Technologies for Decentralized Fluoride Removal: Testing Metallic Iron-based Filters
Abstract
:1. Introduction
2. Existing Technologies for F− Removal and Their Application in the Developing World
2.1. Water Quality Monitoring
2.2. Water Treatment Technologies for Fluoride (F−) Removal
| Material | Comments | Availability |
|---|---|---|
| Metal oxides and hydroxides | Exhibit relatively low efficiency without physico-chemical modification | low |
| Biosorbents | Exhibit relatively low efficiency without physico-chemical modification | very high |
| Geomaterials | Exhibit relatively low efficiency without physico-chemical modification | high |
| Carbonaceous Materials | Typically require expensive physico-chemical activation | low |
| Bone char | Produced by carbonizing animal bones | high |
| Industrial by-Products | Typically require expensive physico-chemical activation | low |
| Metallic iron (Fe0) | Under natural conditions a ubiquitous (hydr)oxide layer is also present | high |
2.3. Biomaterial-Based Filters for F− Removal
2.4. Bone Char-Based Filters for F− Removal
3. Fe0 Filters
3.1. Historical Background
3.2. The State-of-the Art
3.3. Designing Fe0 Filters for Fluoride Removal
3.3.1. Physico-Chemical Processes in Fe0 Filters
3.3.2. Biological Mediated Processes in Fe0 Filters
3.3.3. Optimizing the Efficiency Fe0 Filters
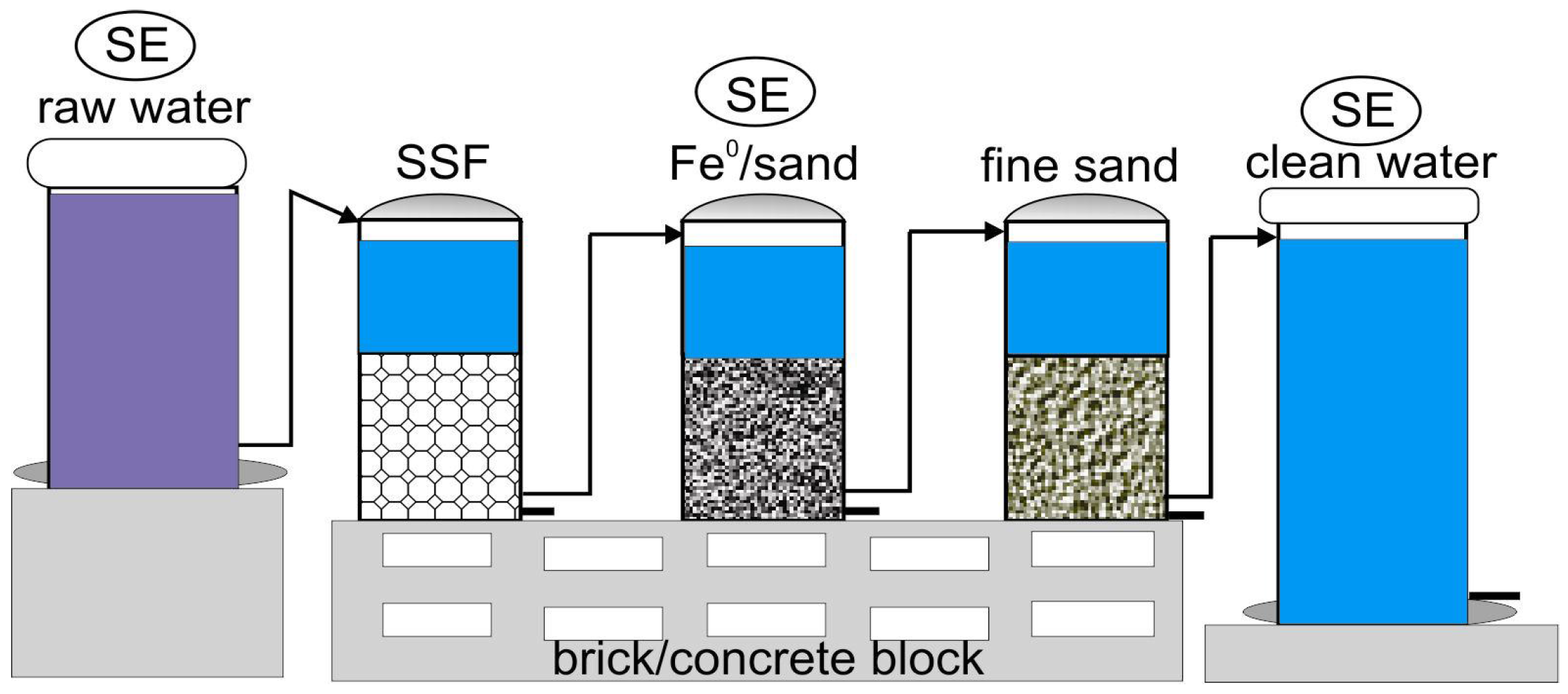
3.3.4. Fe0 Filters for Fluoride Removal
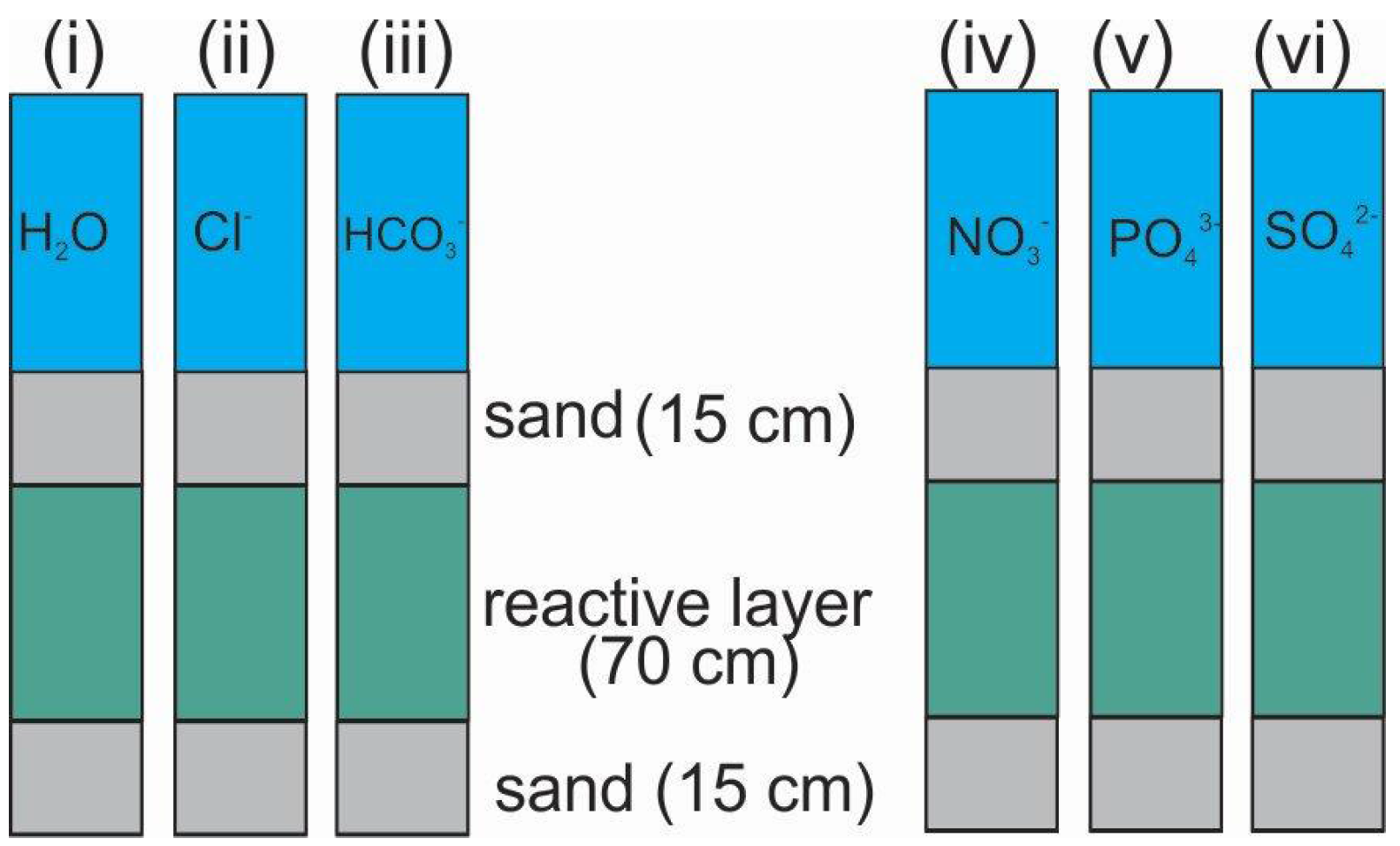

4. Steel Wool for Universal Fluoride Removal
4.1. Availability and Suitability of Steel Wool

4.2. Current Use of Steel Wool for Water Treatment
| Nr | Anno | Ref. | Trade Name | Element (%) | |
|---|---|---|---|---|---|
| Fe | C | ||||
| 1 | 2015 | [141] | Limpano (Brazil) | 99.8 | 0.1 |
| 2 | 2014 | [146] | n.s. | n.s. | n.s. |
| 3 | 2013 | [147] | n.s. | n.s. | n.s. |
| 4 | 2013 | [137] | n.s. | n.s. | n.s. |
| 5 | 2012 | [139] | n.s | 98.6 | n.s |
| 6 | 2011 | [96] | Peerless Metal Powders & Abrasive | n.s. | n.s. |
| 7 | 2010 | [148] | Bombril (Brazil) | n.s. | n.s. |
| 8 | 2009 | [149] | Mapavirulana (Argentina) | 98.5 | 0.1 |
| 9 | 2008 | [150] | n.s. | 98.5 | n.s. |
| 10 | 2007 | [118] | Global Material Technologies (USA) | n.s. | n.s. |
| 11 | 2005 | [140] | Rohit Industries (India) | n.s. | n.s. |
| 12 | 2002 | [133] | n.s. | n.s. | n.s. |
| 13 | 1998 | [132] | Rhodes American Company | n.s. | n.s. |
| 14 | 1997 | [136] | n.s. | n.s. | n.s. |
| 15 | 1992 | [102] | Rhodes American Company | n.s. | n.s. |
| 16 | 1984 | [138] | Rhodes Company (USA) | n.s. | n.s. |
| Nr. | X | [X] (mg/L) | Fe0 (g/L) | pH Value | Duration | Volume (mL) | Mixing | Speed (rpm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Phenol | 200 | 1 to 7 | 5–9 | 300 min | 500 | stirred | n.s. |
