Assessment of Long-Term Evolution of Groundwater Hydrochemical Characteristics Using Multiple Approaches: A Case Study in Cangzhou, Northern China
Abstract
:1. Introduction
2. Materials and Methods
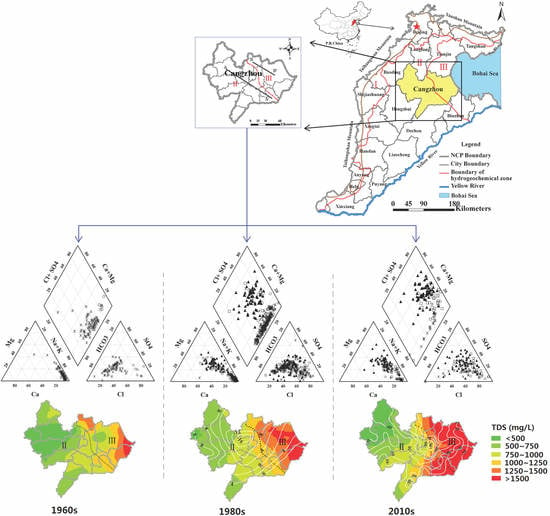
2.1. Study Area Description
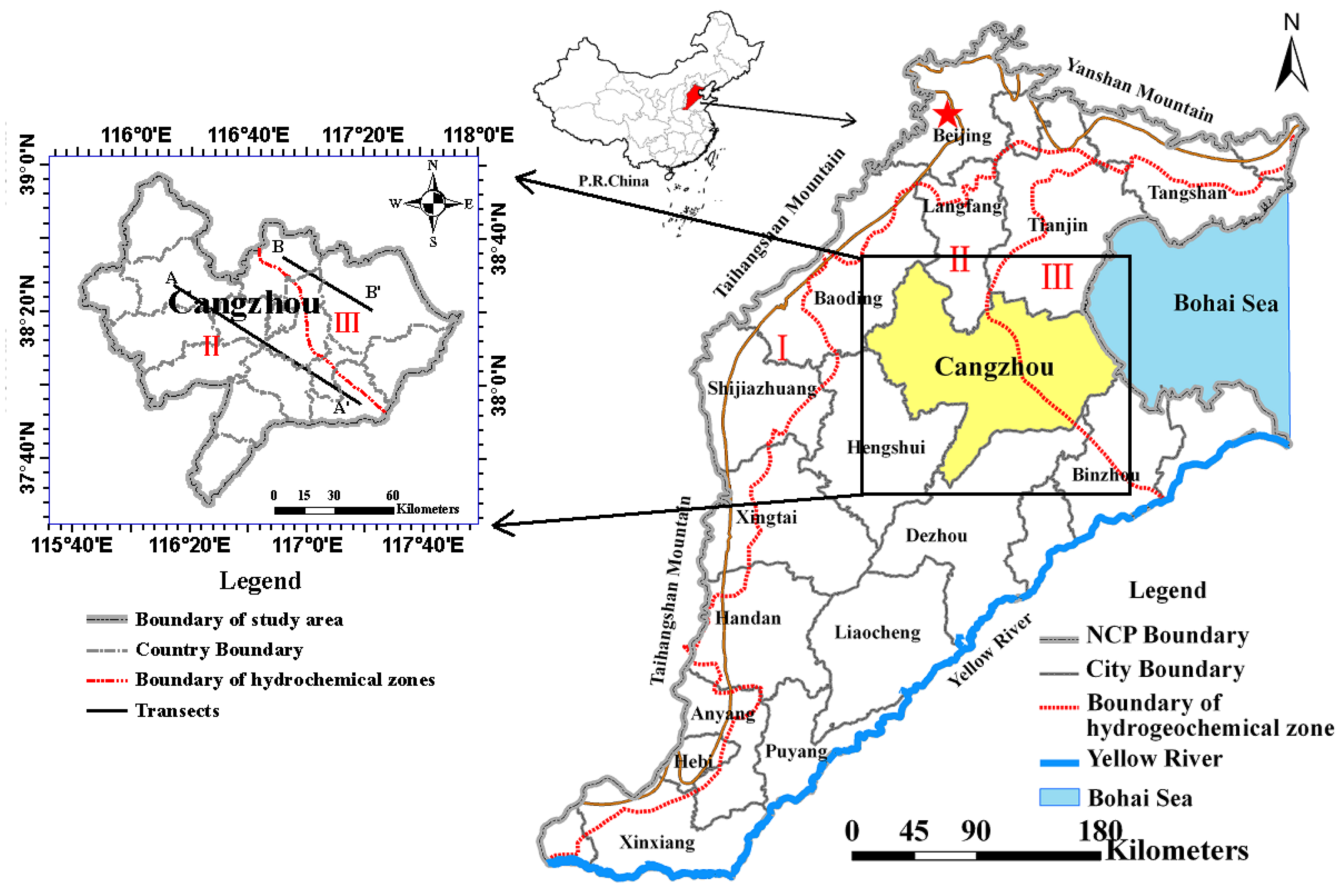
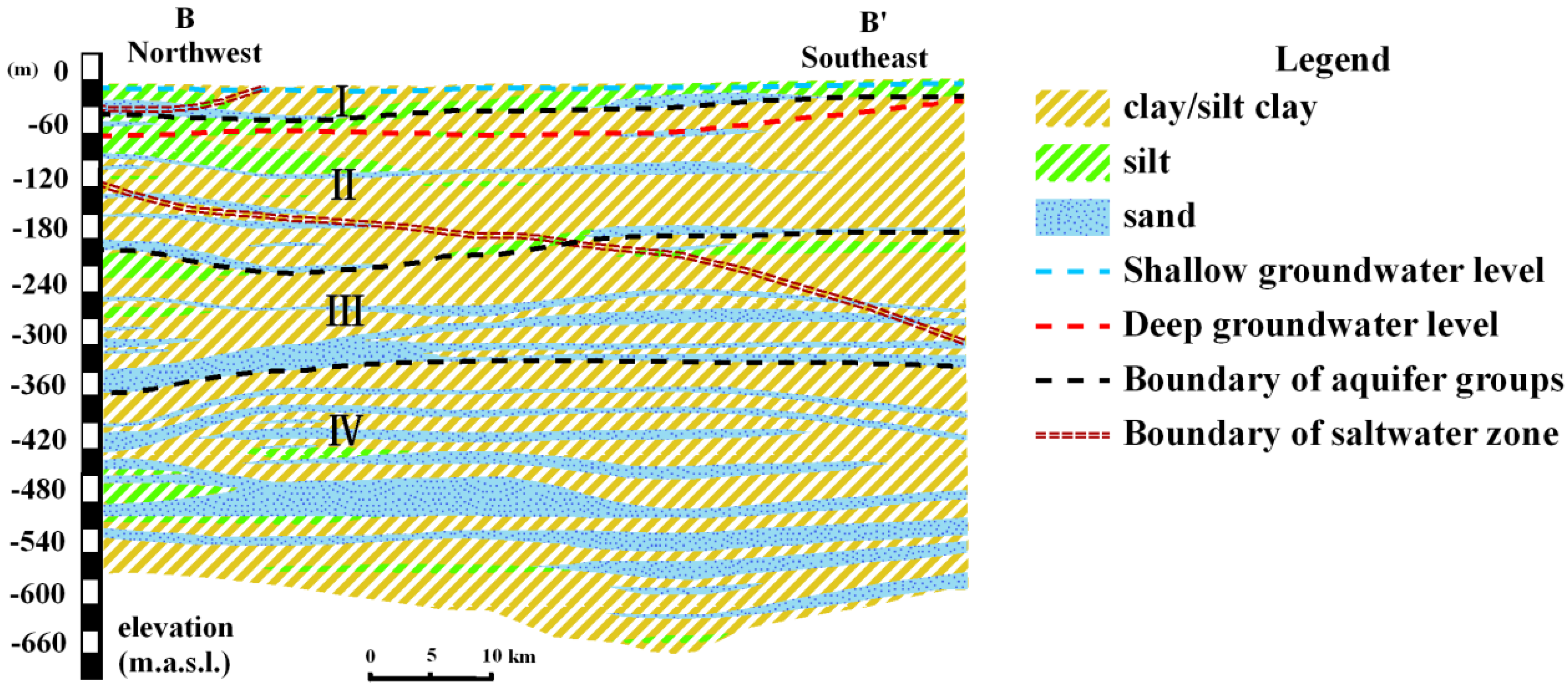
2.2. Geological and Hydrogeological Setting
| Aquifer Division | Geological Time | Depth of Aquifer Bottom (m) | Aquifer Thickness (m) | Stratum Lithology | Aquifer Lithology | Groundwater Type | Groundwater Hydrochemical Types [33] |
|---|---|---|---|---|---|---|---|
| I | Holocene | 10–40 | 5–20 | lacustrine sediments/marine sediment (clay, silt clay) | fine sand | fresh water/saltwater | HCO3.Cl.SO4-cation, HCO3.Cl-cation/Cl.HCO3-cation, Cl-cation |
| II | Upper Pleistocene | 12–250 | 5–70 | alluvium/lacustrine sediments (clay, loam, sandy loam) | silt, fine sand | saltwater/fresh water | SO4.Cl–Na.Mg, Cl–Na.Mg, Cl.SO4–Na.Mg/Cl–Na.Mg |
| III | Middle Pleistocene | 25–350 | 3–70 | alluvium/lacustrine sediments (clay, loam, sandy loam)/pyroclastic | medium sand, fine sand | fresh water | Cl.HCO3–Na, Cl.HCO3.SO4–Na, Cl.SO4–Na (Na.Ca, Na.Mg)/Cl–Na |
| IV | Lower Pleistocene Series | 35–550 | 2–45 | alluvium/lacustrine sediments (clay, silty fine sand) | medium sand, fine sand | saltwater/fresh water |

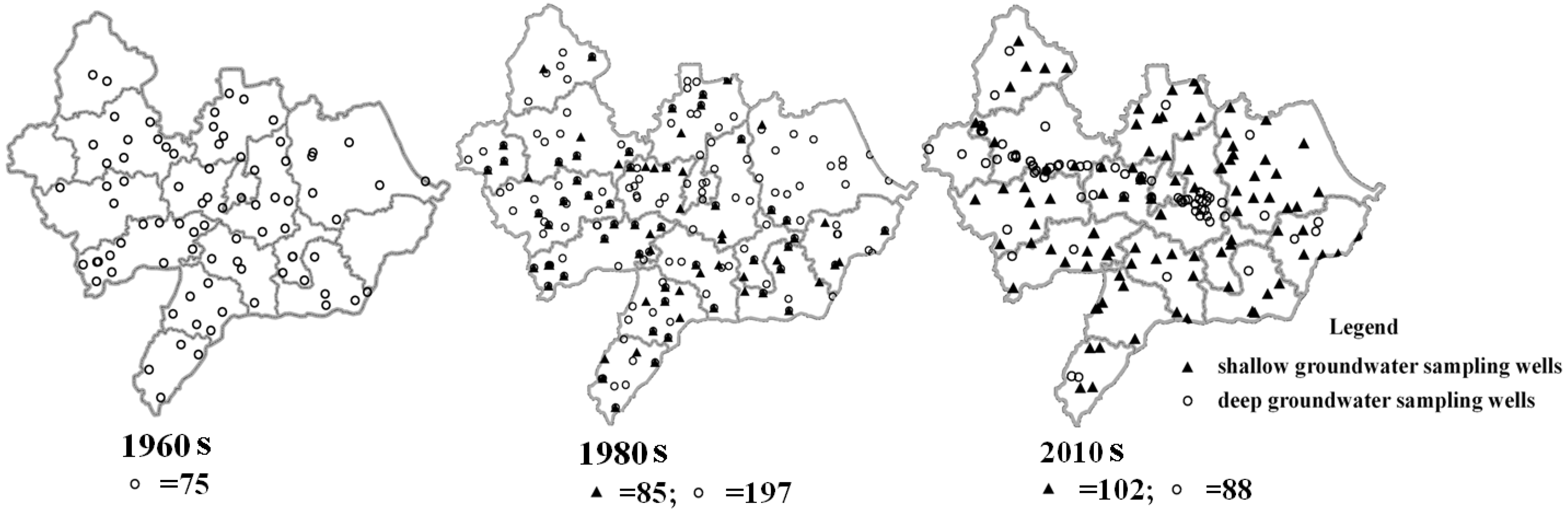
2.3. Data Collection and Groundwater Sampling Test
2.4. Data Analysis Methods
3. Results and Discussion
3.1. Groundwater Hydrochemical Types and Hydrochemical Evolution of Major Ions among Decades
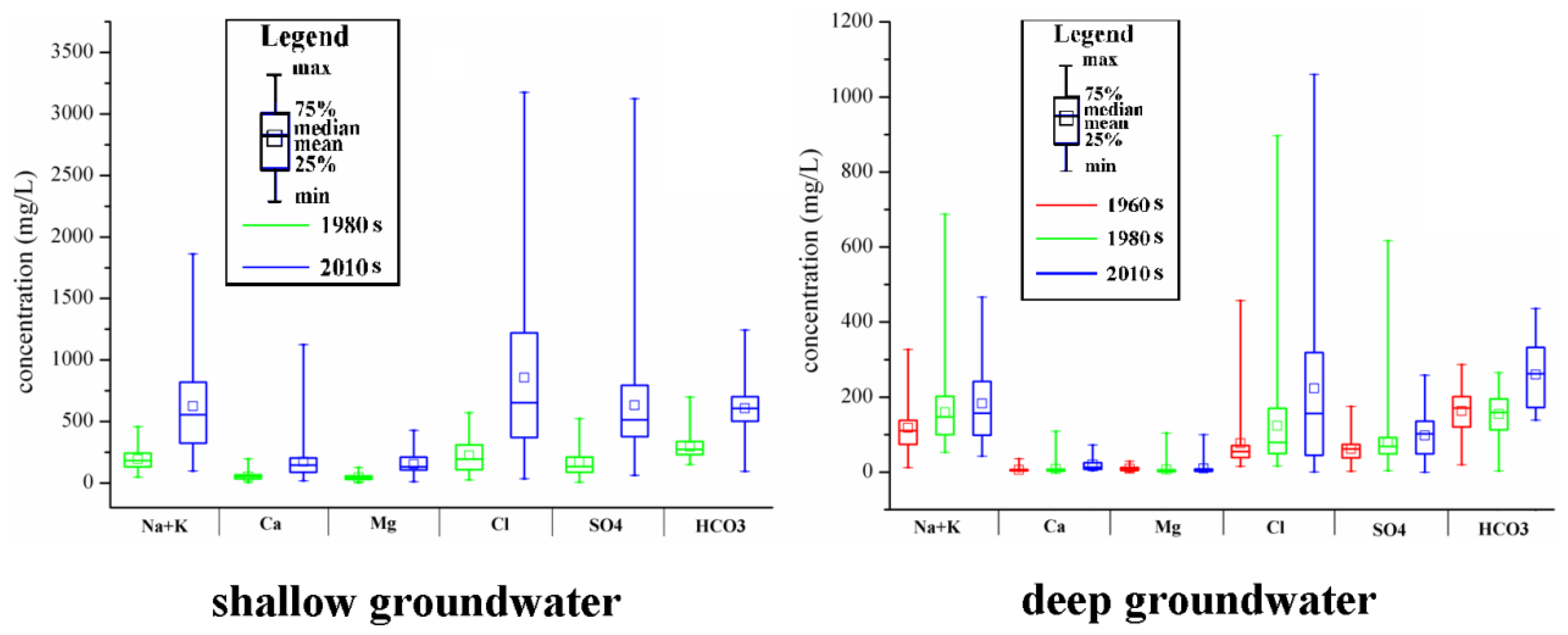
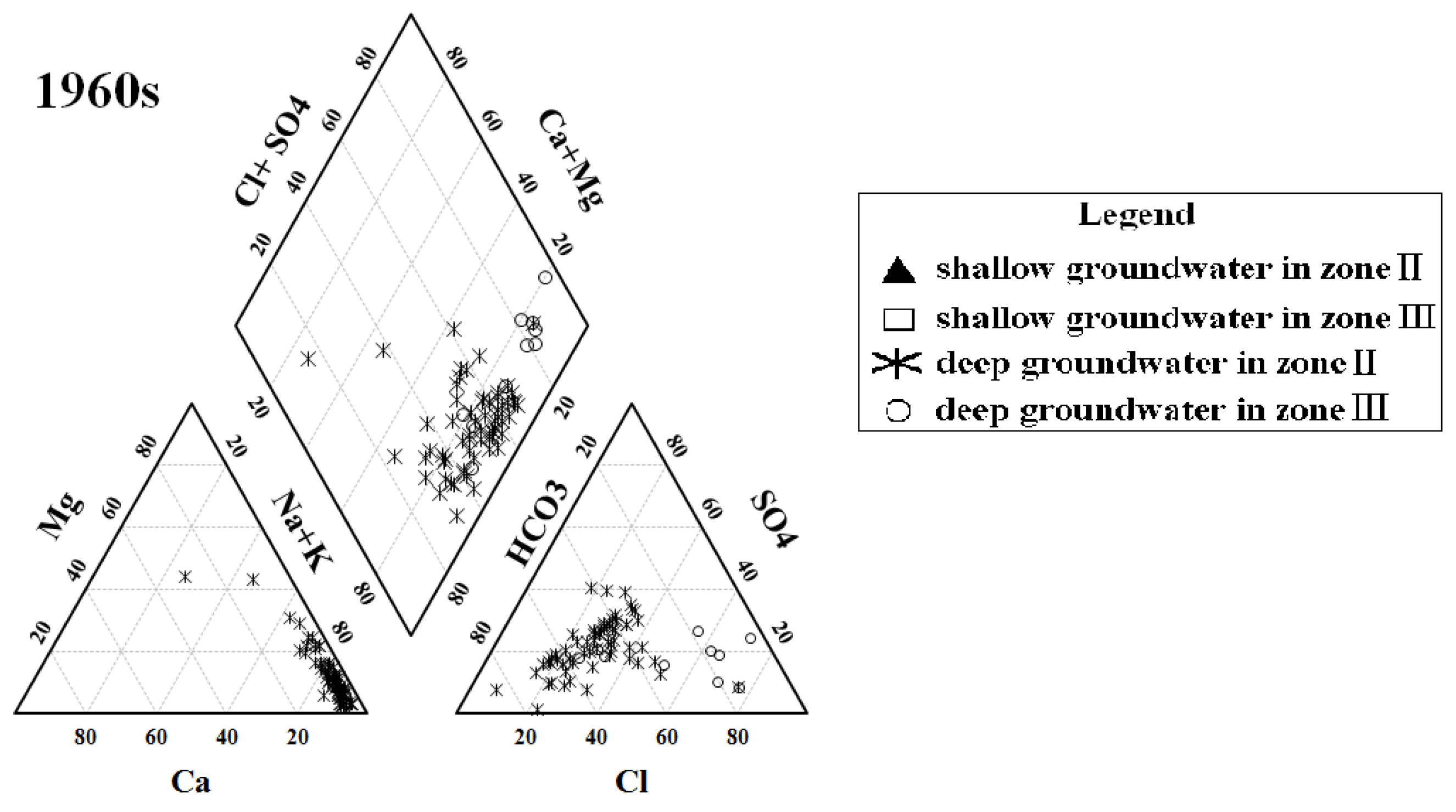


| Decades | Shallow Groundwater | Deep Groundwater | ||
