Nitritation and N2O Emission in a Denitrification and Nitrification Two-Sludge System Treating High Ammonium Containing Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental System and Its Operating Conditions
2.2. Batch Experiments
2.3. Analytical Methods
3. Results and Discussion
3.1. Long-Term Operation and Typical Cycle Examination
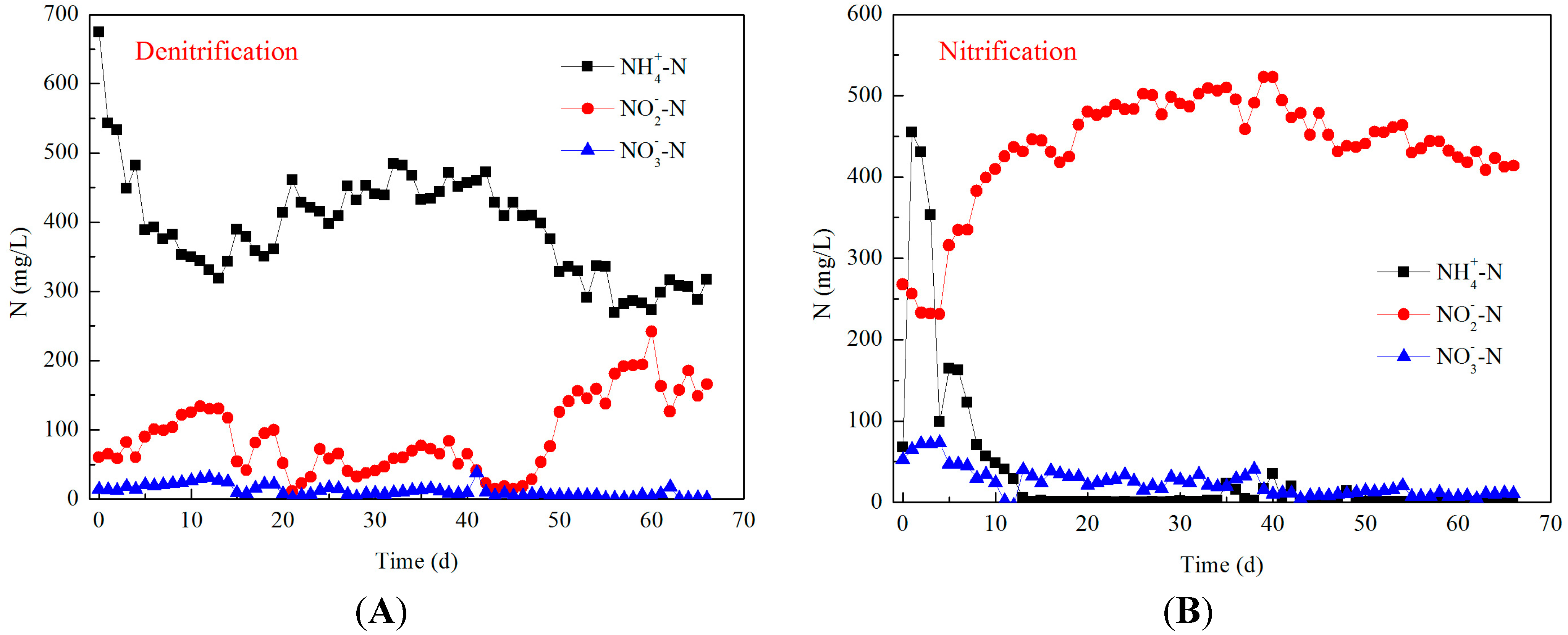
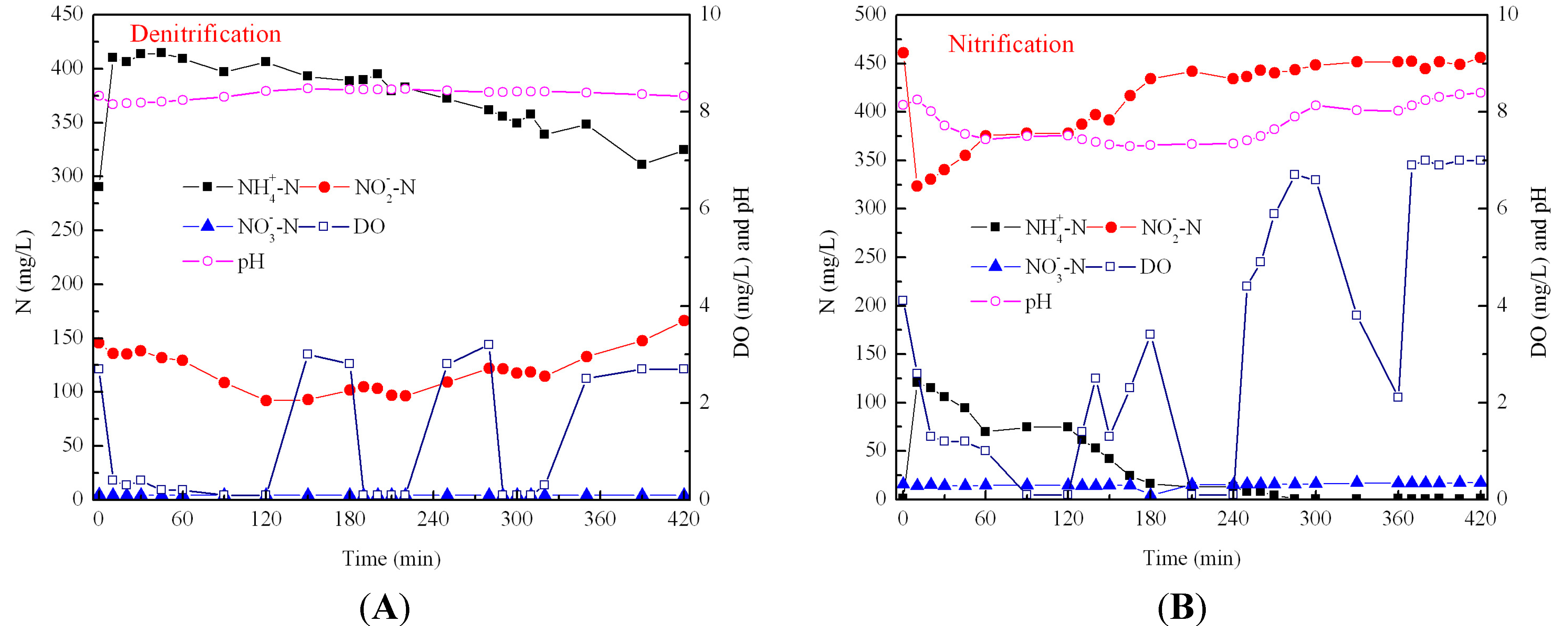
