Redox Dynamics and Oxygen Reduction Rates of Infiltrating Urban Stormwater beneath Low Impact Development (LID)
Abstract
:1. Introduction
2. Materials and Methods
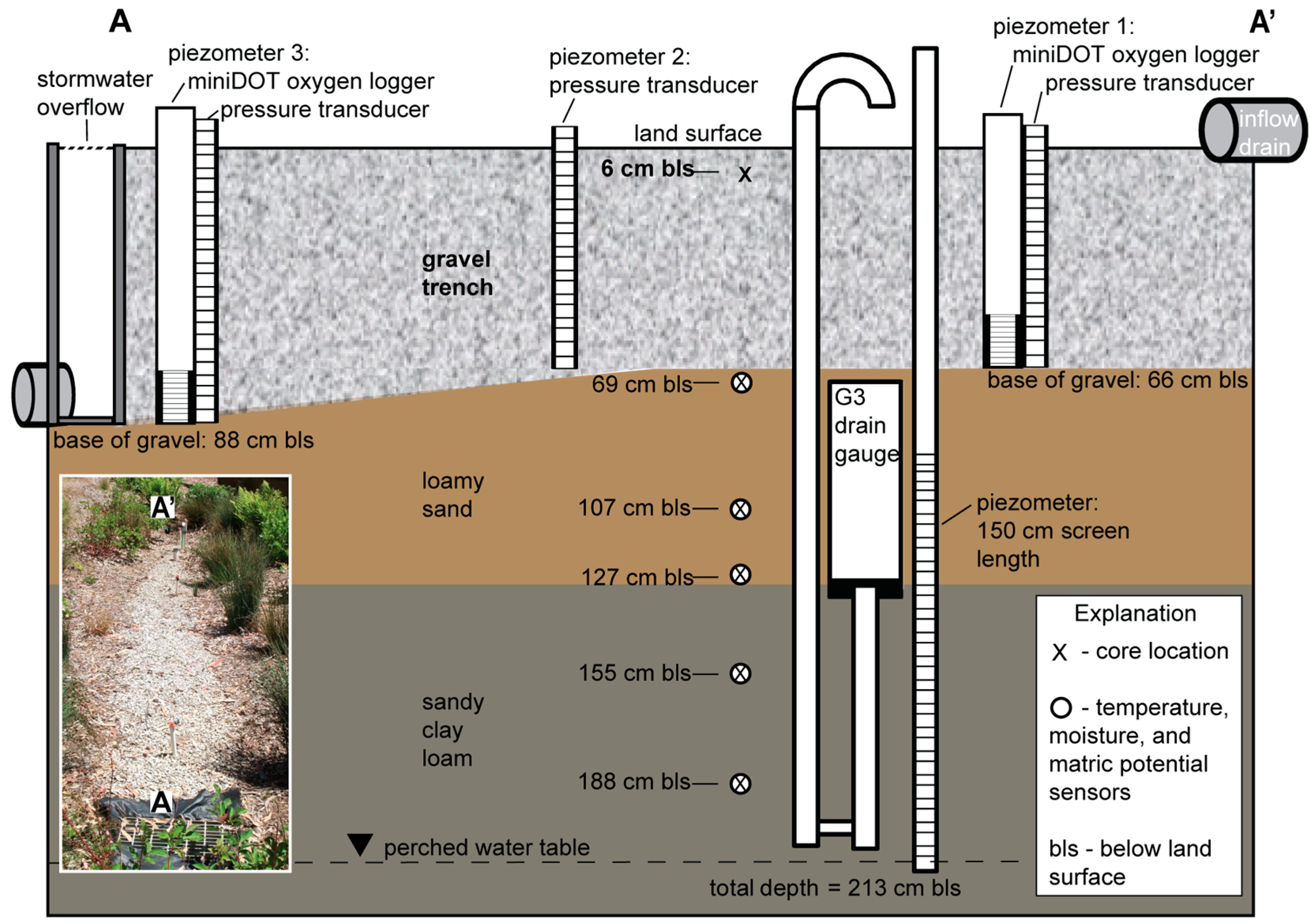
2.1. Study Area
2.2. LID Data Collection
2.3. Saturation of the Gravel Trench
2.4. Precipitation
2.5. Runoff
2.6. Rate of O2 Reduction
2.7. LID Oxygen Demand (LIDOD) Rate
3. Results and Discussion
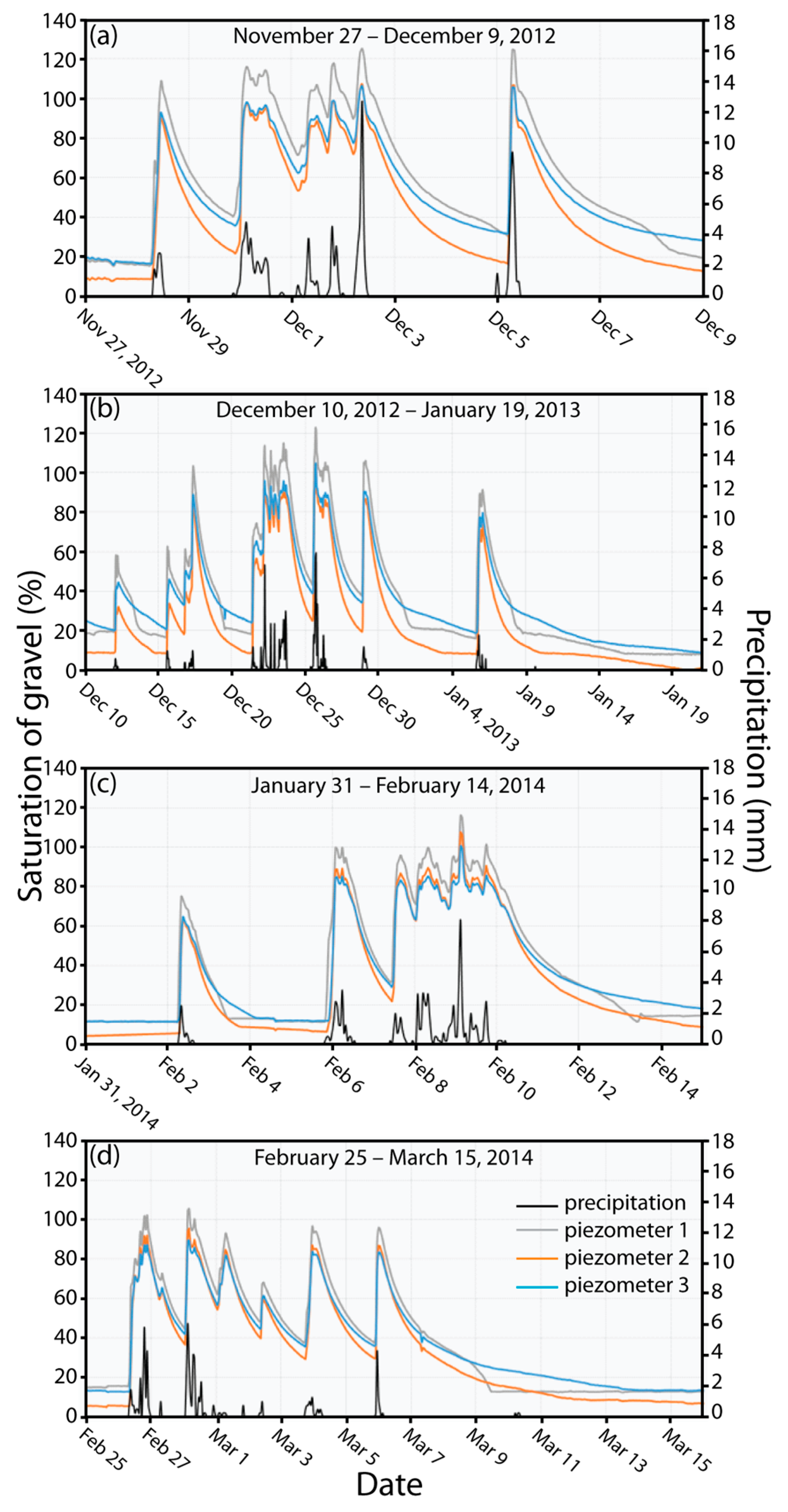
3.1. Water Levels, Saturation of Gravel, Temperature, and Conductivity in Response to Stormwater Runoff
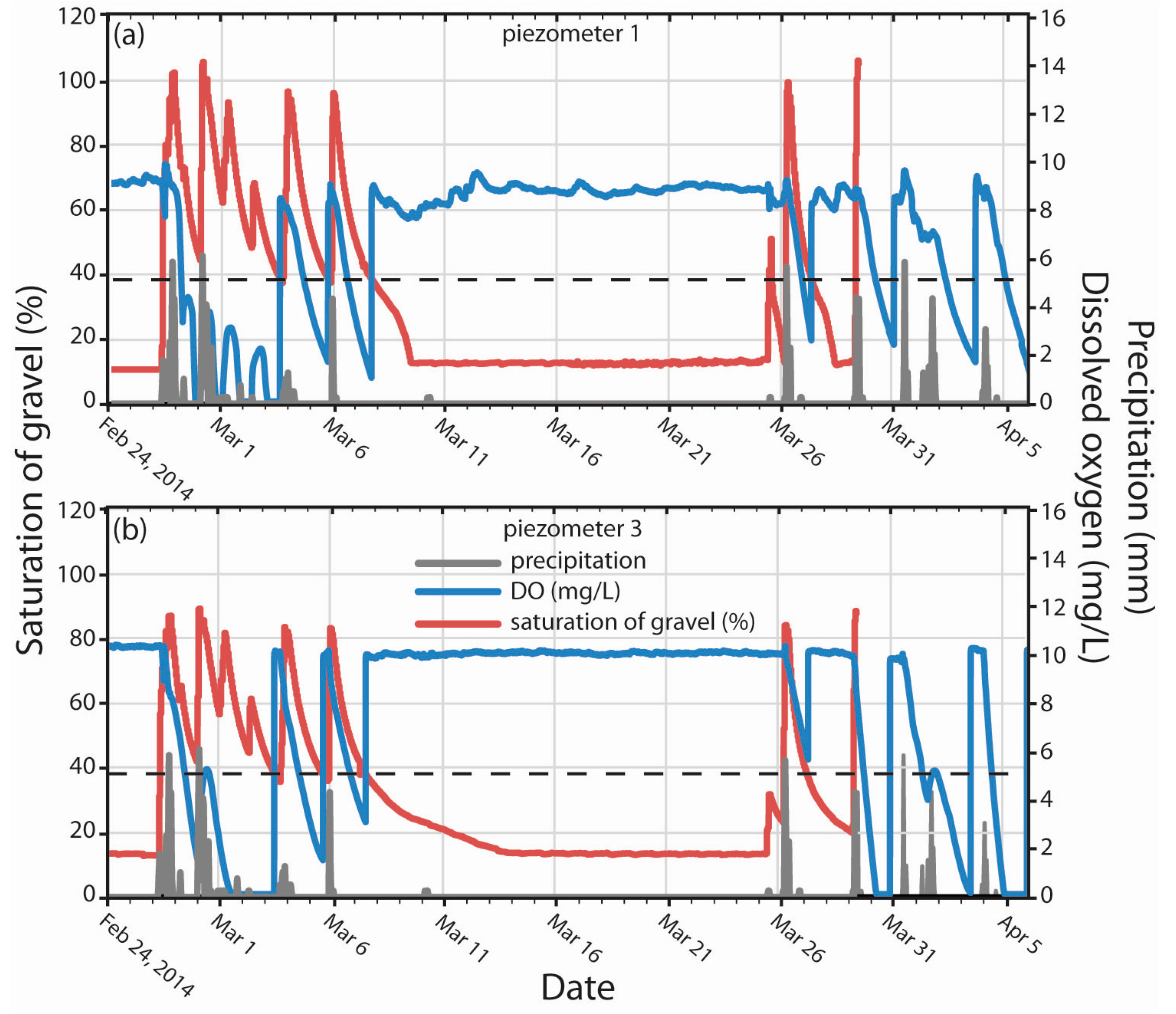
3.2. DO Dynamics in Response to Saturation of Gravel Magnitude and Duration