|---|---|---|---|---|
| Na+/Cl− (meq/meq) | HCO3−/Cl− (meq/meq) | Na+/Cl− (meq/meq) | HCO3−/Cl− (meq/meq) | |
| 1960s | - | - | 2.691453 | 1.833077985 |
| 1980s | 1.518161 | 1.218300033 | 2.520137 | 1.252170605 |
| 2010s | 1.244109 | 0.720123542 | 1.350762 | 1.163695914 |
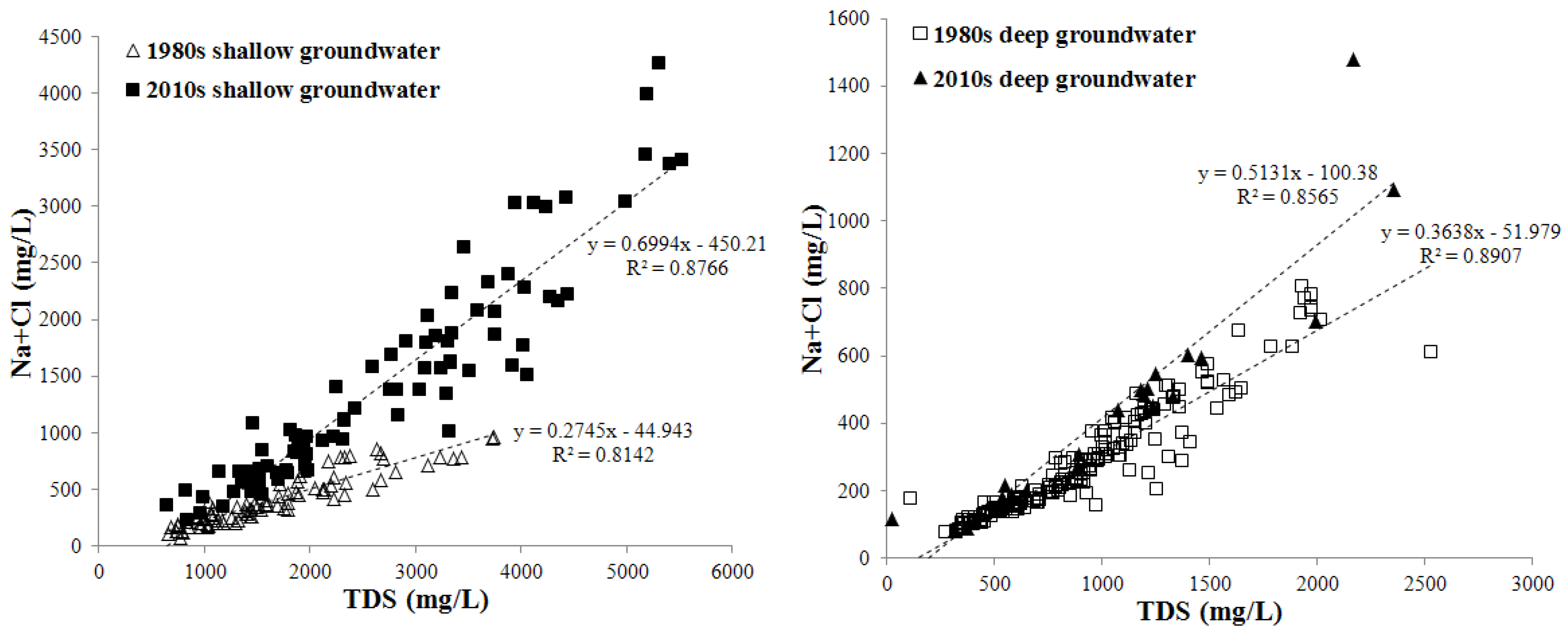
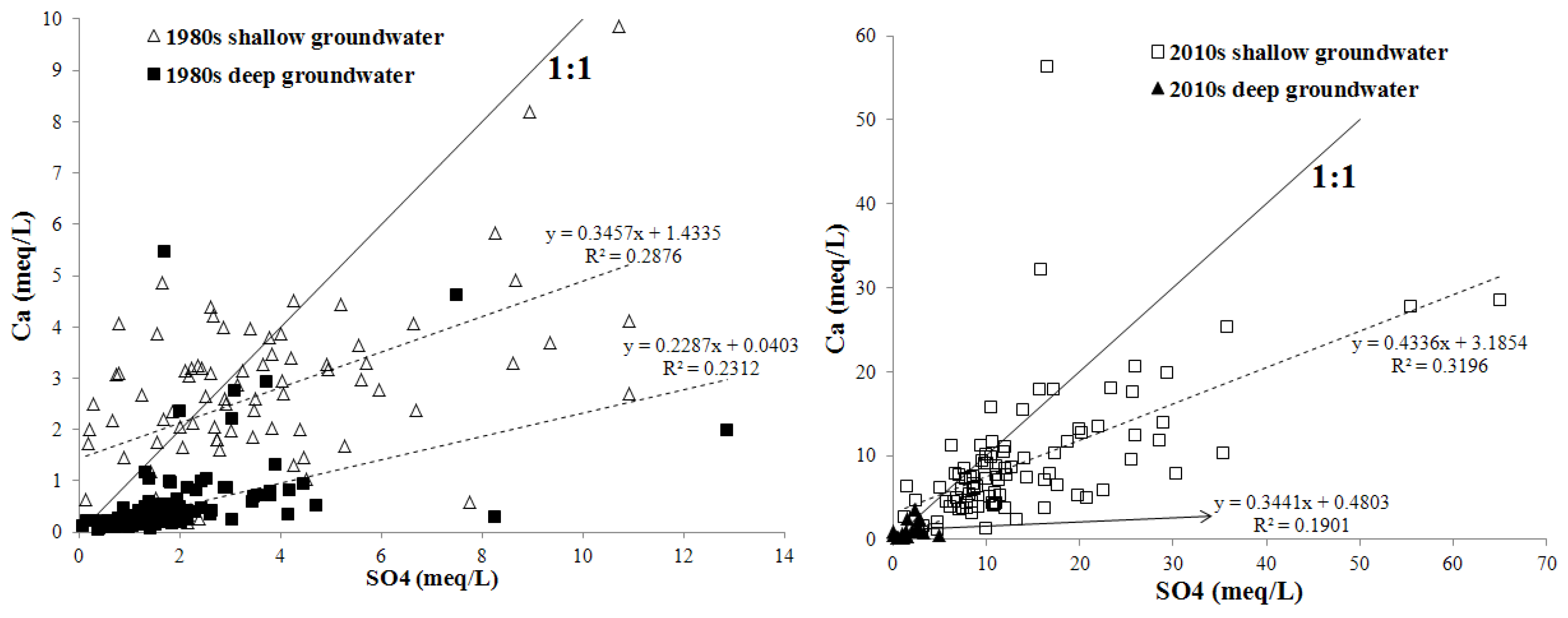
3.2. Statistical Analysis of Groundwater Major Ions and TDS
| Variable | 1960s | 1980s | 2010s | |||||
|---|---|---|---|---|---|---|---|---|
| Deep Groundwater | Shallow Groundwater | Deep Groundwater | Shallow Groundwater | Deep Groundwater | ||||
| F1 | F2 | F1 | F2 | F1 | F2 | F1 | F1 | |
| Na + K | 0.907 | −0.351 | 0.736 | 0.635 | 0.875 | −0.342 | 0.872 | 0.96 |
| Ca | 0.453 | 0.716 | 0.613 | −0.667 | 0.616 | 0.562 | 0.66 | 0.778 |
| Mg | 0.597 | 0.659 | 0.826 | −0.349 | 0.848 | 0.245 | 0.83 | 0.699 |
| Cl | 0.856 | −0.278 | 0.86 | 0.204 | 0.888 | −0.371 | 0.863 | 0.928 |
| SO4 | 0.706 | −0.256 | 0.786 | −0.282 | 0.829 | 0.017 | 0.728 | 0.812 |
| HCO3 | 0.366 | 0.509 | 0.281 | 0.385 | 0.358 | 0.618 | 0.345 | 0.627 |
| TDS | 0.974 | −0.172 | 0.965 | 0.175 | 0.977 | −0.164 | 0.983 | 0.953 |
| Eigen value | 3.697 | 1.502 | 3.967 | 1.271 | 4.424 | 1.04 | 4.246 | 4.835 |
| % of variance | 52.809 | 21.459 | 56.670 | 18.158 | 63.203 | 14.852 | 60.658 | 69.067 |
3.3. Spatial-Temporal Distribution of TDS in Shallow and Deep Groundwater

| Decades | Groundwater Type | OK | COK | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ME | MSE | MSSE | Optimal Model | ME | MSE | MSSE | Optimal Model | ||
| 1960s | deep groundwater | −0.0197 | 220.3 | 1.13 | Spherical model | −0.01756 | 188.2 | 1.005 | Stable model |
