Immunocytokines
Abstract
:1. Introduction
2. Molecular Formats
2.1. Targeting Properties of Antibody Formats
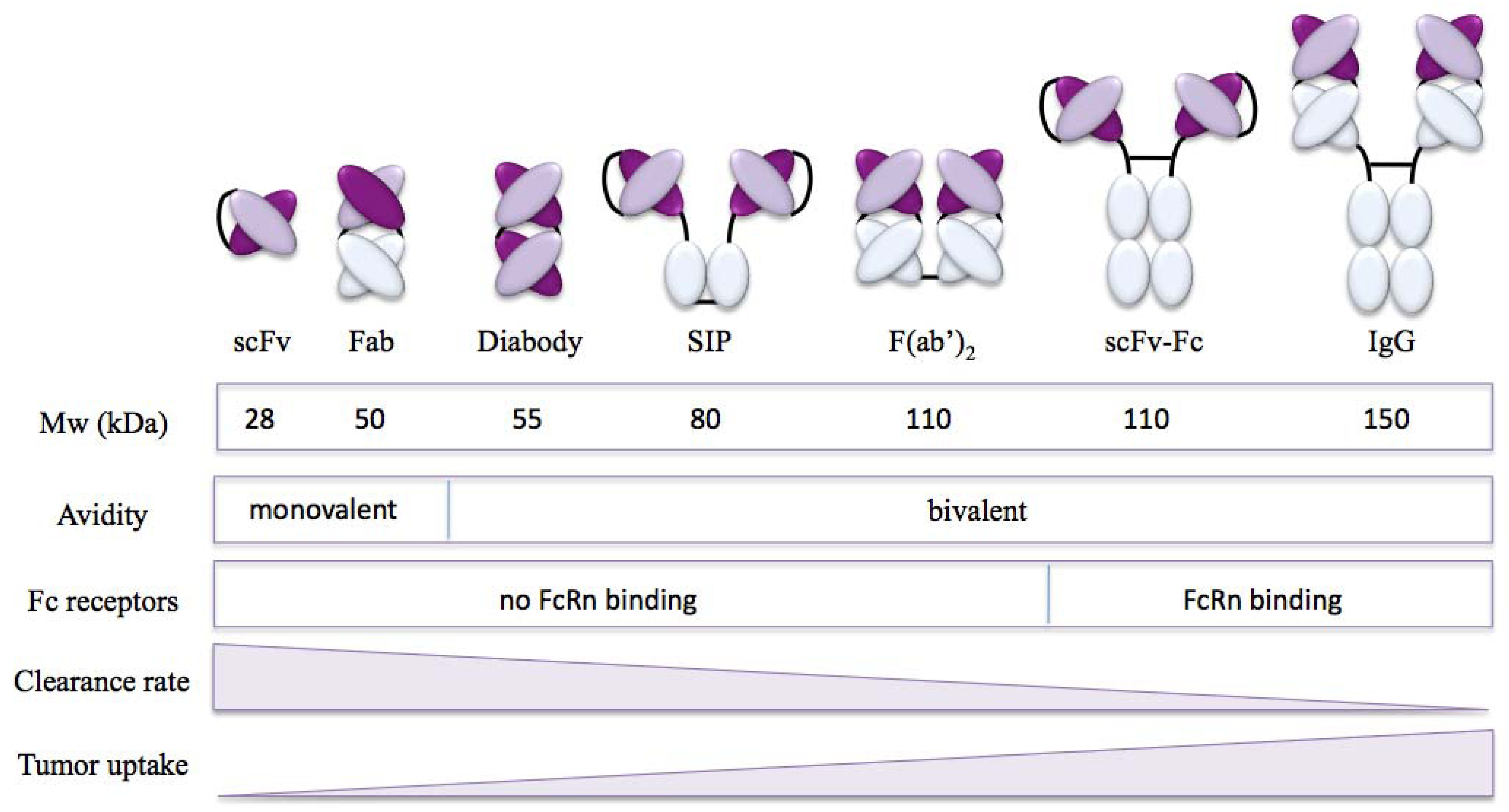
2.2. Targeting Properties of Immunocytokines
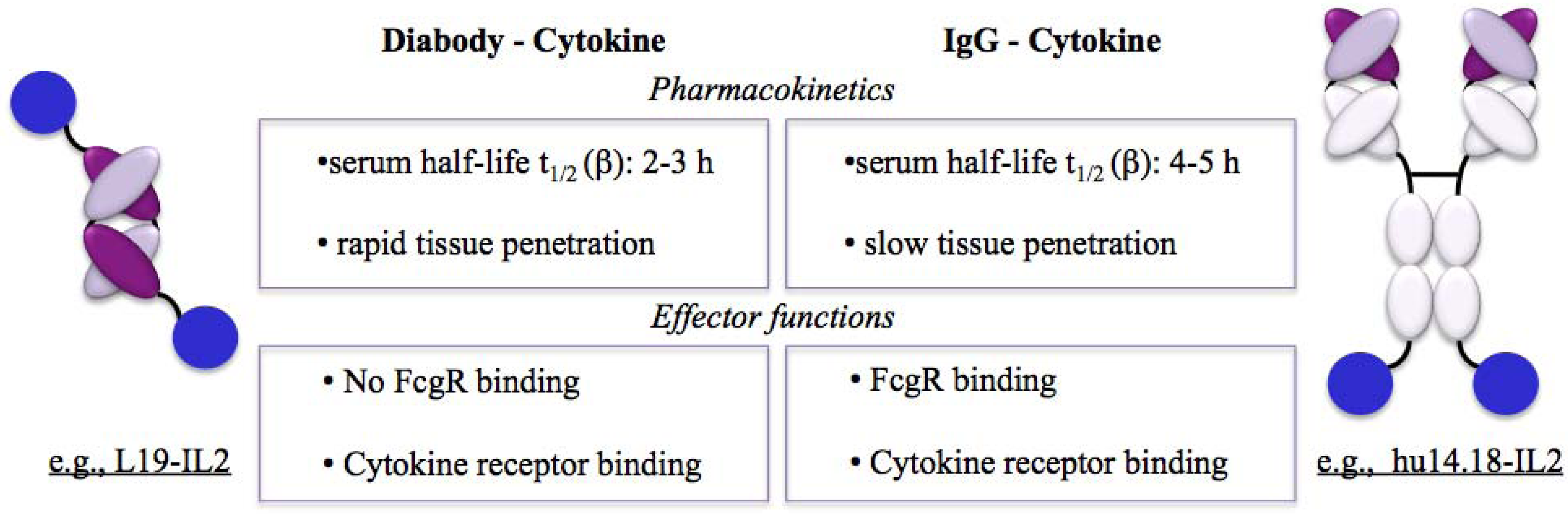
3. Targets
| Antigen | Expression profile | Antibody |
|---|---|---|
| Disease cell surface | ||
| EpCAM Epithelial cell adhesion molecule | Physiologically expressed in epithelia Over-expressed in breast, colorectal and pancreatic carcinomas. | KS |
| GD2 Disialoganglioside | Physiological expression restricted to cerebellum and peripheral nerves. Expressed in neuroectodermal tumors including melanoma, neuroblastoma. | Ch14.18 |
| Disease environment | ||
| EDA / EDB of fibronectin Extra-domains EDA and EDB of fibronectin | Physiological expression restricted to endometrium in the proliferating phase. Expressed around the neo-vasculature and in the stroma of aggressive tumours and in chronic inflammation. | F8 / L19 and BC1 |
| A1 domain of Tenascin-C | Physiological expression restricted to endometrium in the proliferating phase. Expressed around the neo-vasculature and in the stroma of aggressive tumours and in chronic inflammation. | F16 |
| DNA / Histone complexes | Normally not accessible. Exposed in necrotic tissue. | NHS |
3.1. Targeting the Cell Surface
3.2. Targeting the Disease Environment
3.3. Target Identification
4. Fusion Partners
4.1. Cytokines in Cancer
| Compound | Antibody format | Target antigen | Indications | Clinical phase | Company |
|---|---|---|---|---|---|
| Pro-inflammatory | |||||
| F16-IL2 | Diabody | A1 domain of tenascin-C | Breast cancer and lung cancer | Phase II | Philogen |
| L19-IL2 | Diabody | EDB of fibronectin | Melanoma | Phase II | Philogen |
| hu14.18-IL2 | IgG | GD2 | Melanoma and neuroblastoma | Phase I/II | Merck KGaA |
| KS-IL2 | IgG | EpCAM | Ovarian cancer, colorectal cancer, prostate cancer and NSCL carcinoma | Phase I | Merck KGaA |
| NHS-IL2LT | IgG | DNA/ histone | Non-Hodgkin lymphoma and NSCL carcinoma | Phase I | Merck KGaA |
| NHS-IL12 | IgG | DNA/ histone | Epithelial and mesenchymal malignant tumors | Phase I | Merck KGaA |
| BC1-IL12 | IgG | EDB of fibronectin | Melanoma | Phase I/II | Antisoma |
| L19-TNF | Trimeric scFv | EDB of fibronectin | Melanoma (isolated limb perfusion) | Phase I/II | Philogen |
| Anti-inflammatory | |||||
| F8-IL10 | Diabody | EDA of fibronectin | Rheumatoid arthritis | Phase I | Philogen |
4.1.1. IL2-Based Immunocytokines
4.1.2. IL12- and TNF-Based Immunocytokines
4.2. Cytokines in Chronic Inflammatory Diseases
4.2.1. IL10 Immunocytokines
5. Combination Studies
5.1. Selected Preclinical Combination Therapies
5.2. Combination Therapies in Clinical Development
6. Concluding Remarks
Acknowledgments
Conflict of Interest
References
- Miller, P.W.; Sharma, S.; Stolina, M.; Butterfield, L.H.; Luo, J.; Lin, Y.; Dohadwala, M.; Batra, R.K.; Wu, L.; et al. Intratumoral administration of adenoviral interleukin 7 gene-modified dendritic cells augments specific antitumor immunity and achieves tumor eradication. Hum. Gene Ther. 2000, 11, 53–65. [Google Scholar] [CrossRef]
