Antibody Glycosylation and Inflammation
Abstract
:1. Introduction
2. IgG and FcγRs

3. The Contribution of the Fc Glycan to IgG
3.1. Pro-Inflammatory IgG Fc Glycans
3.2. Anti-Inflammatory IgG Fc Glycans
4. IgG Glycovariants under Physiological and Patho-Physiological Conditions
5. The Role of Glycans in IgE Biology
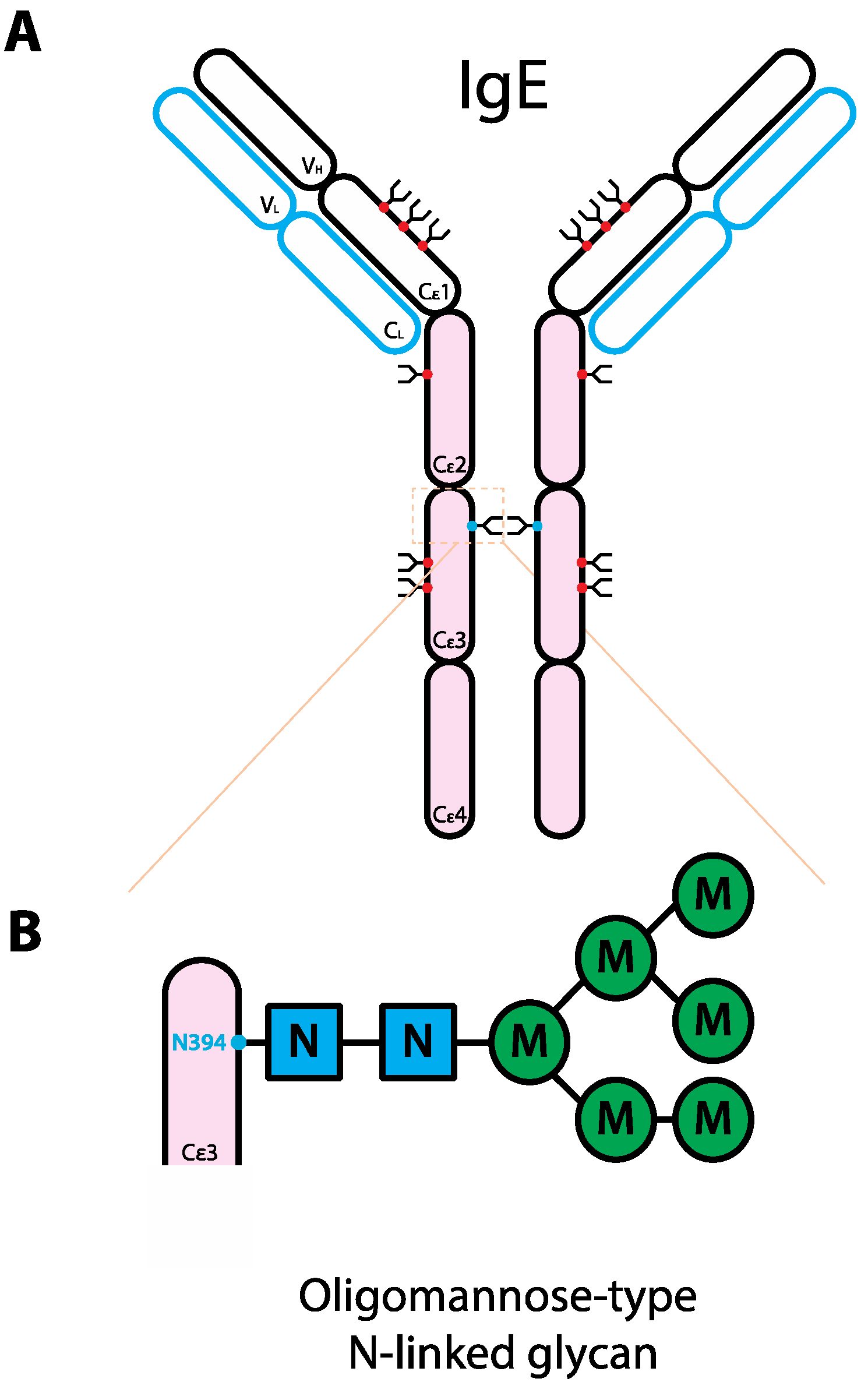
6. The Role of Glycans in IgA Biology
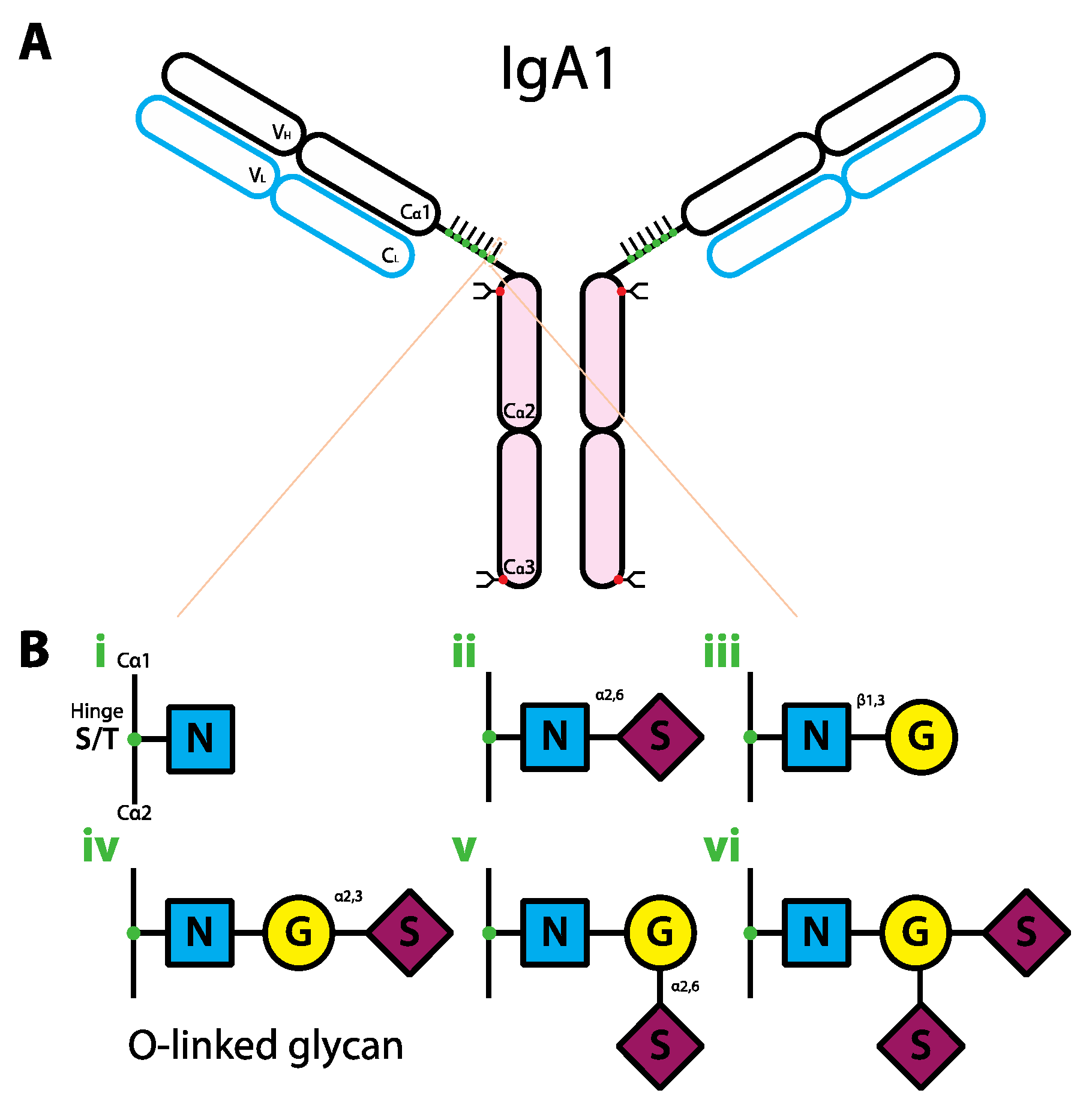
7. The Role of Glycans in IgM Biology

8. Summary
Acknowledgment
Conflict of Interest
References
- Chang, T.W.; Wu, P.C.; Hsu, C.L.; Hung, A.F. Anti-IgE Antibodies for the treatment of IgE-mediated allergic diseases. Adv Immunol. 2007, 93, 63–119. [Google Scholar] [CrossRef]
- Kempeni, J. Preliminary results of early clinical trials with the fully human anti-TNFα monoclonal antibody D2E7. Ann. Rheum. Dis. 1999, 58, I70–I72. [Google Scholar] [CrossRef]
- Maini, R.; St Clair, E.W.; Breedveld, F.; Furst, D.; Kalden, J.; Weisman, M.; Smolen, J.; Emery, P.; Harriman, G.; Feldmann, M.; et al. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. Lancet 1999, 354, 1932–1939. [Google Scholar] [CrossRef]
- Targan, S.R.S.; Hanauer, S.B.S.; van Deventer, S.J.S.; Mayer, L.L.; Present, D.H.D.; Braakman, T.T.; DeWoody, K.L.K.; Schaible, T.F.T.; Rutgeerts, P.J.P. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn“s disease. Crohn”s Disease cA2 Study Group. N. Engl. J. Med. 1997, 337, 1029–1035. [Google Scholar]
- Hudis, C.A. Trastuzumab—Mechanism of action and use in clinical practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef]
- Maloney, D.G.; Grillo-López, A.J.; White, C.A.; Bodkin, D.; Schilder, R.J.; Neidhart, J.A.; Janakiraman, N.; Foon, K.A.; Liles, T.M.; Dallaire, B.K.; et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 1997, 90, 2188–2195. [Google Scholar]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar]
- Harris, L.J.L.; Larson, S.B.S.; Hasel, K.W.K.; Day, J.J.; Greenwood, A.A.; McPherson, A.A. The three-dimensional structure of an intact monoclonal antibody for canine lymphoma. Nature 1992, 360, 369–372. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Gould, H.J.; Sutton, B.J.; Beavil, A.J.; Beavil, R.L.; McCloskey, N.; Coker, H.A.; Fear, D.; Smurthwaite, L. The biology of IGE and the basis of allergic disease. Annu. Rev. Immunol. 2003, 21, 579–628. [Google Scholar] [CrossRef]
- Daëron, M. Fc receptor biology. Annu. Rev. Immunol. 1997, 15, 203–234. [Google Scholar] [CrossRef]
- Takai, T. Roles of Fc receptors in autoimmunity. Nat. Rev. Immunol. 2002, 2, 580–592. [Google Scholar]
- Nimmerjahn, F.; Ravetch, J.V. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Antibody-mediated modulation of immune responses. Immunol. Rev. 2010, 236, 265–275. [Google Scholar] [CrossRef]
- Schwab, I.; Nimmerjahn, F. Intravenous immunoglobulin therapy: How does IgG modulate the immune system? Nat. Rev. Immunol. 2013, 13, 176–189. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310, 1510–1512. [Google Scholar] [CrossRef]
- Wu, J.; Edberg, J.C.; Redecha, P.B.; Bansal, V.; Guyre, P.M.; Coleman, K.; Salmon, J.E.; Kimberly, R.P. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J. Clin. Invest. 1997, 100, 1059–1070. [Google Scholar] [CrossRef]
- Shields, R.L.; Namenuk, A.K.; Hong, K.; Meng, Y.G.; Rae, J.; Briggs, J.; Xie, D.; Lai, J.; Stadlen, A.; Li, B.; et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J. Biol. Chem. 2001, 276, 6591–6604. [Google Scholar] [CrossRef]