| 1980s | shallow groundwater | 0.00283 | 736.2 | 1.011 | Spherical model | −0.001413 | 734.1 | 1.003 | Spherical model |
| deep groundwater | 0.004293 | 460.8 | 1.018 | Exponential model | 0.001011 | 445.6 | 0.9958 | J-Bessel model | |
| 2010s | shallow groundwater | 0.004137 | 4862 | 1.181 | Spherical model | −0.001908 | 1485 | 0.9939 | K-Bessel model |
| deep groundwater | 0.04642 | 358.1 | 1.011 | K-Bessel model | 0.03716 | 316.6 | 0.9915 | J-Bessel model | |
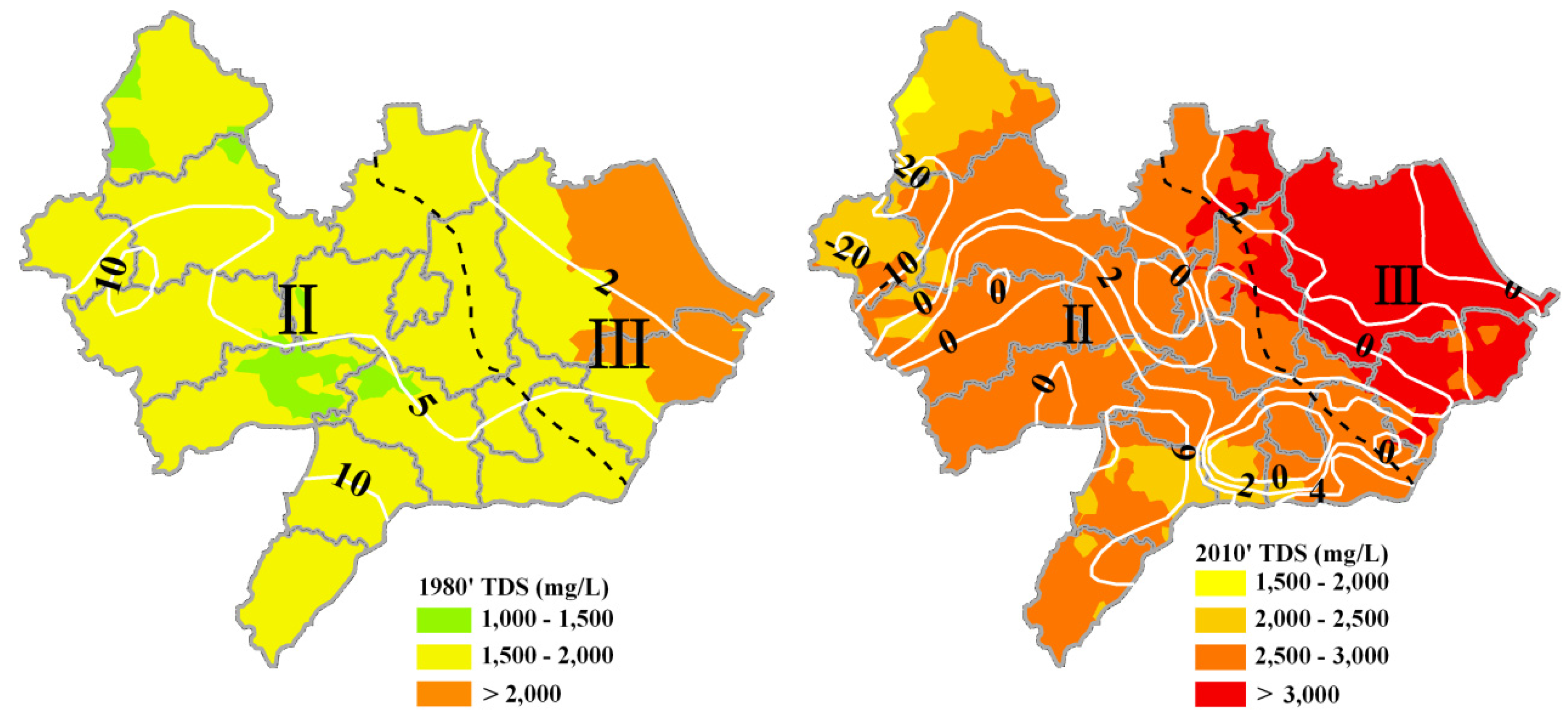

3.4. Discussion on the Major Factors Affecting Areal Groundwater Quality
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations Educational, Scientific and Cultural Organization (UNESCO). Groundwater Resources of the World and Their Use; UNESCO: Paris, France, 2004. [Google Scholar]
- World Health Orgnization (WHO). UN-Water Global Analysis and Assessment of Sanitation and Drinking-Water; The Challenge of Extending and Sustaining Services; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- United Nations Educational, Scientific and Cultural Organization (UNESCO). Water, Cultural Diversity and Global Environmental Change; UNESCO: Paris, France, 2012. [Google Scholar]
- Ravenscroft, P.; McArthur, J.M.; Hoque, M.A. Stable groundwater quality in deep aquifers of Southern Bangladesh: The case against sustainable abstraction. Sci. Total Environ. 2013, 454–455, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Mtoni, Y.; Mjemah, I.C.; Bakundukize, C.; van Camp, M.; Martens, K.; Walraevens, K. Saltwater intrusion and nitrate pollution in the coastal aquifer of Dares Salaam, Tanzania. Environ. Earth Sci. 2013, 70, 1091–1111. [Google Scholar] [CrossRef]