- Aoki, T.; Tashiro, K.; Miyatake, S.; Kinashi, T.; Nakano, T.; Oda, Y.; Kikuchi, H.; Honjo, T. Expression of murine interleukin 7 in a murine glioma cell line results in reduced tumorigenicity in vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 3850–3854. [Google Scholar]
- Koshita, Y.; Lu, Y.; Fujii, S.; Neda, H.; Matsuyama, T.; Satoh, Y.; Itoh, Y.; Takahashi, M.; Kato, J.; Sakamaki, S.; et al. Efficacy of TNF-alpha gene-transduced tumor cells in treatment of established in vivo tumor. Int. J. Cancer 1995, 63, 130–135. [Google Scholar] [CrossRef]
- Barker, S.E.; Grosse, S.M.; Siapati, E.K.; Kritz, A.; Kinnon, C.; Thrasher, A.J.; Hart, S.L. Immunotherapy for neuroblastoma using syngeneic fibroblasts transfected with IL-2 and IL-12. Br. J. Cancer 2007, 97, 210–217. [Google Scholar] [CrossRef]
- Jackaman, C.; Bundell, C.S.; Kinnear, B.F.; Smith, A.M.; Filion, P.; van Hagen, D.; Robinson, B.W.; Nelson, D.J. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: A novel mechanism for IL-2. J. Immunol. 2003, 171, 5051–5063. [Google Scholar]
- Pasche, N.; Neri, D. Immunocytokines: A novel class of potent armed antibodies. Drug Discov. Today 2012.
- Schrama, D.; Reisfeld, R.A.; Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 147–159. [Google Scholar]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef]
- Kuo, T.T.; Aveson, V.G. Neonatal Fc receptor and IgG-based therapeutics. MAbs 2011, 3, 422–430. [Google Scholar] [CrossRef]
- Woof, J.M.; Burton, D.R. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat. Rev. Immunol. 2004, 4, 89–99. [Google Scholar] [CrossRef]
- Ward, E.S.; Martinez, C.; Vaccaro, C.; Zhou, J.; Tang, Q.; Ober, R.J. From sorting endosomes to exocytosis: Association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol. Biol. Cell 2005, 16, 2028–2038. [Google Scholar] [CrossRef]
- Vaccaro, C.; Zhou, J.; Ober, R.J.; Ward, E.S. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat. Biotechnol. 2005, 23, 1283–1288. [Google Scholar] [CrossRef]
- Pop, L.M.; Liu, X.; Ghetie, V.; Vitetta, E.S. The generation of immunotoxins using chimeric anti-CD22 antibodies containing mutations which alter their serum half-life. Int. Immunopharmacol. 2005, 5, 1279–1290. [Google Scholar] [CrossRef]
- Jain, R.K.; Baxter, L.T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: Significance of elevated interstitial pressure. Cancer Res. 1988, 48, 7022–7032. [Google Scholar]
- Yokota, T.; Milenic, D.E.; Whitlow, M.; Schlom, J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992, 52, 3402–3408. [Google Scholar]
- Graff, C.P.; Wittrup, K.D. Theoretical analysis of antibody targeting of tumor spheroids: Importance of dosage for penetration, and affinity for retention. Cancer Res. 2003, 63, 1288–1296. [Google Scholar]
- Milenic, D.E.; Yokota, T.; Filpula, D.R.; Finkelman, M.A.; Dodd, S.W.; Wood, J.F.; Whitlow, M.; Snoy, P.; Schlom, J. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991, 51, 6363–6371. [Google Scholar]