| 3 | Cr | 100 | 0.0083 | 3 | 2 h | 50 | n.s. | - |
| 4 | As(V) | 0.3 | n.s. | 3–12 | 480 min | 100 | shaken | 35–40 |
| 5 | PCBs | <12,800 | 500 | n.s. | 4 h | 2 | stirred | n.s. |
| 7 | Cr(VI) | 9.44 | 0.015 | n.s. | 3 h | 30 | n.s. | n.s. |
| 8 | As | 1 | 2 to 3 | 7.1 | 150 min | 500 | stirred | 300 |
| 9 | As | 1–1.3 | 1.3 | <8 | 24 h | 1000 | quiescient | - |
| 10 | PO43− | 0.5 | n.s. | 5.7 | 24 h | 150 | shaken | - |
| 11 | NO3− | 42 | 2.5–25 | n.s. | 60 d | 200 | n.s. | n.s. |
| 12 | As | 0.5 to 10 | n.s. | 6.1 to 6.8 | 15 min | 50 | n.s. | n.s. |
| 13 | NO3− | 50 | 40 | 5 to 11 | 12 d | 250 | shaken | 100 |
| 15 | PO43− | 4 | 7.41 | 5 | 60 min | 25 | agitated | - |
| 16 | 60Co | 200–1000 | 10 | 6.84 | 30 s | 20 | n.s. | n.s. |
| Nr. | X | [X] (mg/L) | Fe0 (g) | Dimensions (cm) | Porosity (%) | pH | Duration | Flow (mL/h) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | L | RZ | ||||||||
| 2 | Cr | <100 | 10 | 3 | 30 | n.s. | n.s. | 3–7 | 120 min | 20 |
| 4 | As (V) | 0.3 | 47 | 4 | 0.1 | n.s. | n.s. | n.s. | 90 h | <600 |
| 5 | Virus | 1010 PFU | 260 | 2.5 | 8.9 | n.s. | n.s. | 6.2 | 170 d | <38.4 |
| 9 | PO43− | <0.8 | <5.5 | 5.08 | 84 | n.s. | n.s. | n.s. | <2 d | n.s. |
| 10 | NO3− | 40 | <1.5 | 4 | n.s. | 12 | 50 | n.s. | n.s. | n.s. |
| 13 | NO3− | 50 | 8 | 2.5 | 26.5 | n.s. | 90 | 6.7 | 131 d | 3 and 10 |
| 14 | Cr(VI) | 500 | 1 | 3.5 | 20 | n.s. | n.s. | n.s. | n.s. | 15,000 |
| 15 | PO43− | 50 | 0.3 | n.s. | <2.9 | n.s. | n.s. | n.s. | 22 d | 4.2 |
| 16 | 60Co | <180 | 40 | 5 | n.s. | 7 | n.s. | 6.84 | <24 h | 72,000 |
| Nr. | Recent Articles | ||||||
|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| 1 | no | no | no | no | no | no | no |
| 2 | no | no | no | no | no | no | yes |
| 3 | no | no | no | no | no | no | yes |
| 4 | no | no | no | no | no | no | no |
| 5 | no | no | no | no | no | no | no |
| 6 | no | no | no | no | no | no | no |
4.3. Lessons from the Peer-Reviewed Articles on “SW for Water Treatment”
4.4. Steel Wool-Based Fe0 Filters for Water Treatment at the Household Level
4.5. Proposed Design Procedure
4.5.1. Required Water Production Rate
4.5.2. Media and Water Properties
4.5.3. Filter Design
- (i)
- Select a SW material for its intrinsic reactivity using available tools [165];
- (ii)
- Characterize the selected SW material for its elemental composition;
- (iii)
- Test the suitability of selected SW materials at a small scale with a design comparable to the one described by Biswas and Bose [140];
- (iv)
- Test relevant SW materials with the modular filter described in this section (or a well-described modification).