- Yakirevich, A.; Weisbrod, N.; Kuznetsov, M.; Rivera Villarreyes, C.A.; Benavent, I.; Chavez, A.M.; Ferrando, D. Modeling the impact of solute recycling on groundwater salinization under irrigated lands: A study of the Alto Piura aquifer, Peru. J. Hydrol. 2013, 482, 25–39. [Google Scholar] [CrossRef]
- Onodera, S.-I.; Saito, M.; Sawano, M.; Hosono, T.; Taniguchi, M.; Shimada, J.; Umezawa, Y.; Lubis, R.F.; Buapeng, S.; Delinom, R.; et al. Erratum to “effects of intensive urbanization on the intrusion of shallow groundwater into deep groundwater: Examples from Bangkok and Jakarta”. Sci. Total Environ. 2009, 407, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shahnaz, M.; Jehan, N.; Rehman, S.; Shah, M.T.; Din, I. Drinking water quality and human health risk in Charsadda district, Pakistan. J. Clean. Prod. 2013, 60, 93–101. [Google Scholar] [CrossRef]
- Parameswari, K.; Mudgal, B.V. Geochemical investigation of groundwater contamination in Perungudi dumpsite, South India. Arab. J. Geosci. 2013, 7, 1363–1371. [Google Scholar] [CrossRef]
- Ma, L.; Qin, X.; Sun, N.; Yang, G. Human health risk of metals in drinking-water source areas from a forest zone after long-term excessive deforestation. Hum. Ecol. Risk Assess. 2014, 20, 1200–1212. [Google Scholar] [CrossRef]
- Triki, I.; Trabelsi, N.; Zairi, M.; Dhia, H.B. Multivariate statistical and geostatistical techniques for assessing groundwater salinization in Sfax, a coastal region of eastern Tunisia. Desalin. Water Treat. 2014, 52, 1980–1989. [Google Scholar] [CrossRef]
- Trabelsi, R.; Zairi, M.; Dhia, H.B. Groundwater salinization of the Sfax superficial aquifer, Tunisia. Hydrogeol. J. 2007, 15, 1341–1355. [Google Scholar] [CrossRef]