- Borsi, L.; Balza, E.; Bestagno, M.; Castellani, P.; Carnemolla, B.; Biro, A.; Leprini, A.; Sepulveda, J.; Burrone, O.; Neri, D.; Zardi, L. Selective targeting of tumoral vasculature: Comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int. J. Cancer 2002, 102, 75–85. [Google Scholar] [CrossRef]
- Holliger, P.; Prospero, T.; Winter, G. Diabodies": Small bivalent and bispecific antibody fragments. Proc. Natl. Acad. Sci. USA 1993, 90, 6444–6448. [Google Scholar]
- Kendra, K.; Gan, J.; Ricci, M.; Surfus, J.; Shaker, A.; Super, M.; Frost, J.D.; Rakhmilevich, A.; Hank, J.A.; Gillies, S.D.; Sondel, P.M. Pharmacokinetics and stability of the ch14.18-interleukin-2 fusion protein in mice. Cancer Immunol. Immunother. 1999, 48, 219–229. [Google Scholar] [CrossRef]
- Yamane, B.H.; Hank, J.A.; Albertini, M.R.; Sondel, P.M. The development of antibody-IL-2 based immunotherapy with hu14.18-IL2 (EMD-273063) in melanoma and neuroblastoma. Expert Opin. Investig. Drugs 2009, 18, 991–1000. [Google Scholar] [CrossRef]
- Johannsen, M.; Spitaleri, G.; Curigliano, G.; Roigas, J.; Weikert, S.; Kempkensteffen, C.; Roemer, A.; Kloeters, C.; Rogalla, P.; Pecher, G.; et al. The tumour-targeting human L19-IL2 immunocytokine: Preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur. J. Cancer 2010, 46, 2926–2935. [Google Scholar]
- Ebbinghaus, C.; Ronca, R.; Kaspar, M.; Grabulovski, D.; Berndt, A.; Kosmehl, H.; Zardi, L.; Neri, D. Engineered vascular-targeting antibody-interferon-gamma fusion protein for cancer therapy. Int. J. Cancer 2005, 116, 304–313. [Google Scholar] [CrossRef]
- Naramura, M.; Gillies, S.D.; Mendelsohn, J.; Reisfeld, R.A.; Mueller, B.M. Mechanisms of cellular cytotoxicity mediated by a recombinant antibody-IL2 fusion protein against human melanoma cells. Immunol. Lett. 1993, 39, 91–99. [Google Scholar] [CrossRef]
- Alderson, K.L.; Sondel, P.M. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J. Biomed. Biotechnol. 2011, 379123. [Google Scholar]
- Buhtoiarov, I.N.; Neal, Z.C.; Gan, J.; Buhtoiarova, T.N.; Patankar, M.S.; Gubbels, J.A.; Hank, J.A.; Yamane, B.; Rakhmilevich, A.L.; Reisfeld, R.A.; et al. Differential internalization of hu14.18-IL2 immunocytokine by NK and tumor cell: Impact on conjugation, cytotoxicity, and targeting. J. Leukoc. Biol. 2011, 89, 625–638. [Google Scholar] [CrossRef]
- Gubbels, J.A.; Gadbaw, B.; Buhtoiarov, I.N.; Horibata, S.; Kapur, A.K.; Patel, D.; Hank, J.A.; Gillies, S.D.; Sondel, P.M.; Patankar, M.S.; Connor, J. Ab-IL2 fusion proteins mediate NK cell immune synapse formation by polarizing CD25 to the target cell-effector cell interface. Cancer Immunol. Immunother. 2011, 60, 1789–1800. [Google Scholar] [CrossRef]
- Patriarca, C.; Macchi, R.M.; Marschner, A.K.; Mellstedt, H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat. Rev. 2012, 38, 68–75. [Google Scholar] [CrossRef]
- Gires, O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell Mol. Life Sci. 2011, 68, 4009–4022. [Google Scholar] [CrossRef]