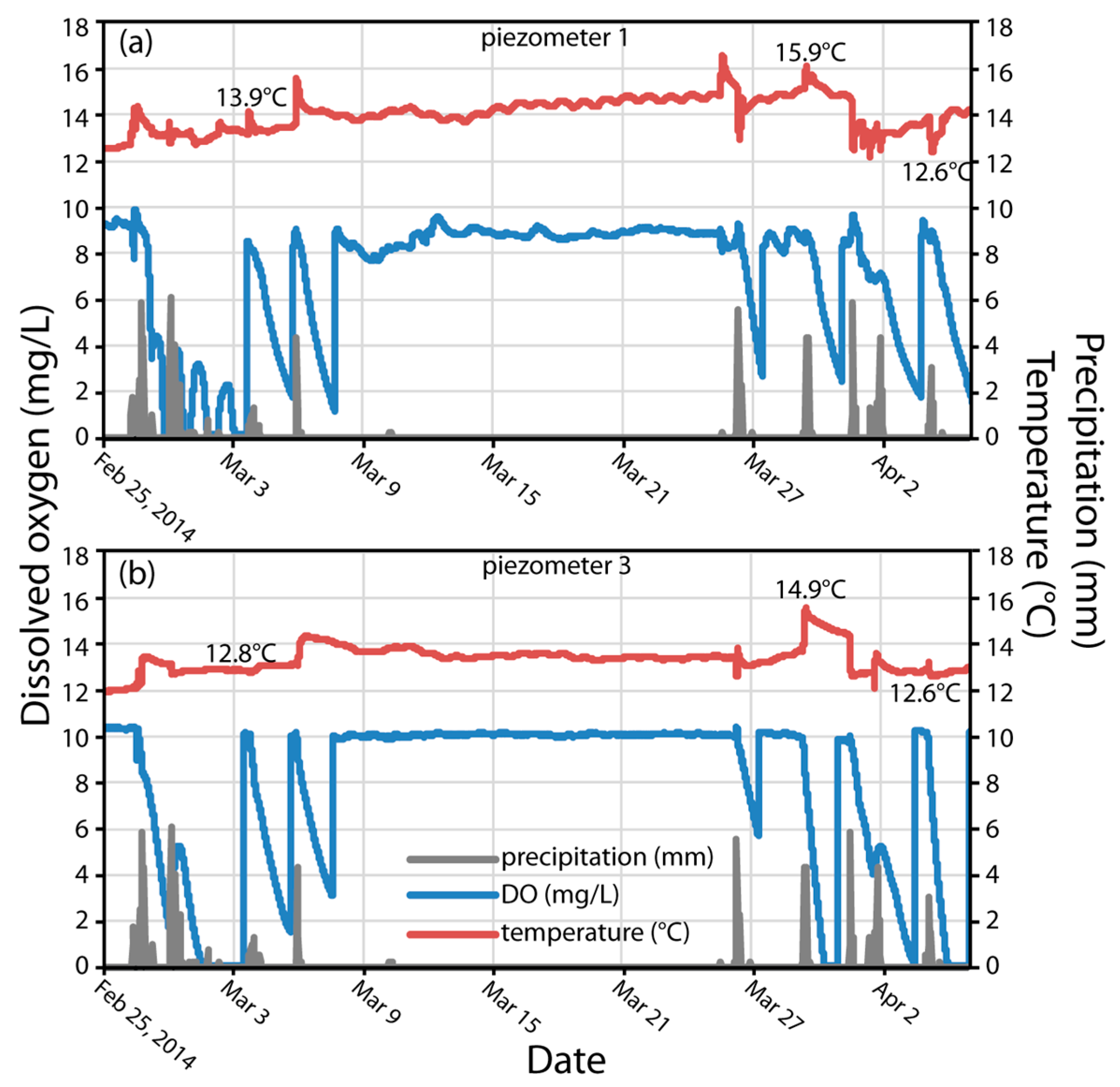
3.3. DO Dynamics in Response to Water Temperature
3.4. Rates of O2 Reduction and LID O2 Demand (LIDOD)
4. Conclusions and Implications for Designing LID BMPs
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paul, M.J.; Meyer, J.L. Streams in the urban landscape. Annu. Rev. Ecol. Evol. Syst. 2001, 32, 333–365. [Google Scholar] [CrossRef]
- Pitt, R.; Field, R.; Lalor, M.; Brown, M. Urban stormwater toxic pollutants: Assessment, sources, and treatability. Water Environ. Res. 1995, 67, 260–275. [Google Scholar] [CrossRef]