- Cruz, J.V.; Coutinho, R.; Pacheco, D.; Cymbron, R.; Antunes, P.; Freire, P.; Mendes, S. Groundwater salinization in the Azores archipelago (Portugal). Environ. Earth Sci. 2010, 62, 1273–1285. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abdel Samie, S.G.; Badawy, H.A. Factors controlling mechanisms of groundwater salinization and hydrogeochemical processes in the Quaternary aquifer of the Eastern Nile Delta, Egypt. Environ. Earth Sci. 2012, 68, 369–394. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Ale, S. Long term (1960–2010) trends in groundwater contamination and salinization in the Ogallala aquifer in Texas. J. Hydrol. 2014, 513, 376–390. [Google Scholar] [CrossRef]
- Guo, H.; Yang, S.; Tang, X.; Li, Y.; Shen, Z. Groundwater geochemistry and its implications for arsenic mobilization in shallow aquifers of the Hetao Basin, Inner Mongolia. Sci. Total Environ. 2008, 393, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Nouri, J.; Mahvi, A.H.; Jahed, G.R.; Babaei, A.A. Regional distribution pattern of groundwater heavy metals resulting from agricultural activities. Environ. Geol. 2007, 55, 1337–1343. [Google Scholar] [CrossRef]
- Shah, B.A. Arsenic in groundwater, Quaternary sediments, and suspended river sediments from the Middle Gangetic Plain, India: Distribution, field relations, and geomorphological setting. Arab. J. Geosci. 2013, 7, 3525–3536. [Google Scholar] [CrossRef]
- Pradhan, J.K.; Kumar, S. Informal e-waste recycling: Environmental risk assessment of heavy metal contamination in Mandoli industrial area, Delhi, India. Environ. Sci. Pollut. Res. Int. 2014, 21, 7913–7928. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Chen, Z.; Liu, J.; Wei, W. Sustainability of intensively exploited aquifer systems in the North China Plain: Insights from multiple environmental tracers. J. Earth Sci. 2014, 25, 605–611. [Google Scholar] [CrossRef]