- Koene, H.R.H.; Kleijer, M.M.; Algra, J.J.; Roos, D.D.; von dem Borne, A.E.A.; de Haas, M.M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 1997, 90, 1109–1114. [Google Scholar]
- Warmerdam, P.A.P.; van de Winkel, J.G.J.; Vlug, A.A.; Westerdaal, N.A.N.; Capel, P.J.P. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J. Immunol. 1991, 147, 1338–1343. [Google Scholar]
- Parren, P.W.; Warmerdam, P.A.; Boeije, L.C.; Arts, J.; Westerdaal, N.A.; Vlug, A.; Capel, P.J.; Aarden, L.A.; van de Winkel, J.G. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J. Clin. Invest. 1992, 90, 1537–1546. [Google Scholar] [CrossRef]
- Musolino, A.; Naldi, N.; Bortesi, B.; Pezzuolo, D.; Capelletti, M.; Missale, G.; Laccabue, D.; Zerbini, A.; Camisa, R.; Bisagni, G.; et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 2008, 26, 1789–1796. [Google Scholar] [CrossRef]
- Weng, W.-K.W.; Levy, R.R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003, 21, 3940–3947. [Google Scholar] [CrossRef]
- Tamura, K.; Shimizu, C.; Hojo, T.; Akashi-Tanaka, S.; Kinoshita, T.; Yonemori, K.; Kouno, T.; Katsumata, N.; Ando, M.; Aogi, K.; et al. FcγR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann. Oncol. 2011, 22, 1302–1307. [Google Scholar] [CrossRef]
- Bibeau, F.; Lopez-Crapez, E.; Di Fiore, F.; Thezenas, S.; Ychou, M.; Blanchard, F.; Lamy, A.; Penault-Llorca, F.; Frebourg, T.; Michel, P.; et al. Impact of Fc RIIa-Fc RIIIa Polymorphisms and KRAS Mutations on the Clinical Outcome of Patients With Metastatic Colorectal Cancer Treated With Cetuximab Plus Irinotecan. J. Clin. Oncol. 2009, 27, 1122–1129. [Google Scholar] [CrossRef]
- Kuo, T.T.; Aveson, V.G. Neonatal Fc receptor and IgG-based therapeutics. MAbs 2011, 3, 422–430. [Google Scholar] [CrossRef]
- Jefferis, R.R. Isotype and glycoform selection for antibody therapeutics. Arch. Biochem. Biophys. 2012, 526, 159–166. [Google Scholar] [CrossRef]
- Idusogie, E.E.E.; Presta, L.G.L.; Gazzano-Santoro, H.H.; Totpal, K.K.; Wong, P.Y.P.; Ultsch, M.M.; Meng, Y.G.Y.; Mulkerrin, M.G.M. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J. Immunol. 2000, 164, 4178–4184. [Google Scholar]
- Deisenhofer, J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9-and 2.8-. ANG. resolution. Biochemistry 1981, 20, 2361–2370. [Google Scholar] [CrossRef]
- Feige, M.J.; Nath, S.; Catharino, S.R.; Weinfurtner, D.; Steinbacher, S.; Buchner, J. Structure of the Murine Unglycosylated IgG1 Fc Fragment. J. Mol. Biol. 2009, 391, 599–608. [Google Scholar] [CrossRef]
- Jefferis, R.R.; Lund, J.J. Interaction sites on human IgG-Fc for FcgammaR: Current models. Immunol. Lett. 2002, 82, 57–65. [Google Scholar] [CrossRef]
- Jefferis, R. The glycosylation of antibody molecules: Functional significance. Glycoconjug. J. 1993, 10, 358–361. [Google Scholar]
- Arnold, J.N.J.; Wormald, M.R.M.; Sim, R.B.R.; Rudd, P.M.P.; Dwek, R.A.R. The impact of glycosylation on the biological function and structure of human immunoglobulins. Immunology 2007, 25, 21–50. [Google Scholar]
- Routier, F.H.; Hounsell, E.F.; Rudd, P.M.; Takahashi, N.; Bond, A.; Hay, F.C.; Alavi, A.; Axford, J.S.; Jefferis, R. Quantitation of the oligosaccharides of human serum IgG from patients with rheumatoid arthritis: A critical evaluation of different methods. J. Immunol. Methods 1998, 213, 113–130. [Google Scholar] [CrossRef]