4.5.4. Beyond Steel Wool Filters
5. Economic Considerations
6. Discussion
6.1. The Major Challenge
6.2. Steel Wool-Based Fe0 Filters as Starting Point
6.3. Filter Design Considerations
6.4. Steel Wool Filters for All
7. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Churchill, H.V. Occurrence of fluorides in some waters of the United States. Ind. Eng. Chem. 1931, 23, 996–998. [Google Scholar] [CrossRef]
- Davey, W.B. The Use of Bone and Other Phosphates for the Removal of Fluorine from Drinking Water. Master’s Thesis, University of Arizona, Tucson, AZ, USA, 1939. [Google Scholar]
- Mckee, R.H.; Johnston, W.S. Removal of flourides from drinking water. Ind. Eng. Chem. 1934, 26, 849–851. [Google Scholar] [CrossRef]
- Fink, G.J.; Lindsay, F.K. Activated alumina for removing fluorides from drinking water. Ind. Eng. Chem. 1936, 28, 947–948. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Leyva-Ramos, R.; Ocampo-Perez, R.; Garcia de la Cruz, R.F.; Aragon-Piña, A.; Martinez-Rosales, J.M.; Guerrero-Coronado, R.M.; Fuentes-Rubio, L. Adsorption of fluoride from water solution on bone char. Ind. Eng. Chem. Res. 2007, 46, 9205–9212. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, I.A. Fluorosis varied treatment options. J. Conserv. Dent. 2010, 13, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Smittakorn, S.; Jirawongboonrod, N.; Mongkolnchai-Arunya, S.; Durnford, D. Homemade bone charcoal adsorbent for defluoridation of groundwater in Thailand. J. Water Health 2010, 8, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Yenkie, M.K.; Labhsetwar, N.; Rayalu, S. Fluoride in drinking water and defluoridation of water. Chem. Rev. 2012, 112, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sharma, R.; Singh, V.K.; Steele, P.; Pittman, C.U., Jr. Fluoride removal from water using bio-char, a green waste, low-cost adsorbent: Equilibrium uptake and sorption dynamics modeling. Ind. Eng. Chem. Res. 2012, 51, 900–914. [Google Scholar] [CrossRef]
- Thole, B. Chapter 4: Ground Water Contamination with Fluoride and Potential Fluoride Removal Technologies for East and Southern Africa. Available online: http://www.intechopen.com/books/perspectives-in-water-pollution/ground-water-contamination-with-fluoride-and-potential-fluoride-removal-technologies-for-east-and-so (accessed on 20 November 2015).
- Indermitte, E.; Saava, A.; Karro, E. Reducing exposure to high fluoride drinking water in Estonia—A Countrywide study. Int. J. Environ. Res. Public Health 2014, 11, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Mulugeta, E.; Zewge, F.; Johnson, C.A.; Chandravanshi, B.S. Aluminium hydro(oxide)-based (AO) adsorbent for defluoridation of drinking water: Optimisation, performance comparison, and field testing. Water SA 2015, 41, 121–128. [Google Scholar] [CrossRef]
- Onyango, M.S.; Leswifi, T.Y.; Ochieng, A.; Kuchar, D.; Otieno, F.O.; Matsuda, H. Breakthrough analysis for water defluoridation using surface-tailored zeolite in a fixed bed column. Ind. Eng. Chem. Res. 2009, 48, 931–937. [Google Scholar] [CrossRef]
- Jing, C.; Cui, J.; Huang, Y.; Li, A. Fabrication, characterization, and application of a composite adsorbent for simultaneous removal of arsenic and fluoride. ACS Appl. Mater. Interfaces 2012, 4, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, S.; Sharma, S.; Kulshrestha, V. Electrodeionization: An efficient way for removal of fluoride from tap water using an aluminum form of phosphomethylated resin. Ind. Eng. Chem. Res. 2015, 54, 4664–4671. [Google Scholar] [CrossRef]
- Shen, J.; Mkongo, G.; Abbt-Braun, G.; Ceppi, S.L.; Richards, B.S.; Schäfer, A.I. Renewable energy powered membrane technology: Fluoride removal in a rural community in northern Tanzania. Sep. Purif. Technol. 2015, 149, 349–361. [Google Scholar] [CrossRef]
- Boruff, C.S. Removal of fluorides from drinking waters. Ind. Eng. Chem. 1934, 26, 69–71. [Google Scholar] [CrossRef]
- Adler, H.; Klein, G.; Lindsay, F.K. Removal of fluorides from potable water by tricalcium phosphate. Ind. Eng. Chem. 1938, 30, 163–165. [Google Scholar] [CrossRef]
- Goldberg, S.; Davis, J.A.; Hem, J.D. The surface chemistry of aluminium oxides and hydroxides. In The Environmental Chemistry of Aluminium, 2nd ed.; Sposito, G., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1996; pp. 271–328. [Google Scholar]
- Tripathy, S.S.; Raichur, A.M. Abatement of fluoride from water using manganese dioxide coated activated alumina. J. Hazard. Mater. 2008, 153, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Saha, S.K.; Ghosh, U.C. Adsorption of fluoride from aqueous solution by a synthetic iron(III)-aluminium(III) mixed oxide. Ind. Eng. Chem. Res. 2007, 46, 5346–5356. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.K.; Dutta, R.K. Fluoride removal from water using crushed limestone. Indian J. Chem. Technol. 2010, 17, 120–125. [Google Scholar]
- Murutu, C.; Onyango, M.S.; Ochieng, A.; Otieno, F.A.O. Fluoride removal performance of phosphoric acid treated lime: Breakthrough analysis and point-of-use system performance. Water SA 2012, 38, 279–285. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967. [Google Scholar] [CrossRef]
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 1998, 36, 164–170. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Antia, D.D.J. Desalination of water using ZVI (Fe0). Water 2015, 7, 3671–3831. [Google Scholar] [CrossRef]
- Ghauch, A. Iron-based Metallic Systems: An Excellent Choice for Sustainable Water Treatment. Freib. Online Geosci. 2015, 38, 80. [Google Scholar]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Nkundimana, E.; Noubactep, C.; Uwamariya, V. Metallic iron for water treatment and environmental remediation: A handout to young researchers. Fresenius Environ. Bull. 2015, 24, 1–14. [Google Scholar]