- Mujoo, K.; Cheresh, D.A.; Yang, H.M.; Reisfeld, R.A. Disialoganglioside GD2 on human neuroblastoma cells: Target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res. 1987, 47, 1098–1104. [Google Scholar]
- Chang, H.R.; Cordon-Cardo, C.; Houghton, A.N.; Cheung, N.K.; Brennan, M.F. Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas. Cancer 1992, 70, 633–638. [Google Scholar] [CrossRef]
- Svennerholm, L.; Bostrom, K.; Fredman, P.; Jungbjer, B.; Lekman, A.; Mansson, J.E.; Rynmark, B.M. Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim. Biophys. Acta 1994, 1214, 115–123. [Google Scholar]
- Allen, M.; Louise Jones, J. Jekyll and Hyde: The role of the microenvironment on the progression of cancer. J. Pathol. 2011, 223, 162–176. [Google Scholar]
- Filer, A.; Pitzalis, C.; Buckley, C.D. Targeting the stromal microenvironment in chronic inflammation. Curr. Opin. Pharmacol. 2006, 6, 393–400. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Schliemann, C.; Neri, D. Antibody-based vascular tumor targeting. Recent Results Cancer Res. 2010, 180, 201–216. [Google Scholar] [CrossRef]
- Neri, D.; Bicknell, R. Tumour vascular targeting. Nat. Rev. Cancer 2005, 5, 436–446. [Google Scholar] [CrossRef]
- Neri, D.; Carnemolla, B.; Nissim, A.; Leprini, A.; Querze, G.; Balza, E.; Pini, A.; Tarli, L.; Halin, C.; Neri, P.; Zardi, L.; Winter, G. Targeting by affinity-matured recombinant antibody fragments of an angiogenesis associated fibronectin isoform. Nat. Biotechnol. 1997, 15, 1271–1275. [Google Scholar]
- Schwager, K.; Villa, A.; Rosli, C.; Neri, D.; Rosli-Khabas, M.; Moser, G. A comparative immunofluorescence analysis of three clinical-stage antibodies in head and neck cancer. Head Neck Oncol. 2011, 3, 25. [Google Scholar] [CrossRef]
- Santimaria, M.; Moscatelli, G.; Viale, G.L.; Giovannoni, L.; Neri, G.; Viti, F.; Leprini, A.; Borsi, L.; Castellani, P.; Zardi, L.; Neri, D.; Riva, P. Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cance. Clin. Cancer Res. 2003, 9, 571–579. [Google Scholar]
- Tarli, L.; Balza, E.; Viti, F.; Borsi, L.; Castellani, P.; Berndorff, D.; Dinkelborg, L.; Neri, D.; Zardi, L. A high-affinity human antibody that targets tumoral blood vessels. Blood 1999, 94, 192–198. [Google Scholar]
- Sharifi, J.; Khawli, L.A.; Hu, P.; King, S.; Epstein, A.L. Characterization of a phage display-derived human monoclonal antibody (NHS76) counterpart to chimeric TNT-1 directed against necrotic regions of solid tumors. Hybrid. Hybridomics 2001, 20, 305–312. [Google Scholar] [CrossRef]
- Chen, S.; Yu, L.; Jiang, C.; Zhao, Y.; Sun, D.; Li, S.; Liao, G.; Chen, Y.; Fu, Q.; Tao, Q.; et al. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J. Clin. Oncol. 2005, 23, 1538–1547. [Google Scholar]
- St Croix, B.; Rago, C.; Velculescu, V.; Traverso, G.; Romans, K.E.; Montgomery, E.; Lal, A.; Riggins, G.J.; Lengauer, C.; Vogelstein, B.; Kinzler, K.W. Genes expressed in human tumor endothelium. Science 2000, 289, 1197–1202. [Google Scholar]
- Oh, P.; Li, Y.; Yu, J.; Durr, E.; Krasinska, K.M.; Carver, L.A.; Testa, J.E.; Schnitzer, J.E. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 2004, 429, 629–635. [Google Scholar]