- Zauner, G.; Selman, M.H.J.; Bondt, A.; Rombouts, Y.; Blank, D.; Deelder, A.M.; Wuhrer, M. Glycoproteomic analysis of antibodies. Mol. Cell. Proteomics 2013, 12, 856–865. [Google Scholar] [CrossRef]
- Stadlmann, J.; Weber, A.; Pabst, M.; Anderle, H.; Kunert, R.; Ehrlich, H.; Peter Schwarz, H.; Altmann, F. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics 2009, 9, 4143–4153. [Google Scholar] [CrossRef]
- Wuhrer, M.; Stam, J.C.; van de Geijn, F.E.; Koeleman, C.A.M.; Verrips, C.T.; Dolhain, R.J.E.M.; Hokke, C.H.; Deelder, A.M. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics 2007, 7, 4070–4081. [Google Scholar] [CrossRef]
- Anthony, R.M.; Wermeling, F.; Ravetch, J.V. Novel roles for the IgG Fc glycan. Ann. NY Acad. Sci. 2012, 1253, 170–180. [Google Scholar] [CrossRef]
- Shinkawa, T.T.; Nakamura, K.K.; Yamane, N.N.; Shoji-Hosaka, E.E.; Kanda, Y.Y.; Sakurada, M.M.; Uchida, K.K.; Anazawa, H.H.; Satoh, M.M.; Yamasaki, M.M.; et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2003, 278, 3466–3473. [Google Scholar]
- Shields, R.L.; Lai, J.; Keck, R.; O'Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar]
- Forthal, D.N.; Gach, J.S.; Landucci, G.; Jez, J.; Strasser, R.; Kunert, R.; Steinkellner, H. Fc-glycosylation influences Fcγ receptor binding and cell-mediated anti-HIV activity of monoclonal antibody 2G12. J. Immunol. 2010, 185, 6876–6882. [Google Scholar]
- Zeitlin, L.L.; Pettitt, J.J.; Scully, C.C.; Bohorova, N.N.; Do, D.K.; Pauly, M.M.; Hiatt, A.A.; Ngo, L.L.; Steinkellner, H.H.; Whaley, K.J.K.; et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl. Acad. Sci. USA 2011, 108, 20690–20694. [Google Scholar]
- Junttila, T.T.; Parsons, K.; Olsson, C.; Lu, Y.; Xin, Y.; Theriault, J.; Crocker, L.; Pabonan, O.; Baginski, T.; Meng, G.; et al. Superior In vivo Efficacy of Afucosylated Trastuzumab in the Treatment of HER2-Amplified Breast Cancer. Cancer Res. 2010, 70, 4481–4489. [Google Scholar] [CrossRef]
- Ferrara, C.; Grau, S.; Jäger, C.; Sondermann, P.; Brünker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M.; et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [CrossRef]
- Ferrara, C.C.; Stuart, F.F.; Sondermann, P.P.; Brünker, P.P.; Umaña, P.P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 2006, 281, 5032–5036. [Google Scholar]
- Davies, J.; Jiang, L.; Pan, L.Z.; LaBarre, M.J.; Anderson, D.; Reff, M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol. Bioeng. 2001, 74, 288–294. [Google Scholar] [CrossRef]
- Umaña, P.; Jean-Mairet, J.; Moudry, R.; Amstutz, H.; Bailey, J.E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 1999, 17, 176–180. [Google Scholar] [CrossRef]
- Hodoniczky, J.; Zheng, Y.Z.; James, D.C. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol. Prog. 2005, 21, 1644–1652. [Google Scholar] [CrossRef]
- Boyd, P.N.; Lines, A.C.; Patel, A.K. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol. Immunol. 1995, 32, 1311–1318. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Anthony, R.M.; Ravetch, J.V. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc. Natl. Acad. Sci. USA 2007, 104, 8433–8437. [Google Scholar] [CrossRef]
- Malhotra, R.; Wormald, M.R.; Rudd, P.M.; Fischer, P.B.; Dwek, R.A.; Sim, R.B. Glycosylation changes of IgG associated with rheumatooid arthritis can activate complement via the mannose-binding protein. Nat. Med. 1995, 1, 237–243. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Crispin, M.; Yu, X.; Baruah, K.; Boesch, A.W.; Harvey, D.J.; Dugast, A.-S.; Heizen, E.L.; Ercan, A.; Choi, I.; et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013, 123, 2183–2192. [Google Scholar] [CrossRef]