- Mudiam, M.K.; Pathak, S.P.; Gopal, K.; Murthy, R.C. Studies on urban drinking water quality in a tropical zone. Environ. Monit. Assess. 2012, 184, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Ale, S. Temporal evolution of depth-stratified groundwater salinity in municipal wells in the major aquifers in Texas, USA. Sci. Total Environ. 2014, 472, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Alley, W.M.; Healy, R.W.; LaBaugh, J.W.; Reilly, T.E. Flow and storage in groundwater systems. Science 2002, 296, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, J.; Cao, G.; Kendy, E.; Wang, H.; Jia, Y. Can China cope with its water crisis? Perspectives from the North China Plain. Ground Water 2010, 48, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zheng, C.; Scanlon, B.R.; Liu, J.; Li, W. Use of flow modeling to assess sustainability of groundwater resources in the North China Plain. Water Resour. Res. 2013, 49, 159–175. [Google Scholar] [CrossRef]
- Hydrogeological Information of Hebei Province, China. Available online: http://www.hbsw.net/news/wenxuezuopin/2008711/0871110JDAH43I81JG12K8689AJ.html (accessed on 25 September 2014).
- United Nations Educational, Scientific and Cultural Organization (UNESCO). World Water Resources, a New Appraisal and Assessment for the 21st Century; UNESCO: Paris, France, 1998. [Google Scholar]
- Han, Z.T.; Wang, P.; Zhang, W.; Xia, W.Z.; Ma, L.S.; Li, Y.S.; Zhang, F.W. Analysis of the evolvement of deep confined groundwater depression cone and water supply strategy for Cangzhou area. Hydrol. Eng. Geo. 2013, 40, 29–33. (In Chinese) [Google Scholar]