- Chiu, P.C. Applications of zero-valent iron (ZVI) and nanoscale ZVI to municipal and decentralized drinking water systems—A review. In Novel Solutions to Water Pollution; Ahuja, S., Hristovski, K., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2013; Volume 1123, pp. 237–249. [Google Scholar]
- Hussam, A.; Munir, A.K.M. A simple and effective arsenic filter based on composite iron matrix: Development and deployment studies for groundwater of Bangladesh. J. Environ. Sci. Health A 2007, 42, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Tepong-Tsindé, R.; Crane, R.; Noubactep, C.; Nassi, A.; Ruppert, H. Testing metallic iron filtration systems for decentralized water treatment at pilot scale. Water 2015, 7, 868–897. [Google Scholar] [CrossRef]
- Fakhri, A.; Adami, S. Response surface methodology for adsorption of fluoride ion using nanoparticle of zero valent iron from aqueous solution. J. Chem. Eng. Process. Technol. 2013, 4. [Google Scholar] [CrossRef]
- Jahin, H.S. Fluoride removal from water using nanoscale zero-valent iron (NZVI). Int. Water Technol. J. 2014, 4, 173–182. [Google Scholar]
- Jeong, J.-Y.; Song, Y.-H.; Kim, J.-H.; Park, J.-Y. Simultaneous removal of nitrate, phosphate, and fluoride using a ZVI-packed bed electrolytic cell. Desal. Water Treat. 2014, 52, 737–743. [Google Scholar] [CrossRef]
- Kalt, P.; Birzer, C.; Evans, H.; Liew, A.; Padovan, M.; Watchman, M. A solar disinfection water treatment for remote communities. Procedia Eng. 2014, 78, 250–258. [Google Scholar] [CrossRef]
- Wambu, E.W.; Onindo, C.O.; Ambusso, W.; Muthakia, G.K. Removal of fluoride from aqueous solutions by adsorption using a siliceous mineral of a Kenyan origin. Clean Soil Air Water 2013, 41, 340–348. [Google Scholar] [CrossRef]
- Habuda-Stani, M.; Ravanèi, M.E.; Flanagan, A. A review on adsorption of fluoride from aqueous solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef]
- Kumar, N.P.; Kumar, N.S.; Krishnaiah, A. Defluoridation of water using Tamarind (Tamarindus indica) fruit cover: Kinetics and equilibrium studies. J. Chil. Chem. Soc. 2012, 57, 1224–1131. [Google Scholar] [CrossRef]
- Pandey, P.K.; Pandey, M.; Sharma, R. Defluoridation of water by biomass: Tinospora cordifolia. J. Environ. Prot. 2012, 3, 610–616. [Google Scholar] [CrossRef]
- Harikumar, P.S.P.; Jaseela, C.; Megha, T. Defluoridation of water using biosorbents. Nat. Sci. 2012, 4, 245–251. [Google Scholar] [CrossRef]
- Yadav, A.K.; Abbassi, R.L.; Gupta, A.; Dadashzadeh, M. Removal of fluoride from aqueous solution and ground water by wheat straw, sawdust and activated bagasse carbon of sugarcane. Ecol. Eng. 2013, 52, 211–218. [Google Scholar] [CrossRef]
- Balouch, A.; Kolachi, M.; Talpur, F.N.; Khan, H.; Bhanger, M.I. Sorption kinetics isotherm and thermodynamic modelling of defluoridation of groundwater using natural adsorbents. Am. J. Anal. Chem. 2013, 4, 221–228. [Google Scholar] [CrossRef]
- Mwakabona, H.T.; Machunda, R.L.; Njau, K.N. Defluoridation of water by sisal leaf biomass: The influence of stereochemistry of the active compounds. Am. J. Chem. Eng. 2014, 2, 42–47. [Google Scholar] [CrossRef]
- Vardhan, C.M.V.; Karthkeyan, J. Removal of fluoride from water using low-cost materials. Int. Water Technol. J. 2011, 1, 120–131. [Google Scholar]
- Bhatnagar, A.; Kumar, E.; Sillanpaa, M. Fluoride removal from water by adsorption: A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Malde, M.K.; Greiner-Simonsen, R.; Julshamn, K.; Bjorvatn, K. Tealeaves may release or absorb fluoride, depending on the fluoride content of water. Sci. Total Environ. 2006, 366, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Mjengera, H.; Mkongo, G. Appropriate defluoridation technology for use in fluorotic areas in Tanzania. Phys. Chem. Earth 2003, 28, 1097–1104. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Rivera-Utrilla, J.; Medellin-Castillo, N.A.; Sanchez-Polo, M. Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. Chem. Eng. J. 2010, 158, 458–467. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Hernández-Montoya, V.; Moreno-Virgena, M.R.; Tovar-Gómez, R.; Montes-Morán, M.A. Optimization of pyrolysis conditions and adsorption properties of bone char for fluoride removal from water. J. Anal. Appl. Pyrol. 2013, 104, 10–18. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Silvestre-Albero, J.; Aguayo-Villarreal, I.A.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. A new synthesis route for bone chars using CO2 atmosphere and their application as fluoride adsorbents. Microporous Mesoporous Mater. 2015, 209, 38–44. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Awwad, N.S.; Aboterika, A.H.A. Removal of mercury from wastewater using camel bone charcoal. J. Hazard. Mater. 2008, 154, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Gwala, P.; Andey, S.; Nagarnaik, P.; Ghosh, S.P.; Pal, P.; Deshmukh, P.; Labhasetwar, P. Design and development of sustainable remediation process for mitigation of fluoride contamination in ground water and field application for domestic use. Sci. Total Environ. 2014, 488–489, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Gómez, R.; Moreno-Virgen, M.R.; Dena-Aguilar, J.A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A. Modeling of fixed-bed adsorption of fluoride on bone char using a hybrid neural network approach. Chem. Eng. J. 2013, 228, 1098–1109. [Google Scholar] [CrossRef]
- Kariuki, S.M.; Ngari, M.S.; Mavura, W.J.; Ollengo, M.S.; Ongoma, P.O. Effect of essential mineral ions from aqueous media on adsorption of fluoride by bone char. J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 9–17. [Google Scholar]
- Khan, A.H.; Rasul, S.B.; Munir, A.K.M.; Habibuddowla, M.; Alauddin, M.; Newaz, S.S.; Hussam, A. Appraisal of a simple arsenic removal method for groundwater of bangladesh. J. Environ. Sci. Health A 2000, 35, 1021–1041. [Google Scholar] [CrossRef]
- You, Y.; Han, J.; Chiu, P.C.; Jin, Y. Removal and inactivation of waterborne viruses using zerovalent iron. Environ. Sci. Technol. 2005, 39, 9263–9269. [Google Scholar] [CrossRef] [PubMed]