- Karsten, C.M.C.; Pandey, M.K.M.; Figge, J.J.; Kilchenstein, R.R.; Taylor, P.R.P.; Rosas, M.M.; McDonald, J.U.J.; Orr, S.J.S.; Berger, M.M.; Petzold, D.D.; et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar]
- Anthony, R.M.; Nimmerjahn, F.; Ashline, D.J.; Reinhold, V.N.; Paulson, J.C.; Ravetch, J.V. Recapitulation of IVIG Anti-Inflammatory Activity with a Recombinant IgG Fc. Science 2008, 320, 373–376. [Google Scholar] [CrossRef]
- Anthony, R.M.; Kobayashi, T.; Wermeling, F.; Ravetch, J.V. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 2011, 475, 110–113. [Google Scholar] [CrossRef]
- Anthony, R.M.R.; Wermeling, F.F.; Karlsson, M.C.I.M.; Ravetch, J.V.J. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. USA 2008, 105, 19571–19578. [Google Scholar]
- Schwab, I.; Biburger, M.; Krönke, G.; Schett, G.; Nimmerjahn, F. IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur. J. Immunol. 2012, 42, 826–830. [Google Scholar]
- Scallon, B.J.; Tam, S.H.; McCarthy, S.G.; Cai, A.N.; Raju, T.S. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 2007, 44, 1524–1534. [Google Scholar]
- Imbach, P.; Wagner, H.P.; Berchtold, W.; Gaedicke, G.; Hirt, A.; Joller, P.; Mueller-Eckhardt, C.; Müller, B.; Rossi, E.; Barandun, S. Intravenous immunoglobulin versus oral corticosteroids in acute immune thrombocytopenic purpura in childhood. Lancet 1985, 2, 464–468. [Google Scholar]
- Levy, Y.; Sherer, Y.; Ahmed, A.; Langevitz, P.; George, J.; Fabbrizzi, F.; Terryberry, J.; Meissner, M.; Lorber, M.; Peter, J.B.; et al. A study of 20 SLE patients with intravenous immunoglobulin—Clinical and serologic response. Lupus 1999, 8, 705–712. [Google Scholar] [CrossRef]
- Hughes, R.A.C.R.; Donofrio, P.P.; Bril, V.V.; Dalakas, M.C.M.; Deng, C.C.; Hanna, K.K.; Hartung, H.-P.H.; Latov, N.; Merkies, I.S.J.I.; van Doorn, P.A.P. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): A randomised placebo-controlled trial. Lancet Neurol. 2008, 7, 136–144. [Google Scholar] [CrossRef]
- Kaneko, Y.Y.; Nimmerjahn, F.F.; Madaio, M.P.M.; Ravetch, J.V.J. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J. Exp. Med. 2006, 203, 789–797. [Google Scholar] [CrossRef]
- Guhr, T.T.; Bloem, J.J.; Derksen, N.I.L.N.; Wuhrer, M.M.; Koenderman, A.H.L.A.; Aalberse, R.C.R.; Rispens, T.T. Enrichment of sialylated IgG by lectin fractionation does not enhance the efficacy of immunoglobulin G in a murine model of immune thrombocytopenia. PLoS One 2011, 6, e21246. [Google Scholar] [CrossRef]
- Leontyev, D.; Katsman, Y.; Ma, X.-Z.; Miescher, S.; Käsermann, F.; Branch, D.R. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion 2012, 52, 1799–1805. [Google Scholar] [CrossRef]
- Sondermann, P.; Pincetic, A.; Maamary, J.; Lammens, K.; Ravetch, J.V. General mechanism for modulating immunoglobulin effector function. Proc. Natl. Acad. Sci. USA 2013, 110, 9868–9873. [Google Scholar] [CrossRef]
- Richards, M.L.M.; Katz, D.H.D. The binding of IgE to murine Fc epsilon RII is calcium-dependent but not inhibited by carbohydrate. J. Immunol. 1990, 144, 2638–2646. [Google Scholar]