- Elliott, A.; Trowsdale, S. A review of models for low impact urban stormwater drainage. Environ. Model. Softw. 2007, 22, 394–405. [Google Scholar] [CrossRef]
- Maniquiz, M.C.; Lee, S.-Y.; Kim, L.-H. Long-Term Monitoring of Infiltration Trench for Nonpoint Source Pollution Control. Water Air Soil Pollut. 2010, 212, 13–26. [Google Scholar] [CrossRef]
- Fletcher, T.D.; Andrieu, H.; Hamel, P. Understanding, management and modelling of urban hydrology and its consequences for receiving waters: A state of the art. Adv. Water Resour. 2013, 51, 261–279. [Google Scholar] [CrossRef]
- Eriksson, E.; Baun, A.; Scholes, L.; Ledin, A.; Ahlman, S.; Revitt, M.; Noutsopoulos, C.; Mikkelsen, P.S. Selected stormwater priority pollutants—A European perspective. Sci. Total Environ. 2007, 383, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Joshi, U.M.; Balasubramanian, R. Characteristics and environmental mobility of trace elements in urban runoff. Chemosphere 2010, 80, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.E.; Fernandes, J.N.; David, L.M. Key issues for sustainable urban stormwater management. Water Res. 2012, 46, 6787–6798. [Google Scholar] [CrossRef] [PubMed]
- Gurdak, J.J.; Hanson, R.T.; Green, T.R. Effects of Climate Variability and Change on Groundwater Resources of the United States; U.S. Geological Survey: Lakewood, CO, USA, 2009; p. 4.