- Strassberger, V.; Fugmann, T.; Neri, D.; Roesli, C. Chemical proteomic and bioinformatic strategies for the identification and quantification of vascular antigens in cancer. J. Proteomics 2010, 73, 1954–1973. [Google Scholar] [CrossRef]
- Rybak, J.N.; Ettorre, A.; Kaissling, B.; Giavazzi, R.; Neri, D.; Elia, G. In vivo protein biotinylation for identification of organ-specific antigens accessible from the vasculature. Nat. Methods 2005, 2, 291–298. [Google Scholar]
- Borgia, B.; Roesli, C.; Fugmann, T.; Schliemann, C.; Cesca, M.; Neri, D.; Giavazzi, R. A proteomic approach for the identification of vascular markers of liver metastasis. Cancer Res. 2010, 70, 309–318. [Google Scholar]
- Frey, K.; Fiechter, M.; Schwager, K.; Belloni, B.; Barysch, M.J.; Neri, D.; Dummer, R. Different patterns of fibronectin and tenascin-C splice variants expression in primary and metastatic melanoma lesions. Exp. Dermatol. 2011, 20, 685–688. [Google Scholar] [CrossRef]
- Schliemann, C.; Roesli, C.; Kamada, H.; Borgia, B.; Fugmann, T.; Klapper, W.; Neri, D. In vivo biotinylation of the vasculature in B-cell lymphoma identifies BST-2 as a target for antibody-based therapy. Blood 2010, 115, 736–744. [Google Scholar]
- Seruga, B.; Zhang, H.; Bernstein, L.J.; Tannock, I.F. Cytokines and their relationship to the symptoms and outcome of cancer. Nat. Rev. Cancer 2008, 8, 887–899. [Google Scholar] [CrossRef]
- Douglas, M.R.; Morrison, K.E.; Salmon, M.; Buckley, C.D. Why does inflammation persist: A dominant role for the stromal microenvironment. Expert Rev. Mol. Med. 2002, 4, 1–18. [Google Scholar]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef]
- McDermott, D.F.; Regan, M.M.; Atkins, M.B. Interleukin-2 therapy of metastatic renal cell carcinoma: Update of phase III trials. Clin. Genitourin. Cancer 2006, 5, 114–119. [Google Scholar] [CrossRef]
- Philip, P.A. Interleukin-2 in the treatment of malignant melanoma. Expert Opin. Investig. Drugs 1998, 7, 361–371. [Google Scholar] [CrossRef]
- Carnemolla, B.; Borsi, L.; Balza, E.; Castellani, P.; Meazza, R.; Berndt, A.; Ferrini, S.; Kosmehl, H.; Neri, D.; Zardi, L. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood 2002, 99, 1659–1665. [Google Scholar]
- Schliemann, C.; Palumbo, A.; Zuberbuhler, K.; Villa, A.; Kaspar, M.; Trachsel, E.; Klapper, W.; Menssen, H.D.; Neri, D. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood 2009, 113, 2275–2283. [Google Scholar]
- Marlind, J.; Kaspar, M.; Trachsel, E.; Sommavilla, R.; Hindle, S.; Bacci, C.; Giovannoni, L.; Neri, D. Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin. Cancer Res. 2008, 14, 6515–6524. [Google Scholar]
- King, D.M.; Albertini, M.R.; Schalch, H.; Hank, J.A.; Gan, J.; Surfus, J.; Mahvi, D.; Schiller, J.H.; Warner, T.; Kim, K.; et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J. Clin. Oncol. 2004, 22, 4463–4473. [Google Scholar]