- Yu, X.; Vasiljevic, S.; Mitchell, D.A.; Crispin, M.; Scanlan, C.N. Dissecting the Molecular Mechanism of IVIg Therapy: The Interaction between Serum IgG and DC-SIGN is Independent of Antibody Glycoform or Fc Domain. J. Mol. Biol. 2013, 425, 1253–1258. [Google Scholar] [CrossRef]
- Oaks, M.; Taylor, S.; Shaffer, J. Autoantibodies targeting tumor-associated antigens in metastatic cancer: Sialylated IgGs as candidate anti-inflammatory antibodies. OncoImmunology 2013, 2, eLocation ID: e24841. [Google Scholar]
- Käsermann, F.F.; Boerema, D.J.D.; Rüegsegger, M.M.; Hofmann, A.A.; Wymann, S.S.; Zuercher, A.W.A.; Miescher, S.S. Analysis and functional consequences of increased Fab-sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS One 2012, 7, e37243. [Google Scholar] [CrossRef]
- Barb, A.W.A.; Prestegard, J.H.J. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat. Chem. Biol. 2011, 7, 147–153. [Google Scholar] [CrossRef]
- Gerdes, C.A.; Nicolini, V.; Herter, S.; van Puijenbroek, E.; Lang, S.; Roemmele, M.; Moessner, E.; Freytag, O.; Friess, T.; Ries, C.H.; et al. GA201 (RG7160): A novel, humanised, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin. Cancer Res. 2012, 19, 1126. [Google Scholar]
- Gasdaska, J.R.; Sherwood, S.; Regan, J.T.; Dickey, L.F. An afucosylated anti-CD20 monoclonal antibody with greater antibody-dependent cellular cytotoxicity and B-cell depletion and lower complement-dependent cytotoxicity than rituximab. Mol. Immunol. 2012, 50, 134–141. [Google Scholar] [CrossRef]
- van de Geijn, F.E.; Wuhrer, M.; Selman, M.H.J.; Willemsen, S.P.; de Man, Y.A.; Deelder, A.M.; Hazes, J.M.W.; Dolhain, R.J. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: Results from a large prospective cohort study. Arthritis Res. Ther. 2009, 11, R193–R193. [Google Scholar] [CrossRef]
- Rook, G.A.W.; Steele, J.; Brealey, R.; Whyte, A.; Isenberg, D.; Sumar, N.; Nelson, J.L.; Bodman, K.B.; Young, A.; Roitt, I.M.; et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. Trends Biotechnol. 1991, 4, 779–794. [Google Scholar]
- Parekh, R.R.; Roitt, I.I.; Isenberg, D.D.; Dwek, R.R.; Rademacher, T.T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J. Exp. Med. 1988, 167, 1731–1736. [Google Scholar] [CrossRef]
- Shikata, K.K.; Yasuda, T.T.; Takeuchi, F.; Konishi, T.T.; Nakata, M.M.; Mizuochi, T.T. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconjug. J. 1998, 15, 683–689. [Google Scholar] [CrossRef]
- Parekh, R.B.; Dwek, R.A.; Sutton, B.J.; Fernandes, D.L.; Leung, A.; Stanworth, D.; Rademacher, T.W. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 1985, 316, 452–457. [Google Scholar] [CrossRef]
- Parekh, R.B.; Roitt, I.M.; Isenberg, D.A.; Dwek, R.A.; Ansell, B.M.; Rademacher, T.W. Galactosylation of IgG associated oligosaccharides: Reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet 1988, 1, 966–969. [Google Scholar]
- Espy, C.C.; Morelle, W.W.; Kavian, N.N.; Grange, P.P.; Goulvestre, C.C.; Viallon, V.V.; Chéreau, C.C.; Pagnoux, C.C.; Michalski, J.-C.J.; Guillevin, L.L.; et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener's). Arthritis Rheum. 2011, 63, 2105–2115. [Google Scholar] [CrossRef]
- Parekh, R.; Isenberg, D.; Rook, G.; Roitt, I.; Dwek, R.; Rademacher, T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. Trends Biotechnol. 1989, 2, 101–114. [Google Scholar]
- Mehta, A.S.; Long, R.E.; Comunale, M.A.; Wang, M.; Rodemich, L.; Krakover, J.; Philip, R.; Marrero, J.A.; Dwek, R.A.; Block, T.M. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J. Virol. 2008, 82, 1259–1270. [Google Scholar] [CrossRef]