- Kuss, A.J.M.; Gurdak, J.J. Groundwater level response in U.S. principal aquifers to ENSO, NAO, PDO, and AMO. J. Hydrol. 2014, 519, 1939–1952. [Google Scholar] [CrossRef]
- Barbu, I.A.; Ballestero, T.P.; Roseen, R.M. LID-SWM Practices as a Means of Resilience to Climate Change and Its Effects on Groundwater Recharge. In Proceedings of the World Environmental and Water Resources Congress, Great Rivers, Kansas City, MO, USA, 17–21 May 2009; pp. 1–10.
- U.S. Environmental Protection Agency. Low Impact Development (LID)—A Literature Review; USEPA, Office of Water: Washington, DC, USA, 2000; p. 35.
- Department of Environmental Resources. Low-Impact Development Hydrologic Analysis; Prince George’s County, Department of Environmental Resources: Marlboro, MD, USA, 1999.
- Facility for Advancing Water Biofiltration (FAWB). Adoption Guidelines for Stormwater Biofiltration Systems; FAWB, Monash University: Melbourne, Australia, 2009. [Google Scholar]
- Dietz, M.E.; Clausen, J.C. Saturation to improve pollutant retention in a rain garden. Environ. Sci. Technol. 2006, 40, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Prince George’s County. Design Manual for Use of Bioretention in Stormwater Management; Prince George’s County (MD) Government, Department of Environmental Protection, Watershed Protection Branch: Landover, MD, USA, 1993.
- Kim, H.; Seagren, E.A.; Davis, A.P. Engineered bioretention for removal of nitrate from stormwater runoff. Water Environ. Res. 2003, 75, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Zinger, Y.; Blecken, G.-T.; Fletcher, T.D.; Viklander, M.; Deletić, A. Optimising nitrogen removal in existing stormwater biofilters: Benefits and tradeoffs of a retrofitted saturated zone. Ecol. Eng. 2013, 51, 75–82. [Google Scholar] [CrossRef]
- Zhang, Z.; Rengel, Z.; Liaghati, T.; Antoniette, T.; Meney, K. Influence of plant species and submerged zone with carbon addition on nutrient removal in stormwater biofilter. Ecol. Eng. 2011, 37, 1833–1841. [Google Scholar] [CrossRef]
- Payne, E.G.I.; Fletcher, T.D.; Cook, P.L.M.; Deletic, A.; Hatt, B.E. Processes and drivers of nitrogen removal in stormwater biofiltration. Crit. Rev. Environ. Sci. Technol. 2013, 44, 796–846. [Google Scholar] [CrossRef]
- Blecken, G.-T.; Zinger, Y.; Deletić, A.; Fletcher, T.D.; Viklander, M. Impact of a submerged zone and a carbon source on heavy metal removal in stormwater biofilters. Ecol. Eng. 2009, 35, 769–778. [Google Scholar] [CrossRef]
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C.; Winogradoff, D. Water quality improvement through bioretention: Lead, copper, and zinc removal. Water Environ. Res. 2003, 75, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chandrasena, G.I.; Pham, T.; Payne, E.G.; Deletic, A.; McCarthy, D.T. E. E. coli removal in laboratory scale stormwater biofilters: Influence of vegetation and submerged zone. J. Hydrol. 2014, 519, 814–822. [Google Scholar] [CrossRef]
- Rippy, M.A. Meeting the criteria: Linking biofilter design to fecal indicator bacteria removal. Wiley Interdiscip. Rev. Water 2015, 2, 577–592. [Google Scholar] [CrossRef]
- Li, Y.L.; Deletic, A.; Alcazar, L.; Bratieres, K.; Fletcher, T.D.; McCarthy, D.T. Removal of Clostridium perfringens, Escherichia coli and F-RNA coliphages by stormwater biofilters. Ecol. Eng. 2012, 49, 137–145. [Google Scholar] [CrossRef]