- Delowar, H.K.M.; Uddin, I.; Abou el Hassan, W.H.; Perveen, M.F.; Irshad, M.; Islam, A.F.M.S.; Yoshida, I. A comparative study of household groundwater arsenic removal technologies and their water quality parameters. J. Appl. Sci. 2006, 6, 2193–2200. [Google Scholar]
- Litter, M.I.; Morgada, M.E.; Bundschuh, J. Possible treatments for arsenic removal in Latin American waters for human consumption. Environ. Pollut. 2010, 158, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Metallic iron for safe drinking water worldwide. Chem. Eng. J. 2010, 165, 740–749. [Google Scholar] [CrossRef]
- Noubactep, C. Metallic iron for safe drinking water production. Freib. Online Geosci. 2011, 27, 38. [Google Scholar]
- Deng, Y.; Englehardt, J.D.; Abdul-Aziz, S.; Bataille, T.; Cueto, J.; de Leon, O.; Wright, M.E.; Gardinali, P.; Narayanan, A.; Polar, J.; et al. Ambient iron-mediated aeration (IMA) for water reuse. Water Res. 2013, 47, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Kaegi, R.; Voegelin, A.; Hussam, A.; Munir, A.K.M.; Hug, S.J. Arsenic removal with composite iron matrix filters in Bangladesh: A field and laboratory study. Environ. Sci. Technol. 2013, 47, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.S.; Chaudhari, S.K. Arsenic removal from simulated groundwater using household filter columns containing iron filings and sand. J. Water Proc. Eng. 2015, 6, 151–157. [Google Scholar] [CrossRef]
- Noubactep, C.; Schöner, A.; Woafo, P. Metallic iron filters for universal access to safe drinking water. Clean Soil Air Water 2009, 37, 930–937. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. Enhancing sustainability of household water filters by mixing metallic iron with porous materials. Chem. Eng. J. 2010, 162, 635–642. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S. Designing laboratory metallic iron columns for better result comparability. J. Hazard. Mater. 2011, 189, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Temgoua, E.; Rahman, M.A. Designing iron-amended biosand filters for decentralized safe drinking water provision. Clean Soil Air Water 2012, 40, 798–807. [Google Scholar] [CrossRef]
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.-B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Murcott, S.; Shrestha, R.R.; Dangol, B.; Maharjan, M. Development and dissemination of Kanchan™ arsenic filter in rural Nepal. Water Sci. Technol. Water Supply 2006, 6, 137–146. [Google Scholar] [CrossRef]
- Ngai, T.K.K.; Shrestha, R.R.; Dangol, B.; Maharjan, M.; Murcott, S.E. Design for sustainable development—Household drinking water filter for arsenic and pathogen treatment in Nepal. J. Environ. Sci. Health A 2007, 42, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Chiew, H.; Sampson, M.L.; Huch, S.; Ken, S.; Bostick, B.C. Effect of groundwater iron and phosphate on the efficacy of arsenic removal by iron-amended biosand filters. Environ. Sci. Technol. 2009, 43, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Hussam, A. Contending with a development disaster: SONO filters remove arsenic from well water in Bangladesh. Innovations 2009, 4, 89–102. [Google Scholar] [CrossRef]
- Gottinger, A.M. Chemical-free Arsenic Removal from Potable Water with a ZVI-amended Biofilter. Master’s Thesis, University of Regina, Regina, SK, Canada, 2010; p. 90. [Google Scholar]
- Gottinger, A.M.; McMartin, D.W.; Wild, D.J.; Moldovan, B. Integration of zero valent iron sand beds into biological treatment systems for uranium removal from drinking water wells in rural Canada. Can. J. Civ. Eng. 2013, 40, 945–950. [Google Scholar] [CrossRef]
- Kowalski, K.P.; Søgaard, E.G. Implementation of zero-valent iron (ZVI) into drinking water supply—Role of the ZVI and biological processes. Chemosphere 2014, 117, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.K.K.; Fenner, R.A. Designing programme implementation strategies to increase the adoption and use of biosand water filters in rural India. Water Altern. 2014, 7, 320–341. [Google Scholar]
- Wenk, C.B.; Kaegi, R.; Hug, S.J. Factors affecting arsenic and uranium removal with zero-valent iron: Laboratory tests with Kanchan-type iron nail filter columns with different groundwaters. Environ. Chem. 2014, 11, 547–557. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J.; Badruzzaman, A.B.M. Arsenic removal from Bangladesh tube well water with filter columns containing zerovalent iron filings and sand. Environ. Sci. Technol. 2005, 39, 8032–8037. [Google Scholar] [CrossRef] [PubMed]
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Metals 1923, 29, 529–591. [Google Scholar]
- Cox, G.L.; Roetheli, B.E. Effect of oxygen concentration on corrosion rates of steel and composition of corrosion products formed in oxygenated water. Ind. Eng. Chem. 1931, 23, 1012–1016. [Google Scholar] [CrossRef]
- Caré, S.; Crane, R.; Calabro, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modelling the permeability loss of metallic iron water filtration systems. Clean Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Westerhoff, P.; James, J. Nitrate removal in zero-valent iron packed columns. Water Res. 2003, 37, 1818–1830. [Google Scholar] [CrossRef]
- Lackovic, J.A.; Nikolaidis, N.P.; Dobbs, G.M. Inorganic arsenic removal by zero-valent iron. Environ. Eng. Sci. 2000, 17, 29–39. [Google Scholar] [CrossRef]