- Ribas, A.; Kirkwood, J.M.; Atkins, M.B.; Whiteside, T.L.; Gooding, W.; Kovar, A.; Gillies, S.D.; Kashala, O.; Morse, M.A. Phase I/II open-label study of the biologic effects of the interleukin-2 immunocytokine EMD 273063 (hu14.18-IL2) in patients with metastatic malignant melanom. J. Transl. Med. 2009, 7, 68. [Google Scholar]
- Osenga, K.L.; Hank, J.A.; Albertini, M.R.; Gan, J.; Sternberg, A.G.; Eickhoff, J.; Seeger, R.C.; Matthay, K.K.; Reynolds, C.P.; Twist, C.; et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: A study of the Children's Oncology Group. Clin. Cancer Res. 2006, 12, 1750–1759. [Google Scholar]
- Shusterman, S.; London, W.B.; Gillies, S.D.; Hank, J.A.; Voss, S.D.; Seeger, R.C.; Reynolds, C.P.; Kimball, J.; Albertini, M.R.; Wagner, B.; et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: A Children's Oncology Group (COG) phase II study. J. Clin. Oncol. 2010, 28, 4969–4975. [Google Scholar]
- Ko, Y.J.; Bubley, G.J.; Weber, R.; Redfern, C.; Gold, D.P.; Finke, L.; Kovar, A.; Dahl, T.; Gillies, S.D. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): Results of a phase I trial in patients with prostate cance. J. Immunother. 2004, 27, 232–239. [Google Scholar]
- Gillies, S.D.; Lan, Y.; Hettmann, T.; Brunkhorst, B.; Sun, Y.; Mueller, S.O.; Lo, K.M. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin. Cancer Res. 2011, 17, 3673–3685. [Google Scholar]
- Yang, J.C.; Sherry, R.M.; Steinberg, S.M.; Topalian, S.L.; Schwartzentruber, D.J.; Hwu, P.; Seipp, C.A.; Rogers-Freezer, L.; Morton, K.E.; White, D.E.; et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J. Clin. Oncol. 2003, 21, 3127–3132. [Google Scholar]
- Rosenberg, S.A.; Yang, J.C.; White, D.E.; Steinberg, S.M. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: Identification of the antigens mediating response. Ann. Surg. 1998, 228, 307–319. [Google Scholar]
- Atkins, M.B.; Robertson, M.J.; Gordon, M.; Lotze, M.T.; DeCoste, M.; DuBois, J.S.; Ritz, J.; Sandler, A.B.; Edington, H.D.; Garzone, P.D.; et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 1997, 3, 409–417. [Google Scholar]
- Halin, C.; Rondini, S.; Nilsson, F.; Berndt, A.; Kosmehl, H.; Zardi, L.; Neri, D. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat. Biotechnol. 2002, 20, 264–269. [Google Scholar] [CrossRef]
- Li, J.; Hu, P.; Khawli, L.A.; Yun, A.; Epstein, A.L. chTNT-3/hu IL-12 fusion protein for the immunotherapy of experimental solid tumors. Hybrid. Hybridomics 2004, 23, 1–10. [Google Scholar] [CrossRef]
- Rudman, S.M.; Jameson, M.B.; McKeage, M.J.; Savage, P.; Jodrell, D.I.; Harries, M.; Acton, G.; Erlandsson, F.; Spicer, J.F. A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin. Cancer Res. 2011, 17, 1998–2005. [Google Scholar]
- Lejeune, F.J.; Lienard, D.; Matter, M.; Ruegg, C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006, 6, 6. [Google Scholar]
- Borsi, L.; Balza, E.; Carnemolla, B.; Sassi, F.; Castellani, P.; Berndt, A.; Kosmehl, H.; Biro, A.; Siri, A.; Orecchia, P.; et al. Selective targeted delivery of TNFalpha to tumor blood vessels. Blood 2003, 102, 4384–4392. [Google Scholar]