- Holman-Dodds, J.K.; Bradley, A.A.; Potter, K.W. Evaluation of hydrologic benefits of infiltration based urban storm water management. J. Am. Water Resour. Assoc. 2007, 39, 205–215. [Google Scholar] [CrossRef]
- Newcomer, M.E.; Gurdak, J.J.; Sklar, L.S.; Nanus, L. Urban recharge beneath low impact development and effects of climate variability and change. Water Resour. Res. 2014, 50, 1716–1734. [Google Scholar] [CrossRef]
- Dietz, M.E.; Clausen, J.C. A field evaluation of rain garden flow and pollutant treatment. Water Air Soil Pollut. 2005, 167, 123–138. [Google Scholar] [CrossRef]
- Lu, P.; Yuan, T. Low impact development design for urban stormwater management—A case study in USA. In Proceedings of the International Symposium on Water Resource and Environmental Protection (ISWREP), Xi’an, China, 20–22 May 2011; Volume 4, pp. 2741–2744.
- U.S. Environmental Protection Agency. Incorporating Low Impact Development into Municipal Stormwater Programs; U.S. Environmental Protection Agency: Washington, DC, USA, 2009; p. 11.
- Korom, S.F. Natural denitrification in the saturated zone: A review. Water Resour. Res. 1992, 28, 1657–1668. [Google Scholar] [CrossRef]
- McMahon, P.B.; Chapelle, F.H. Redox processes and water quality of selected Principal Aquifer systems. Ground Water 2008, 46, 259–271. [Google Scholar] [CrossRef] [PubMed]
- McMahon, P.B.; Cowdery, T.K.; Chapelle, F.H.; Jurgens, B.C. Redox Conditions in Selected Principal Aquifers of the United States; U.S. Geological Survey: Lakewood, CO, USA, 2009; p. 6.
- Kumar, A.R.; Riyazuddin, P. Seasonal variation of redox species and redox potentials in shallow groundwater: A comparison of measured and calculated redox potentials. J. Hydrol. 2012, 444–445, 187–198. [Google Scholar] [CrossRef]
- Datry, T.; Malard, F.; Gibert, J. Dynamics of solutes and dissolved oxygen in shallow urban groundwater below a stormwater infiltration basin. Sci. Total Environ. 2004, 329, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Von Rohr, M.R.; Hering, J.G.; Kohler, H.-P.E.; von Gunten, U. Column studies to assess the effects of climate variables on redox processes during riverbank filtration. Water Res. 2014, 61, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Greskowiak, J.; Prommer, H.; Massmann, G.; Johnston, C.D.; Nützmann, G.; Pekdeger, A. The impact of variably saturated conditions on hydrogeochemical changes during artificial recharge of groundwater. Appl. Geochem. 2005, 20, 1409–1426. [Google Scholar] [CrossRef]
- Vaughan, K.L.; Rabenhorst, M.C.; Needelman, B.A. Saturation and temperature effects on the development of reducing conditions in soils. Soil Sci. Soc. Am. J. 2009, 73, 663. [Google Scholar] [CrossRef]
- Tesoriero, A.J.; Puckett, L.J. O2 reduction and denitrification rates in shallow aquifers. Water Resour. Res. 2011, 47. [Google Scholar] [CrossRef]
- Fan, A.M.; Steinberg, V.E. Health implications of nitrate and nitrite in drinking water: An update on methemoglobinemia occurrence and reproductive and developmental toxicity. Regul. Toxicol. Pharmacol. 1996, 23, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Spalding, R.F.; Exner, M.E. Occurrence of nitrate in groundwater—A Review. J. Environ. Qual. 1993, 22, 392–402. [Google Scholar] [CrossRef]