- Noubactep, C. Processes of contaminant removal in “Fe0–H2O” systems revisited: The importance of co-precipitation. Open Environ. Sci. 2007, 1, 9–13. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the mechanism of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Bradley, I.; Straub, A.; Maraccini, P.; Markazi, S.; Nguyen, T.H. Iron oxide amended biosand filters for virus removal. Water Res. 2011, 45, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.B.; Popescu, I.C.; Crane, R.A.; Noubactep, C. Nano-scale metallic iron for the treatment of solutions containing multiple inorganic contaminants. J. Hazard. Mater. 2011, 186, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S.; Crane, R. Nanoscale metallic iron for environmental remediation: Prospects and limitations. Water Air Soil Pollut. 2012, 223, 1363–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allred, B.J.; Racharaks, R. Laboratory comparison of four iron-based filter materials for drainage water phosphate treatment. Water Environ. Res. 2014, 86, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.J.; Tost, B.C. Laboratory comparison of four iron-based filter materials for water treatment of trace element contaminants. Water Environ. Res. 2014, 86, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705. [Google Scholar] [CrossRef]
- Giles, D.E.; Mohapatra, M.; Issa, T.B.; Anand, S.; Singh, P. Iron and aluminium based adsorption strategies for removing arsenic from water. J. Environ. Manag. 2011, 92, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Noubactep, C. Elemental metals for environmental remediation: Lessons from hydrometallurgy. Fresenius Environ. Bull. 2012, 215, 1192–1196. [Google Scholar]
- Phukan, M. Characterizing the Fe0/sand system by the extent of dye discoloration. Freib. Online Geosci. 2015, 42, 80. [Google Scholar]
- Phukan, M.; Noubactep, C.; Licha, T. Characterizing the ion-selective nature of Fe0-based filters using azo dyes. Chem. Eng. J. 2015, 259, 481–491. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3050–3056. [Google Scholar] [CrossRef]
- Crawford, R.J.; Harding, I.H.; Mainwaring, D.E. Adsorption and coprecipitation of multiple heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir 1993, 9, 3057–3062. [Google Scholar] [CrossRef]
- London, M.R.; Wahman, D.G.; Katz, L.E.; Speitel, G.E., Jr. Zero-valent iron/biotic treatment system for perchlorate-contaminated water: Lab-scale performance, modeling, and full-scale implications. J. Environ. Eng. 2013, 139, 1361–1367. [Google Scholar] [CrossRef]
- White, A.F.; Peterson, M.L. Reduction of aqueous transition metal species on the surfaces of Fe(II)-containing oxides. Geochim. Cosmochim. Acta 1996, 60, 3799–3814. [Google Scholar] [CrossRef]
- Charlet, L.; Liger, E.; Gerasimo, P. Decontamination of TCE- and U-rich waters by granular iron: Role of sorbed Fe (II). J. Environ. Eng. 1998, 124, 25–30. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D. Degradation of toxic halogenated organic compounds by iron-containing mono-, bi- and tri-metallic particles in water. Inorg. Chim. Acta 2015, 431, 48–60. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Duff, M.C.; Coughlin, J.U.; Hunter, B.D. Uranium co-precipitation with iron oxide minerals. Geochim. Cosmochim. Acta 2002, 66, 3533–3547. [Google Scholar] [CrossRef]
- Tepong-Tsindé, R.; Phukan, M.; Nassi, A.; Noubactep, C.; Ruppert, H. Validating the efficiency of the MB discoloration method for the characterization of Fe0/H2O systems using accelerated corrosion by chloride ions. Chem. Eng. J. 2015, 279, 353–362. [Google Scholar] [CrossRef]
- Sikora, E.; Macdonald, D.D. The passivity of iron in the presence of ethylenediaminetetraacetic acid I. General electrochemical behavior. J. Electrochem. Soc. 2000, 147, 4087–4092. [Google Scholar] [CrossRef]
- Erickson, A.J. Enhanced sand Filtration for Storm Water Phosphorus Removal. Master’s Thesis, University of Minnesota, Minneapolis, MN, USA, 2005. [Google Scholar]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. ASCE 2007, 133, 485–497. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Capturing phosphates with iron enhanced sand filtration. Water Res. 2012, 46, 3032–3042. [Google Scholar] [CrossRef] [PubMed]
- Masse, J.P.; Salvo, L.; Rodney, D.; Bréchet, Y.; Bouaziz, O. Influence of relative density on the architecture and mechanical behaviour of a steel metallic wool. Scr. Mater. 2006, 54, 1379–1383. [Google Scholar] [CrossRef]
- Noubactep, C.; Caré, S.; Togue-Kamga, F.; Schöner, A.; Woafo, P. Extending service life of household water filters by mixing metallic iron with sand. Clean Soil Air Water 2010, 38, 951–959. [Google Scholar] [CrossRef]
- Rahman, M.A.; Karmakar, S.; Salama, H.; Gactha-Bandjun, N.; Btatkeu-K, B.D.; Noubactep, C. Optimising the design of Fe0-based filtration systems for water treatment: The suitability of porous iron composites. J. Appl. Solut. Chem. Model. 2013, 2, 165–177. [Google Scholar]
- Haig, S.-J. Characterising the Functional Ecology of Slow sand Filters through Environmental Genomics. Ph.D. Thesis, School of Engineering, University of Glasgow, Glasgow, UK, 2014. [Google Scholar]
- Campos, L.C.; Su, M.F.J.; Graham, N.J.D.; Smith, S.R. Biomass development in slow sand filters. Water Res. 2002, 36, 4543–4551. [Google Scholar] [CrossRef]
- Pokhrel, D.; Viraraghavan, T.; Braul, L. Evaluation of treatment systems for the removal of arsenic from groundwater. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2005, 9, 152–157. [Google Scholar] [CrossRef]
- Pokhrel, D.; Viraraghavan, T. Biological filtration for removal of arsenic from drinking water. J. Environ. Manag. 2009, 90, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. Flaws in the design of Fe0-based filtration systems? Chemosphere 2014, 117, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, K. Optimizing the design of metallic iron filters for water treatment. Freib. Online Geosci. 2012, 32, 60. [Google Scholar]
- Miyajima, K.; Noubactep, C. Impact of Fe0 amendment on methylene blue discoloration by sand columns. Chem. Eng. J. 2013, 217, 310–319. [Google Scholar] [CrossRef]
- Salama, H.; Rahman, M.A.; Noubactep, C. Solar energy sustains universal self-reliance in water supply. In Presented at the First International Conference on Solar Energy Solutions for Electricity and Water Supply in Rural Areas, Cairo, Egyt, 7–10 October 2015.