- Kodar, K.K.; Stadlmann, J.J.; Klaamas, K.K.; Sergeyev, B.B.; Kurtenkov, O.O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: Relation to tumor progression and survival. Glycoconjug. J. 2012, 29, 57–66. [Google Scholar] [CrossRef]
- Saldova, R.R.; Royle, L.L.; Radcliffe, C.M.C.; Hamid, U.M.U.A.; Evans, R.R.; Arnold, J.N.J.; Banks, R.E.R.; Hutson, R.R.; Harvey, D.J.D.; Antrobus, R.R.; et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology 2007, 17, 1344–1356. [Google Scholar] [CrossRef]
- Scherer, H.U.H.; van der Woude, D.D.; Ioan-Facsinay, A.A.; Bannoudi, el, H.H.; Trouw, L.A. L.; Wang, J.J.; Häupl, T.T.; Burmester, G.-R.G.; Deelder, A.M.A.; Huizinga, T.W.J.T.; et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010, 62, 1620–1629. [Google Scholar]
- Pekelharing, J.M.; Hepp, E.; Kamerling, J.P.; Gerwig, G.J.; Leijnse, B. Alterations in carbohydrate composition of serum IgG from patients with rheumatoid arthritis and from pregnant women. Ann. Rheum. Dis. 1988, 47, 91–95. [Google Scholar] [CrossRef]
- Gornik, I.I.; Maravić, G.G.; Dumić, J.J.; Flögel, M.M.; Lauc, G.G. Fucosylation of IgG heavy chains is increased in rheumatoid arthritis. Clin. Biochem. 1999, 32, 605–608. [Google Scholar] [CrossRef]
- Guo, N.; Liu, Y.; Masuda, Y.; Kawagoe, M.; Ueno, Y.; Kameda, T.; Sugiyama, T. Repeated immunization induces the increase in fucose content on antigen-specific IgG N-linked oligosaccharides. Clin. Biochem. 2005, 38, 149–153. [Google Scholar] [CrossRef]
- Wang, J.; Balog, C.I.A.; Stavenhagen, K.; Koeleman, C.A.M.; Scherer, H.U.; Selman, M.H.J.; Deelder, A.M.; Huizinga, T.W.J.; Toes, R.E.M.; Wuhrer, M. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol. Cell. Proteomics 2011, 10, M110.004655. [Google Scholar]
- Croce, A.; Firuzi, O.; Altieri, F.; Eufemi, M.; Agostino, R.; Priori, R.; Bombardieri, M.; Alessandri, C.; Valesini, G.; Saso, L. Effect of infliximab on the glycosylation of IgG of patients with rheumatoid arthritis. J. Clin. Lab. Anal. 2007, 21, 303–314. [Google Scholar] [CrossRef]
- Pasek, M.; Duk, M.; Podbielska, M.; Sokolik, R.; Szechiński, J.; Lisowska, E.; Krotkiewski, H. Galactosylation of IgG from rheumatoid arthritis (RA) patients—Changes during therapy. Glycoconjug. J. 2006, 23, 463–471. [Google Scholar] [CrossRef]
- Axford, J.S.J. Decreased B-cell galactosyltransferase activity in rheumatoid arthritis. Rheumatology (Oxford) 1988, 27, 170. [Google Scholar] [CrossRef]
- Furukawa, K.K.; Matsuta, K.K.; Takeuchi, F.F.; Kosuge, E.E.; Miyamoto, T.T.; Kobata, A.A. Kinetic study of a galactosyltransferase in the B cells of patients with rheumatoid arthritis. Int. Immunol. 1990, 2, 105–112. [Google Scholar] [CrossRef]
- Axford, J.S.; Sumar, N.; Alavi, A.; Isenberg, D.A.; Young, A.; Bodman, K.B.; Roitt, I.M. Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J. Clin. Invest. 1992, 89, 1021–1031. [Google Scholar] [CrossRef]
- Alavi, A.; Axford, J.S.; Pool, A.J. Serum galactosyltransferase isoform changes in rheumatoid arthritis. J. Rheumatol. 2004, 31, 1513–1520. [Google Scholar]
- Keusch, J.J.; Lydyard, P.M.P.; Berger, E.G.E.; Delves, P.J.P. B lymphocyte galactosyltransferase protein levels in normal individuals and in patients with rheumatoid arthritis. Glycoconjug. J. 1998, 15, 1093–1097. [Google Scholar] [CrossRef]