- Gurdak, J.J.; Qi, S.L. Vulnerability of recently recharged groundwater in principle aquifers of the United States to nitrate contamination. Environ. Sci. Technol. 2012, 46, 6004–6012. [Google Scholar] [CrossRef] [PubMed]
- California Stormwater Quality Association (CASQA). Stormwater Best Management Practice Handbook: New Development and Redevelopment; California Stormwater Quality Association: Menlo Park, CA, USA, 2003; p. 378. [Google Scholar]
- Clifton, H.E.; Hunter, R.E.; Gardner, J.V. Analysis of eustatic, tectonic, and sedimentologic influences on transgressive and regressive cycles in the Upper Cenozoic Merced Formation, San Francisco, California. In New Perspectives in Basin Analysis; Kleinspehn, K.L., Paola, C., Eds.; Springer: New York, NY, USA, 1988; pp. 109–128. [Google Scholar]
- Nzewi, O.; Gilman, J.A.; Bartow, G. 2009 Annual Groundwater Monitoring Report, Westside Basin; San Francisco Public Utilities Commission: San Francisco, CA, USA, 2010. [Google Scholar]
- Precision Measurement Engineering (PME). miniDOT User’s Manual; Precision Measurement Engineering: Vista, CA, USA, 2014. [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA). National Centers for Environmental Information. Available online: https://www.ncdc.noaa.gov/ (accessed on 13 May 2016).
- Pazwash, H. Urban Storm Water Management; CRC Press, Taylor & Francis Group: Florence, KY, USA, 2011. [Google Scholar]
- Heckathorn, H.A.; Gibs, J. Sediment Oxygen Demand in the Saddle River and Salem River Watersheds, New Jersey, July–August 2008; U.S. Geological Survey: Reston, VA, USA, 2010; p. 10.
- Wilson, T.P. In-Situ Sediment Oxygen Demand Rates in Hammonton Creek, Hammonton, New Jersey, and Crosswicks Creek, near New Egypt, New Jersey, August–October 2009; U.S. Geological Survey: Reston, VA, USA, 2014; p. 18.
- Danfoura, M.N. Effects of Low Impact Development Design on Redox Dynamics in the Vadose Zone and Urban Groundwater. Master’s Thesis, San Francisco State University, San Francisco, CA, USA, 2015. [Google Scholar]
- Appleyard, S.J. Impact of stormwater infiltration basins on groundwater quality, Perth metropolitan region, Western Australia. Environ. Geol. 1993, 21, 227–236. [Google Scholar] [CrossRef]
- Sansalone, J.J.; Hird, J.P.; Cartledge, F.K.; Tittlebaum, M.E. Event-Based Stormwater Quality and Quantity Loadings from Elevated Urban Infrastructure Affected by Transportation. Water Environ. Res. 2005, 77, 348–365. [Google Scholar] [CrossRef] [PubMed]
- Datry, T.; Malard, F.; Vitry, L.; Hervant, F.; Gibert, J. Solute dynamics in the bed sediments of a stormwater infiltration basin. J. Hydrol. 2003, 273, 217–233. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Property of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- McMahon, P.B.; Böhlke, J.K.; Kauffman, L.J.; Kipp, K.L.; Landon, M.K.; Crandall, C.A.; Burow, K.R.; Brown, C.J. Source and transport controls on the movement of nitrate to public supply wells in selected principal aquifers of the United States. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Böhlke, J.K.; Wanty, R.; Tuttle, M.; Delin, G.; Landon, M. Denitrification in the recharge area and discharge area of a transient agricultural nitrate plume in a glacial outwash sand aquifer, Minnesota. Water Resour. Res. 2002, 38. [Google Scholar] [CrossRef]