- Baas-Becking, L.G.M.; Kaplan, I.R.; Moore, D. Limits of the natural environments in terms of pH and oxidation-reduction potentials. J. Geol. 1960, 68, 243–284. [Google Scholar] [CrossRef]
- Till, B.A.; Weathers, L.J.; Alvarez, P.J.J. Fe(0)-supported autotrophic denitrification. Environ. Sci. Technol. 1998, 32, 634–639. [Google Scholar] [CrossRef]
- Campos, V. The effect of carbon steel-wool in removal of arsenic from drinking water. Environ. Geol. 2002, 42, 81–82. [Google Scholar] [CrossRef]
- Campbell, A.D.; Bolton, M. The Vhembe filter: A product for rural South Africa. Image Text J. Des. 2010, 16, 4–20. [Google Scholar]
- Barstow, C.K.; Ngabo, F.; Rosa, G.; Majorin, F.; Boisson, S.; Clasen, T.; Thomas, E.A. Designing and piloting a program to provide water filters and improved cookstoves in Rwanda. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Özer, A.; Altundogan, H.S.; Erdem, M.; Tümen, F. A study on the Cr(VI) removal from aqueous solutions by steel wool. Environ. Pollut. 1997, 97, 107–112. [Google Scholar] [CrossRef]
- Rao, T.S.; Murthy, D.S. Removal of arsenic (V) from water by adsorption onto low-cost and waste materials. Int. J. Res. Eng. Technol. 2013, 2, 206–212. [Google Scholar]
- Tseng, C.L.; Yang, M.H.; Lin, C.C. Rapid determination of cobalt-60 in sea water with steel wool adsorption. J. Radioanal. Nucl. Chem. Lett. 1984, 85, 253–260. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liao, W.; Yak, H.-K. Complete reduction of polychlorinated biphenyls by acid-etched steel wool in supercritical carbon dioxide. Ind. Eng. Chem. Res. 2012, 51, 6625–6630. [Google Scholar] [CrossRef]
- Biswas, S.; Bose, P. Zero-valent iron-assisted autotrophic denitrification. J. Environ. Eng. 2005, 131, 1212–1220. [Google Scholar] [CrossRef]
- Teixeira, L.A.C.; de Abreu Vieira Junior, N.; Yokoyama, L.; da Fonseca, F.V. Degradation of phenol in mine waters using hydrogen peroxide and commercial steel wool. Int. J. Miner. Process. 2015, 138, 15–19. [Google Scholar] [CrossRef]
- Birk, J.P.; McGrath, L.; Gunter, S.K. A general chemistry experiment for the determination of the oxygen content of air. J. Chem. Educ. 1981, 58, 804–805. [Google Scholar] [CrossRef]
- Martins, G.F. Percent oxygen in air. J. Chem. Educ. 1987, 64. [Google Scholar] [CrossRef]
- Najdoski, M.; Petrusevski, V.M.; Alexander, M.D. A novel experiment for fast and simple determination of the oxygen content in the air. J. Chem. Educ. 2000, 77, 1447–1448. [Google Scholar] [CrossRef]
- Vera, F.; Rivera, R.; Núñez, C. A simple experiment to measure the content of oxygen in the air using heated steel wool. J. Chem. Educ. 2011, 88, 1341–1342. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Mitra, P.; Banerjee, P.; Sarkar, D. Reduction of hexavalent chromium present in wastewater by steel wool in a continuous flow system. APCBEE Procedia 2014, 10, 59–63. [Google Scholar] [CrossRef]
- Mitra, P.; Banerjee, P.; Sarkar, D.; Chakrabarti, S. Commercial steel wool for reduction of hexavalent chromium in wastewater: Batch kinetic studies and rate model. Int. J. Environ. Sci. Technol. 2013, 11, 449–460. [Google Scholar] [CrossRef]
- Gromboni, C.F.; Donati, G.L.; Matos, W.O.; Neves, E.F.A.; Nogueira, A.R.A.; Nobrega, J.A. Evaluation of metabisulfite and a commercial steel wool for removing chromium(VI) from wastewater. Environ. Chem. Lett. 2010, 8, 73–77. [Google Scholar] [CrossRef]
- Triszcz, J.M.; Porta, A.; Einschlag, F.S.G. Effect of operating conditions on iron corrosion rates in zero-valent iron systems for arsenic removal. Chem. Eng. J. 2009, 150, 431–439. [Google Scholar] [CrossRef]
- Cornejo, L.; Lienqueo, H.; Arenas, M.; Acarapi, J.; Contreras, D.; Yáñez, J.; Mansilla, H.D. In field arsenic removal from natural water by zero-valent iron assisted by solar radiation. Environ. Pollut. 2008, 156, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.B.; Mason, M.G.; Picken, H.D.; Weathers, L.J.; Parkin, G.F. Bioaugmentation of Fe(0) for the remediation of chlorinated aliphatic hydrocarbons. Environ. Eng. Sci. 2000, 17, 169–181. [Google Scholar] [CrossRef]
- Karschunke, K.; Jekel, M. Arsenic removal by iron hydroxides, produced by enhanced corrosion of iron. Water Sci. Technol. Water Supply 2002, 2, 237–245. [Google Scholar]
- Wang, Y.-H.; Lin, S.-H.; Juang, R.-S. Removal of heavy metal ions from aqueous solutions using various low-cost adsorbents. J. Hazard. Mater. 2003, 102, 291–302. [Google Scholar] [CrossRef]
- Chang, S.-H.; Wang, K.-S.; Hu, P.-I.; Lui, I.-C. Rapid recovery of dilute copper from a simulated Cu–SDS solution with low-cost steel wool cathode reactor. J. Hazard. Mater. 2009, 163, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Antia, D.D.J. Sustainable zero-valent metal (ZVM) water treatment associated with diffusion, infiltration, abstraction, and recirculation. Sustainability 2010, 2, 2988–3073. [Google Scholar] [CrossRef]
- Wei, M.-C.; Wang, K.-S.; Huang, C.-L.; Chiang, C.-W.; Chiang, T.-J.; Lee, S.-S.; Chang, S.-H. Improvement of textile dye removal by electrocoagulation with low-cost steel wool cathode reactor. Chem. Eng. J. 2012, 192, 37–44. [Google Scholar] [CrossRef]
- Yamashita, T.; Yamamoto-Ikemoto, R. Nitrogen and phosphorus removal from wastewater treatment plant effluent via bacterial sulfate reduction in an anoxic bioreactor packed with wood and iron. Int. J. Environ. Res. Public Health 2014, 11, 9835–9853. [Google Scholar] [CrossRef] [PubMed]
- Callery, O.; Brennan, R.B.; Healy, M.G. Use of amendments in a peat soil to reduce phosphorus losses from forestry operations. Ecol. Eng. 2015, 85, 193–200. [Google Scholar] [CrossRef]
- Golder Associates Inc. (Golder) Literature Review of Treatment Technologies to Remove Selenium from Mining Influenced Water. Available online: http://namc.org/docs/00057713.PDF (accessed on 26 November 2015).