- Jeddi, P.A.P.; Bodman-Smith, K.B.K.; Lund, T.T.; Lydyard, P.M.P.; Mengle-Gaw, L.L.; Isenberg, D.A.D.; Youinou, P.P.; Delves, P.J.P. Agalactosyl IgG and beta-1,4-galactosyltransferase gene expression in rheumatoid arthritis patients and in the arthritis-prone MRL lpr/lpr mouse. Immunology 1996, 87, 654–659. [Google Scholar]
- Omtvedt, L.A.; Royle, L.; Husby, G.; Sletten, K.; Radcliffe, C.M.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M. Glycan analysis of monoclonal antibodies secreted in deposition disorders indicates that subsets of plasma cells differentially process IgG glycans. Arthritis Rheum. 2006, 54, 3433–3440. [Google Scholar] [CrossRef]
- Nasirikenari, M.; Chandrasekaran, E.V.; Matta, K.L.; Segal, B.H.; Bogner, P.N.; Lugade, A.A.; Thanavala, Y.; Lee, J.J.; Lau, J.T.Y. Altered eosinophil profile in mice with ST6Gal-1 deficiency: an additional role for ST6Gal-1 generated by the P1 promoter in regulating allergic inflammation. J. Leukoc. Biol. 2010, 87, 457–466. [Google Scholar] [CrossRef]
- Jones, M.B.; Nasirikenari, M.; Lugade, A.A.; Thanavala, Y.; Lau, J.T.Y. Anti-inflammatory IgG Production Requires Functional P1 Promoter in -Galactoside 2,6-Sialyltransferase 1 (ST6Gal-1) Gene. J. Biol. Chem. 2012, 287, 15365–15370. [Google Scholar]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef]
- Dorrington, K.J.; Bennich, H.H. Structure-function relationships in human immunoglobulin E. Immunol. Rev. 1978, 41, 3–25. [Google Scholar] [CrossRef]
- Arnold, J.N.J.; Radcliffe, C.M.C.; Wormald, M.R.M.; Royle, L.L.; Harvey, D.J.D.; Crispin, M.M.; Dwek, R.A.R.; Sim, R.B.R.; Rudd, P.M.P. The glycosylation of human serum IgD and IgE and the accessibility of identified oligomannose structures for interaction with mannan-binding lectin. J. Immunol. 2004, 173, 6831–6840. [Google Scholar]
- Basu, M.M.; Hakimi, J.J.; Dharm, E.E.; Kondas, J.A.J.; Tsien, W.H.W.; Pilson, R.S.R.; Lin, P.P.; Gilfillan, A.A.; Haring, P.P.; Braswell, E.H.E. Purification and characterization of human recombinant IgE-Fc fragments that bind to the human high affinity IgE receptor. J. Biol. Chem. 1993, 268, 13118–13127. [Google Scholar]
- Young, R.J.R.; Owens, R.J.R.; Mackay, G.A.G.; Chan, C.M.C.; Shi, J.J.; Hide, M.M.; Francis, D.M.D.; Henry, A.J.A.; Sutton, B.J.B.; Gould, H.J.H. Secretion of recombinant human IgE-Fc by mammalian cells and biological activity of glycosylation site mutants. Protein Eng. 1995, 8, 193–199. [Google Scholar] [CrossRef]
- Björklund, J.E.M.; Karlsson, T.; Magnusson, C.G.M. N-glycosylation influences epitope expression and receptor binding structures in human IgE. Mol. Immunol. 1999, 36, 213–221. [Google Scholar] [CrossRef]
- Björklund, J.E.; Schmidt, M.; Magnusson, C.G. Characterisation of recombinant human IgE-Fc fragments expressed in baculovirus-infected insect cells. Mol. Immunol. 2000, 37, 169–177. [Google Scholar] [CrossRef]
- Garman, S.C.; Wurzburg, B.A.; Tarchevskaya, S.S.; Kinet, J.P.; Jardetzky, T.S. Structure of the Fc fragment of human IgE bound to its high-affinity receptor Fc epsilonRI alpha. Nature 2000, 406, 259–266. [Google Scholar] [CrossRef]
- Nettleton, M.Y.; Kochan, J.P. Role of Glycosylation Sites in the IgE Fc Molecule. Int. Arch. Allergy Immunol. 1995, 107, 328–329. [Google Scholar] [CrossRef]
- Vercelli, D.; Helm, B.; Marsh, P.; Padlan, E.; Geha, R.S.; Gouid, H. The B-cell binding site on human immunoglobulin E. Nature 1989, 338, 649–651. [Google Scholar] [CrossRef]