- Li, Y.S.; Fei, Y.H.; Qian, Y.; Jian, M.; Han, Z.T.; Wang, P.; Zhang, Z.J. Discussion on evolution characteristics and formation mechanism of deep groundwater depression cone in Cangzhou region. J. Arid Land Resour. Environ. 2013, 27, 181–184. (In Chinese) [Google Scholar]
- Fu, X.G.; Lei, D.L.; Liu, W.C. Relationship and control countermeasures between land subsidence and groundwater depression cones in Cangzhou. In Proceedings of the Chinese Hydraulic Engineering Society Academic Annual Conference in 2003, Beijing, China, 11–13 November 2003; pp. 110–115. (In Chinese)
- Lv, Q.Y. Analysis of annual characteristics and utilization status of water resources in Cangzhou. Groundwater 2014, 36, 157–160. (In Chinese) [Google Scholar]
- Zhang, K.Y.; Zhang, C.H.; Zhamg, L. Hydraulic characteristics of Cangzhou. Water Conserv. Sci. Technol. Econ. 2010, 16, 1211–1212. (In Chinese) [Google Scholar]
- Zhang, Z.H.; Shen, Z.L.; Xue, Y.Q.; Ren, H.F.; Shi, D.H. Evolution of Groundwater Environment in North China Plain; Geology Press: Beijing, China, 2000; pp. 6–7, 35–57, 75–77. (In Chinese) [Google Scholar]
- Zhang, Z.J.; Fei, Y.H.; Chen, Z.Y.; Zhao, Z.Z.; Xie, Z.H. Investigation and Assessment of the Sustainable Utilization of Groundwater Resources in North China Plain; Geology Press: Beijing, China, 2009; pp. 59–81. (In Chinese) [Google Scholar]
- Zhang, Z.J.; Fei, Y.H. Atlas of Sustainable Utilization of Groundwater in North China Plain; China Map Press: Beijing, China, 2009; pp. 50–51. (In Chinese) [Google Scholar]
- National Geological Archives of China. Available online: http://www.ngac.cn/Zlml.aspx?KeyType=1&Key=%u6CA7%u5DDE (accessed on 25 September 2014).
- Ministry of Health, China (MHO). Standard Examination Methods for Drinking Water-General Principles; MHO: Beijing, China, 2006.