- CH2M Hill Inc. (CH2M) Review of Available Technologies for the Removal of Selenium from Water. Final Report Prepared for North American Metals Council. 2010, p. 233. Available online: http://namc.org/docs/00062756.PDF (accessed on 20 November 2015).
- Kane, T.P.; Lovett, R.J.; Bouse, S.A. Element Removal Process. U.S. Patent 8,101,087, 24 January 2002. [Google Scholar]
- Noubactep, C.; Licha, T.; Scott, T.B.; Fall, M.; Sauter, M. Exploring the influence of operational parameters on the reactivity of elemental iron materials. J. Hazard. Mater. 2009, 172, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Kubare, M.; Haarhoff, J. Rational design of domestic biosand filters. J. Water SRT Aqua 2010, 59, 1–15. [Google Scholar] [CrossRef]
- Kandra, H.S.; Deletic, A.; McCarthy, D. Assessment of impact of filter design variables on clogging in stormwater filters. Water Resour. Manag. 2014, 28, 1873–1885. [Google Scholar] [CrossRef]
- Btatkeu-K., B.D.; Miyajima, K.; Noubactep, C.; Caré, S. Testing the suitability of metallic iron for environmental remediation: Discoloration of methylene blue in column studies. Chem. Eng. J. 2013, 215–216, 959–968. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines for Drinking Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Noubactep, C. Affordable safe drinking water for victims of natural disasters. In Natural Disasters and Sustainable Development; Kätsch, C., Meliczek, H., Eds.; Cuvillier Verlag: Göttingen, Germany, 2014; pp. 57–75. [Google Scholar]
- Btatkeu-K, B.D.; Olvera-Vargas, H.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Determining the optimum Fe0 ratio for sustainable granular Fe0/sand water filters. Chem. Eng. J. 2014, 247, 265–274. [Google Scholar] [CrossRef]
- Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Caré, S. Designing metallic iron based water filters: Light from methylene blue discoloration. J. Environ. Manag. 2015. [Google Scholar] [CrossRef] [PubMed]
- Twidwell, L.; McCloskey, J.; Joyce, H.; Dahlgren, E.; Hadden, A. Removal of selenium oxyanions from mine waters utilizing elemental iron and galvanically coupled metals. In Innovations in Natural Resource Processing—Proceedings of the Jan. D. Miller Symposium; Young, C.A., Kellar, J.J., Free, M.J., Drelich, J., King, P.P., Eds.; Society for Mining, Metallurgy, and Exploration, Inc.: Littleton, CO, USA, 2005. [Google Scholar]
- Nicol, M.J.; Schalch, E.; Balestra, P.; Hegedus, H. A modern study of the kinetics and mechanism of the cementation of gold. J. S. Afr. Inst. Min. Metall. 1979, 79, 191–198. [Google Scholar]
- Noubactep, C. Elemental metals for environmental remediation: Learning from cementation process. J. Hazard. Mater. 2010, 181, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Sarr, D. Zero-valent-iron permeable reactive barriers—How long will they last? Remediation 2001, 11, 1–18. [Google Scholar]
- Noubactep, C. Metallic iron for environmental remediation: A review of reviews. Water Res. 2015, 85, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Alamilla, J.L.; Espinosa-Medina, M.A.; Sosa, E. Modelling steel corrosion damage in soil environment. Corros. Sci. 2009, 51, 2628–2638. [Google Scholar] [CrossRef]
- Noubactep, C. Characterizing the reactivity of metallic iron in Fe0/EDTA/H2O systems with column experiments. Chem. Eng. J. 2010, 162, 656–661. [Google Scholar] [CrossRef]
- Stergioudi, F.; Kaprara, E.; Simeonidis, K.; Sagris, D.; Mitrakas, M.; Vourlias, G.; Michailidis, N. Copper foams in water treatment technology: Removal of hexavalent chromium. Mater. Des. 2015, 87, 287–294. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndé-Tchoupé, A.I.; Crane, R.A.; Mwakabona, H.T.; Noubactep, C.; Njau, K.N. Technologies for Decentralized Fluoride Removal: Testing Metallic Iron-based Filters. Water 2015, 7, 6750-6774. https://doi.org/10.3390/w7126657
Ndé-Tchoupé AI, Crane RA, Mwakabona HT, Noubactep C, Njau KN. Technologies for Decentralized Fluoride Removal: Testing Metallic Iron-based Filters. Water. 2015; 7(12):6750-6774. https://doi.org/10.3390/w7126657
Chicago/Turabian StyleNdé-Tchoupé, Arnaud Igor, Richard A. Crane, Hezron T. Mwakabona, Chicgoua Noubactep, and Karoli N. Njau. 2015. "Technologies for Decentralized Fluoride Removal: Testing Metallic Iron-based Filters" Water 7, no. 12: 6750-6774. https://doi.org/10.3390/w7126657
APA StyleNdé-Tchoupé, A. I., Crane, R. A., Mwakabona, H. T., Noubactep, C., & Njau, K. N. (2015). Technologies for Decentralized Fluoride Removal: Testing Metallic Iron-based Filters. Water, 7(12), 6750-6774. https://doi.org/10.3390/w7126657