- Nadesalingam, J.J.; Reid, K.B.M.K.; Palaniyar, N.N. Collectin surfactant protein D binds antibodies and interlinks innate and adaptive immune systems. FEBS Lett. 2005, 579, 4449–4453. [Google Scholar] [CrossRef]
- Rutishauser, U.; Acheson, A.; Hall, A.K.; Mann, D.M.; Sunshine, J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science 1988, 240, 53–57. [Google Scholar]
- Monteiro, R.C. The role of IgA and IgA Fc receptors as anti-inflammatory agents. J. Clin. Immunol. 2010, 30, S61–S64. [Google Scholar] [CrossRef]
- Bakema, J.E.J.; van Egmond, M.M. Immunoglobulin A: A next generation of therapeutic antibodies? J. Biol. Chem. 2011, 3, 352–361. [Google Scholar]
- Novak, J.J.; Julian, B.A.B.; Mestecky, J.J.; Renfrow, M.B.M. Glycosylation of IgA1 and pathogenesis of IgA nephropathy. Semin. Immunopathol. 2012, 34, 365–382. [Google Scholar] [CrossRef]
- Mattu, T.S.; Pleass, R.J.; Willis, A.C.; Kilian, M.; Wormald, M.R.; Lellouch, A.C.; Rudd, P.M.; Woof, J.M.; Dwek, R.A. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J. Biol. Chem. 1998, 273, 2260–2272. [Google Scholar] [CrossRef]
- Carayannopoulos, L.; Max, E.E.; Capra, J.D. Recombinant human IgA expressed in insect cells. Proc. Natl. Acad. Sci. USA 1994, 91, 8348–8352. [Google Scholar] [CrossRef]
- Basset, C.C.; Durand, V.V.; Jamin, C.C.; Clément, J.J.; Pennec, Y.Y.; Youinou, P.P.; Dueymes, M.M.; Roitt, I.M.I. Increased N-linked glycosylation leading to oversialylation of monomeric immunoglobulin A1 from patients with Sjögren's syndrome. Scand. J. Immunol. 2000, 51, 300–306. [Google Scholar] [CrossRef]
- Suzuki, H.; Moldoveanu, Z.; Hall, S.; Brown, R.; Vu, H.L.; Novak, L.; Julian, B.A.; Tomana, M.; Wyatt, R.J.; Edberg, J.C.; et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J. Clin. Invest. 2008, 118, 629–639. [Google Scholar]
- Tomana, M.; Novak, J.; Julian, B.A.; Matousovic, K.; Konecny, K.; Mestecky, J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J. Clin. Invest. 1999, 104, 73–81. [Google Scholar] [CrossRef]
- Coppo, R.; Basolo, B.; Martina, G.; Rollino, C.; De Marchi, M.; Giacchino, F.; Mazzucco, G.; Messina, M.; Piccoli, G. Circulating immune complexes containing IgA, IgG and IgM in patients with primary IgA nephropathy and with Henoch-Schoenlein nephritis. Correlation with clinical and histologic signs of activity. Clin. Nephrol. 1982, 18, 230–239. [Google Scholar]
- Lai, K.N. Pathogenesis of IgA nephropathy. Nat. Rev. Nephrol. 2012, 8, 275–283. [Google Scholar] [CrossRef]
- Arnold, J.N.; Wormald, M.R.; Suter, D.M.; Radcliffe, C.M.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M.; Sim, R.B. Human serum IgM glycosylation: identification of glycoforms that can bind to mannan-binding lectin. J. Biol. Chem. 2005, 280, 29080–29087. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shade, K.-T.C.; Anthony, R.M. Antibody Glycosylation and Inflammation. Antibodies 2013, 2, 392-414. https://doi.org/10.3390/antib2030392
Shade K-TC, Anthony RM. Antibody Glycosylation and Inflammation. Antibodies. 2013; 2(3):392-414. https://doi.org/10.3390/antib2030392
Chicago/Turabian StyleShade, Kai-Ting C., and Robert M. Anthony. 2013. "Antibody Glycosylation and Inflammation" Antibodies 2, no. 3: 392-414. https://doi.org/10.3390/antib2030392
APA StyleShade, K.-T. C., & Anthony, R. M. (2013). Antibody Glycosylation and Inflammation. Antibodies, 2(3), 392-414. https://doi.org/10.3390/antib2030392