- Balza, E.; Mortara, L.; Sassi, F.; Monteghirfo, S.; Carnemolla, B.; Castellani, P.; Neri, D.; Accolla, R.S.; Zardi, L.; Borsi, L. Targeted delivery of tumor necrosis factor-alpha to tumor vessels induces a therapeutic T cell-mediated immune response that protects the host against syngeneic tumors of different histologic origin. Clin. Cancer Res. 2006, 12, 2575–2582. [Google Scholar]
- Huhn, R.D.; Radwanski, E.; O'Connell, S.M.; Sturgill, M.G.; Clarke, L.; Cody, R.P.; Affrime, M.B.; Cutler, D.L. Pharmacokinetics and immunomodulatory properties of intravenously administered recombinant human interleukin-10 in healthy volunteers. Blood 1996, 87, 699–705. [Google Scholar]
- Rosenblum, I.Y.; Johnson, R.C.; Schmahai, T.J. Preclinical safety evaluation of recombinant human interleukin-10. Regul. Toxicol. Pharmacol. 2002, 35, 56–71. [Google Scholar] [CrossRef]
- Schwager, K.; Kaspar, M.; Bootz, F.; Marcolongo, R.; Paresce, E.; Neri, D.; Trachsel, E. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res. Ther. 2009, 11, R142. [Google Scholar] [CrossRef]
- Schwager, K.; Bootz, F.; Imesch, P.; Kaspar, M.; Trachsel, E.; Neri, D. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum. Reprod. 2011, 26, 2344–2352. [Google Scholar] [CrossRef]
- Trachsel, E.; Kaspar, M.; Bootz, F.; Detmar, M.; Neri, D. A human mAb specific to oncofetal fibronectin selectively targets chronic skin inflammation in vivo. J. Invest. Dermatol. 2007, 127, 881–886. [Google Scholar]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar]
- Moschetta, M.; Pretto, F.; Berndt, A.; Galler, K.; Richter, P.; Bassi, A.; Oliva, P.; Micotti, E.; Valbusa, G.; Schwager, K.; et al. Paclitaxel Enhances Therapeutic Efficacy of the F8-IL2 Immunocytokine to EDA-Fibronectin-Positive Metastatic Human Melanoma Xenografts. Cancer Res. 2012, 72, 1814–1824. [Google Scholar] [CrossRef]
- Holden, S.A.; Lan, Y.; Pardo, A.M.; Wesolowski, J.S.; Gillies, S.D. Augmentation of antitumor activity of an antibody-interleukin 2 immunocytokine with chemotherapeutic agents. Clin. Cancer Res. 2001, 7, 2862–2869. [Google Scholar]
- Pedretti, M.; Verpelli, C.; Marlind, J.; Bertani, G.; Sala, C.; Neri, D.; Bello, L. Combination of temozolomide with immunocytokine F16-IL2 for the treatment of glioblastoma. Br. J. Cancer 2010, 103, 827–836. [Google Scholar] [CrossRef]
- Johnson, E.E.; Yamane, B.H.; Buhtoiarov, I.N.; Lum, H.D.; Rakhmilevich, A.L.; Mahvi, D.M.; Gillies, S.D.; Sondel, P.M. Radiofrequency ablation combined with KS-IL2 immunocytokine (EMD 273066) results in an enhanced antitumor effect against murine colon adenocarcinoma. Clin. Cancer Res. 2009, 15, 4875–4884. [Google Scholar]
- Hornick, J.L.; Khawli, L.A.; Hu, P.; Sharifi, J.; Khanna, C.; Epstein, A.L. Pretreatment with a monoclonal antibody/interleukin-2 fusion protein directed against DNA enhances the delivery of therapeutic molecules to solid tumors. Clin. Cancer Res. 1999, 5, 51–60. [Google Scholar]
