | 68 | >10:1 | 6:1 | 97 |
 | 16 | 4 | 37 | 1:0 | 0:1 | 99 |
 | 7 | 0.5 | 63 | 7:1 | 13:1 | 97 |
 | 6 | 0.3 | 43 | 6:1 | 1:0 | 93 |
| C7H15 | 5 | 18 | 44 | >10:1 | 1:0 | 96 |
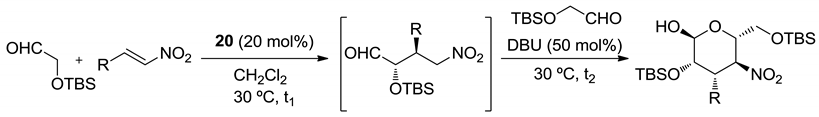
| R | t1 (h) | t2 (h) | Yield (%) | ee (%) |
|---|---|---|---|---|
 | 4 | 1 | 51 | 98 |
 | 4 | 1 | 65 | 96 |
 | 23 | 1 | 48 | 95 |
 | 5 | 1 | 57 | 98 |
 | 7 | 1 | 59 | 96 |
 | 20 | 2 | 66 | 93 |
| C7H15 | 5 | 1 | 50 | 96 |
© 2011 by the authors. licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alvarez-Casao, Y.; Marques-Lopez, E.; Herrera, R.P. Organocatalytic Enantioselective Henry Reactions. Symmetry 2011, 3, 220-245. https://doi.org/10.3390/sym3020220
Alvarez-Casao Y, Marques-Lopez E, Herrera RP. Organocatalytic Enantioselective Henry Reactions. Symmetry. 2011; 3(2):220-245. https://doi.org/10.3390/sym3020220
Chicago/Turabian StyleAlvarez-Casao, Yolanda, Eugenia Marques-Lopez, and Raquel P. Herrera. 2011. "Organocatalytic Enantioselective Henry Reactions" Symmetry 3, no. 2: 220-245. https://doi.org/10.3390/sym3020220
APA StyleAlvarez-Casao, Y., Marques-Lopez, E., & Herrera, R. P. (2011). Organocatalytic Enantioselective Henry Reactions. Symmetry, 3(2), 220-245. https://doi.org/10.3390/sym3020220