- McMahon, P.B.; Böhlke, J.K.; Christenson, S.C. Geochemistry, radiocarbon ages, and paleorecharge conditions along a transect in the central High Plains aquifer, southwestern Kansas, USA. Appl. Geochem. 2004, 19, 1655–1686. [Google Scholar] [CrossRef]




| Precipitation Event | Total Rainfall (mm) | Duration of Saturation of Gravel (Days) | Dissolved Oxygen (DO) (mg·L−1) | Average Temp. (°C) | ||
|---|---|---|---|---|---|---|
| Min. | Avg. (SE) | Max. | ||||
| 26 February–3 March 2014 | ||||||
| piezometer 1 | 54 | 5.24 | 0.13 | 2.78 (0.05) | 9.9 | 13.6 |
| piezometer 3 | 5.16 | 0.10 | 3.12 (0.05) | 10.41 | 12.9 | |
| 3–4 March 2014 | ||||||
| piezometer 1 | 5.3 | 1.99 | 1.77 | 5.06 (0.06) | 8.22 | 13.4 |
| piezometer 3 | 1.93 | 1.51 | 4.92 (0.07) | 10.15 | 13.0 | |
| 5–6 March 2014 | ||||||
| piezometer 1 | 5.8 | 1.81 | 1.12 | 4.71 (0.06) | 8.86 | 14.3 |
| piezometer 3 | 1.63 | 3.09 | 5.76 (0.05) | 9.79 | 14.2 | |
| 25–26 March 2014 | ||||||
| piezometer 1 | 13 | 1.17 | 2.85 | 6.3 (0.07) | 9.26 | 14.3 |
| piezometer 3 | 1.11 | 5.68 | 8.04 (0.05) | 10.39 | 13.2 | |
| 29 March 2014 | ||||||
| piezometer 1 | 12 | 1.69 | 2.47 | 5.57 (0.06) | 8.87 | 15.2 |
| piezometer 3 | 1.64 | 0.10 | 3.1 (0.1) | 9.99 | 14.8 | |
| 4 April 2014 | ||||||
| piezometer 1 | 6.4 | 2.14 | 1.23 | 5.16 (0.06) | 8.96 | 13.8 |
| piezometer 3 | 2 | 0.10 | 2.44 (0.08) | 10.2 | 12.8 | |
| Precipitation Event | Total Rainfall (mm) | Average Temp. (°C) | Zero-Order Reaction Rates (−K0) | LIDODT | LIDOD20 | |
|---|---|---|---|---|---|---|
| (mg·L−1·min−1) | (mg·L−1·h−1) | (mg·m−3·min−1) | (mg·m−3·min−1) | |||
| 26 February 26–3 March 2014 | ||||||
| piezometer 1 | 54 | -- | 0.003 | 0.15 | -- | -- |
| piezometer 3 | 0.003 | 0.18 | -- | -- | ||
| 3–4 March 2014 | ||||||
| piezometer 1 | 5.3 | 13.0 | 0.003 | 0.19 | 1.48 | 2.23 |
| piezometer 3 | 0.003 | 0.16 | 1.80 | 2.80 | ||
| 5–6 March 2014 | ||||||
| piezometer 1 | 5.8 | 14.2 | 0.004 | 0.25 | 1.84 | 2.63 |
| piezometer 3 | 0.003 | 0.18 | 1.52 | 2.20 | ||
| 25–26 March 2014 | ||||||
| piezometer 1 | 13 | 12.6 | 0.003 | 0.17 | 2.49 | 3.59 |
| piezometer 3 | 0.005 | 0.27 | 1.83 | 2.81 | ||
| 29 March 2014 | ||||||
| piezometer 1 | 12 | -- | 0.002 | 0.10 | -- | -- |
| piezometer 3 | 0.002 | 0.12 | -- | -- | ||
| 4 April 2014 | ||||||
| piezometer 1 | 6.4 | -- | 0.003 | 0.16 | -- | -- |
| piezometer 3 | 0.003 | 0.20 | -- | -- | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danfoura, M.N.; Gurdak, J.J. Redox Dynamics and Oxygen Reduction Rates of Infiltrating Urban Stormwater beneath Low Impact Development (LID). Water 2016, 8, 435. https://doi.org/10.3390/w8100435
Danfoura MN, Gurdak JJ. Redox Dynamics and Oxygen Reduction Rates of Infiltrating Urban Stormwater beneath Low Impact Development (LID). Water. 2016; 8(10):435. https://doi.org/10.3390/w8100435
Chicago/Turabian StyleDanfoura, Mays N., and Jason J. Gurdak. 2016. "Redox Dynamics and Oxygen Reduction Rates of Infiltrating Urban Stormwater beneath Low Impact Development (LID)" Water 8, no. 10: 435. https://doi.org/10.3390/w8100435
APA StyleDanfoura, M. N., & Gurdak, J. J. (2016). Redox Dynamics and Oxygen Reduction Rates of Infiltrating Urban Stormwater beneath Low Impact Development (LID). Water, 8(10), 435. https://doi.org/10.3390/w8100435