- Liu, Z.; Smyth, F.E.; Renner, C.; Lee, F.T.; Oosterwijk, E.; Scott, A.M. Anti-renal cell carcinoma chimeric antibody G250: Cytokine enhancement of in vitro antibody-dependent cellular cytotoxicity. Cancer Immunol. Immunother. 2002, 51, 171–177. [Google Scholar] [CrossRef]
- Friedberg, J.W.; Neuberg, D.; Gribben, J.G.; Fisher, D.C.; Canning, C.; Koval, M.; Poor, C.M.; Green, L.M.; Daley, J.; Soiffer, R.; et al. Combination immunotherapy with rituximab and interleukin 2 in patients with relapsed or refractory follicular non-Hodgkin's lymphoma. Br. J. Haematol. 2002, 117, 828–834. [Google Scholar] [CrossRef]
- Frey, K.; Schliemann, C.; Schwager, K.; Giavazzi, R.; Johannsen, M.; Neri, D. The immunocytokine F8-IL2 improves the therapeutic performance of sunitinib in a mouse model of renal cell carcinoma. J. Urol. 2010, 184, 2540–2548. [Google Scholar] [CrossRef]
- Halin, C.; Gafner, V.; Villani, M.E.; Borsi, L.; Berndt, A.; Kosmehl, H.; Zardi, L.; Neri, D. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003, 63, 3202–3210. [Google Scholar]
- Gillies, S.D.; Lan, Y.; Brunkhorst, B.; Wong, W.K.; Li, Y.; Lo, K.M. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol. Immunother. 2002, 51, 449–460. [Google Scholar] [CrossRef]
- Connor, J.P.; Stupp, R.; Cristea, M.C.; Lewis, N.; Lewis, L.D.; Mattiacci, M.R.; Felder, M.; Stewart, S.; Henslee-Downey, J.; Neugebauer, R.; Komarnitsky, P.B. Phase IB trial of EMD 273066 (huKS-IL2) with cyclophosphamide in patients with EpCAM-positive advanced solid tumor. J Clin Oncol 2011, 29 (suppl; abstr 2556), 2011. [Google Scholar]
- De Braud, F.G.; Catania, C.; Onofri, A.; Pierantoni, C.; Cascinu, S.; Maur, M.; Masini, C.; Conte, P.F.; Giovannoni, L.; Tasciotti, A.; Lovato, V.; Neri, D.; Menssen, H.D. Combinations of the immunocytokine F16-IL2 with doxorubicin or with paclitaxel investigated in phase Ib studies in patients with advanced solid tumors. J Clin Oncol 2010, 28 (suppl; abstr e13017), 2010. [Google Scholar]
- Eigentler, T.K.; Weide, B.; de Braud, F.; Spitaleri, G.; Romanini, A.; Pflugfelder, A.; Gonzalez-Iglesias, R.; Tasciotti, A.; Giovannoni, L.; Schwager, K.; et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin. Cancer Res. 2011, 17, 7732–7742. [Google Scholar]
- Tijink, B.M.; Perk, L.R.; Budde, M.; Stigter-van Walsum, M.; Visser, G.W.; Kloet, R.W.; Dinkelborg, L.M.; Leemans, C.R.; Neri, D.; van Dongen, G.A. 124)I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of (131)I-L19-SIP radioimmunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1235–1244. [Google Scholar]
- Bassani-Sternberg, M.; Barnea, E.; Beer, I.; Avivi, I.; Katz, T.; Admon, A. Soluble plasma HLA peptidome as a potential source for cancer biomarkers. Proc. Natl. Acad. Sci. USA 2010, 107, 18769–18776. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gutbrodt, K.L.; Neri, D. Immunocytokines. Antibodies 2012, 1, 70-87. https://doi.org/10.3390/antib1010070
Gutbrodt KL, Neri D. Immunocytokines. Antibodies. 2012; 1(1):70-87. https://doi.org/10.3390/antib1010070
Chicago/Turabian StyleGutbrodt, Katrin L., and Dario Neri. 2012. "Immunocytokines" Antibodies 1, no. 1: 70-87. https://doi.org/10.3390/antib1010070