- Piper, M. A graphical procedure in the geochemical interpretation of water analysis. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Wang, S. Groundwater quality and its suitability for drinking and agricultural use in the Yanqi Basin of Xinjiang Province, Northwest China. Environ. Monit. Assess. 2013, 185, 7469–7484. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, P.; Someshwar Rao, M.; Rawat, Y.S.; Kumar, C.P.; Krishan, G.; Parveen, T. Evaluation of hydrogeochemistry and water quality in Bist-Doab region, Punjab, India. Environ. Earth Sci. 2013, 72, 693–706. [Google Scholar] [CrossRef]
- Alderman, J.H.; Hudak, P.F.; Acevedo, M. Chemical ratios and groundwater contamination in East Texas. Bull. Environ. Contam. Toxicol. 2002, 69, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Song, S.H. Evaluation of groundwater quality in coastal areas: Implications for sustainable agriculture. Environ. Geol. 2007, 52, 1231–1242. [Google Scholar] [CrossRef]
- Kharroubi, A.; Tlahigue, F.; Agoubi, B.; Azri, C.; Bouri, S. Hydrochemical and statistical studies of the groundwater salinization in Mediterranean arid zones: Case of the Jerba coastal aquifer in southeast Tunisia. Environ. Earth Sci. 2012, 67, 2089–2100. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, C.; Pang, J.; Zha, X.; Li, X.; Zhang, Y. Concentration of heavy metals in the modern flood slackwater deposits along the upper Hanjiang River valley, China. Catena 2014, 116, 123–131. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, L.; Li, J.; Wang, C.; Ji, J. Sediment properties and heavy metal pollution assessment in the river, estuary and lake environments of a fluvial plain, China. Catena 2014, 119, 52–60. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, H.; Chen, J.; Qiao, L. Assessment of groundwater chemistry and status in a heavily used semi-arid region with multivariate statistical analysis. Water 2014, 6, 2212–2232. [Google Scholar] [CrossRef]
- Sadat Noori, S.M.; Ebrahimi, K.; Liaghat, A.-M.; Hoorfar, A.-H. Comparison of different geostatistical methods to estimate groundwater level at different climatic periods. Water Environ. J. 2013, 27, 10–19. [Google Scholar] [CrossRef]
- Arslan, H. Estimation of spatial distrubition of groundwater level and risky areas of seawater intrusion on the coastal region in Carsamba Plain, Turkey, using different interpolation methods. Environ. Monit. Assess. 2014, 186, 5123–5134. [Google Scholar] [CrossRef] [PubMed]
- Babak, O.; Deutsch, C.V. Improved spatial modeling by merging multiple secondary data for intrinsic collocated cokriging. J. Petrol. Sci. Eng. 2009, 69, 93–99. [Google Scholar] [CrossRef]
- Wu, C.; Wu, J.; Luo, Y. Spatial prediction of soil organic matter content using cokriging with remotely sensed data. Soil Sci. Soc. Am. J. 2009, 73, 1202–1208. [Google Scholar] [CrossRef]
- Ghadermaz, I.J.; Sayyad, G.; Mohammadi, J. Spatial prediction of nitrate concentration in drinking water using pH as auxiliary cokriging variable. Procedia Environ. Sci. 2013, 3, 130–135. [Google Scholar] [CrossRef]
- Ahmed, S.; de Marsily, G.; Talbot, A. Combined use of hydraulic and electrical properties of an aquifer in a geostatistical estimation of transmissivity. Groundwater 1988, 26, 78–86. [Google Scholar] [CrossRef]
- Xing, L.; Guo, H.; Zhan, Y. Groundwater hydrochemical characteristics and processes along flow paths in the North China Plain. J. Asian Earth Sci. 2013, 70–71, 250–264. [Google Scholar]
- Zhan, Y.; Guo, H.; Wang, Y.; Li, R.; Hou, C.; Shao, J.; Cui, Y. Evolution of groundwater major components in the Hebei Plain: Evidences from 30-year monitoring data. J. Earth Sci. 2014, 25, 563–574. [Google Scholar] [CrossRef]
- Chen, W.H.; Nie, M.Y. Quaternary Geology in Hebei; Geological Publish House: Beijing, China, 1987. (In Chinese) [Google Scholar]
- Spears, D.A. Mineralogical control of the chemical evolution of groundwater. In Solute Processes; Wiley: Chichester, UK, 1986; p. 512. [Google Scholar]
- El Mejri, H.; Ben Moussa, A.; Zouari, K. The use of hydrochemical and environmental isotopic tracers to understand the functioning of the aquifer system in the Bou Hafna and Haffouz regions, Central Tunisia. Quat. Int. 2014, 338, 88–98. [Google Scholar]
- Sajil Kumar, P.J.; Jegathambal, P.; James, E.J. Factors influencing the high fluoride concentration in groundwater of Vellore District, South India. Environ. Earth Sci. 2014, 72, 2437–2446. [Google Scholar]
- Ministry of Environmental Protection, China (MEP). Quality Standard for Ground Water; MEP: Beijing, China, 1993.
- Cao, G.; Han, D.; Song, X. Evaluating actual evapotranspiration and impacts of groundwater storage change in the North China Plain. Hydrol. Process. 2014, 28, 1797–1808. [Google Scholar] [CrossRef]
- Al-Harbi, M.; AL-Ruwaih, F.M.; Alsulaili, A. Statistical and analytical evaluation of groundwater quality in Al-Rawdhatain field. Environ. Prog. Sustain. 2014, 33, 895–904. [Google Scholar] [CrossRef]
- Umar, M.; Waseem, A.; Sabir, M.A.; Kassi, A.M.; Khan, A.S. The impact of geology of recharge areas on groundwater quality: A case study of Zhob River Basin, Pakistan. Clean Soil Air Water 2013, 41, 119–127. [Google Scholar] [CrossRef]
- Fei, Y.H.; Zhang, Z.J.; Song, H.B.; Qian, Y.; Chen, J.S.; Meng, S.H. Discussion of vertical variations of saline groundwater and mechanism in North China Plain. Water Resour. Prot. 2009, 26, 21–23. (In Chinese) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, M.-Y.; Liu, L.-Y.; Yan, Y. Assessment of Long-Term Evolution of Groundwater Hydrochemical Characteristics Using Multiple Approaches: A Case Study in Cangzhou, Northern China. Water 2015, 7, 1109-1128. https://doi.org/10.3390/w7031109
Li W, Wang M-Y, Liu L-Y, Yan Y. Assessment of Long-Term Evolution of Groundwater Hydrochemical Characteristics Using Multiple Approaches: A Case Study in Cangzhou, Northern China. Water. 2015; 7(3):1109-1128. https://doi.org/10.3390/w7031109
Chicago/Turabian StyleLi, Wei, Ming-Yu Wang, Li-Ya Liu, and Yan Yan. 2015. "Assessment of Long-Term Evolution of Groundwater Hydrochemical Characteristics Using Multiple Approaches: A Case Study in Cangzhou, Northern China" Water 7, no. 3: 1109-1128. https://doi.org/10.3390/w7031109