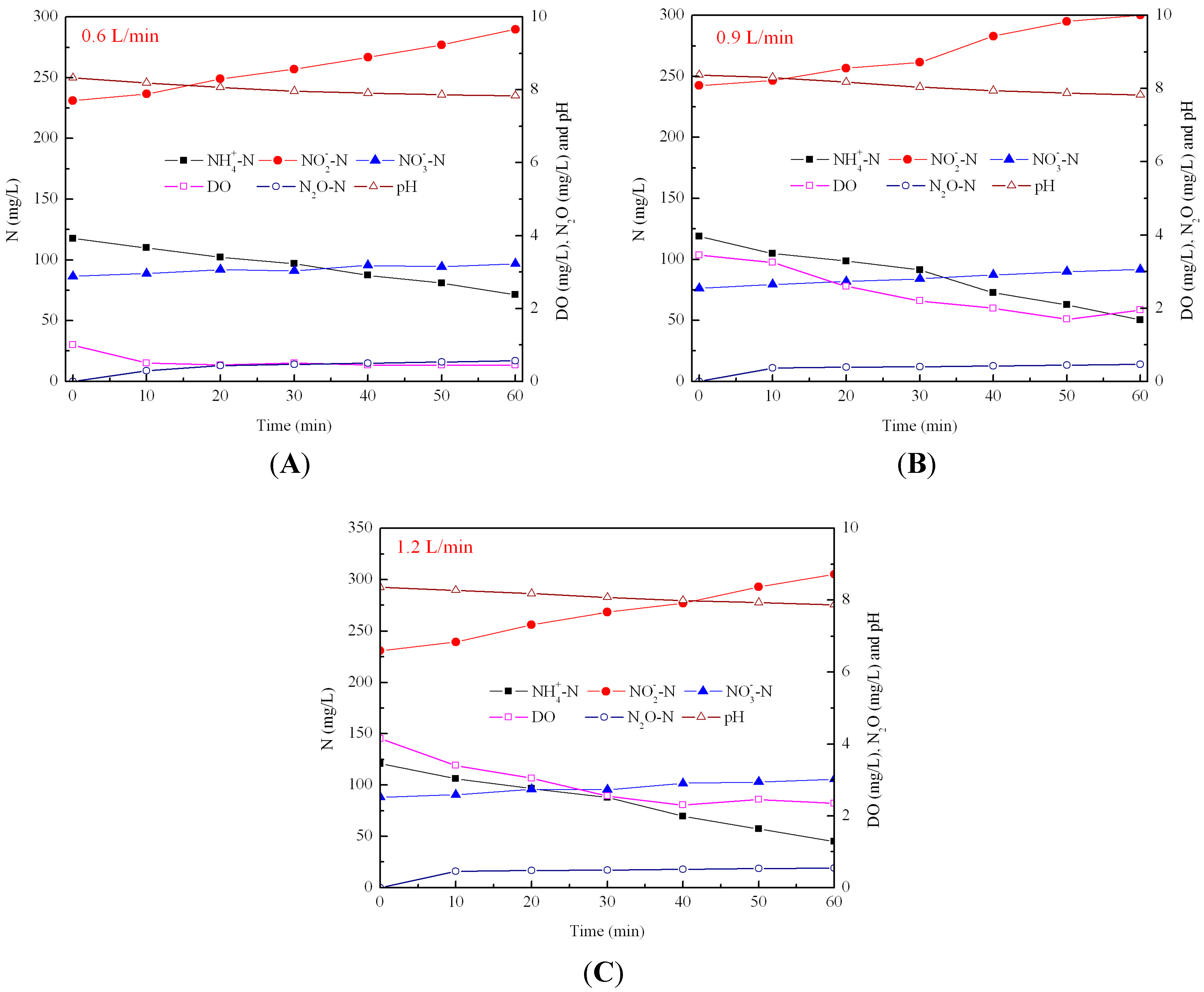
3.2. Effect of Aeration Rates on N2O Emission during Nitrification
| Aeration rate (L/min) | rNH4-N (mg/g·h) | rNO2-N (mg/g·h) | rNO3-N (mg/g·h) | rN2O (mg/g·h) | rNH4-N/rNO3-N | rN2O-N/rNH4-N (%) |
|---|---|---|---|---|---|---|
| 0.6 | 24.85 | 32.24 | 5.30 | 0.19 | 4.69 | 0.78 |
| 0.9 | 36.22 | 34.03 | 8.36 | 0.11 | 4.33 | 0.31 |
| 1.2 | 37.31 | 36.89 | 8.81 | 0.09 | 4.23 | 0.24 |
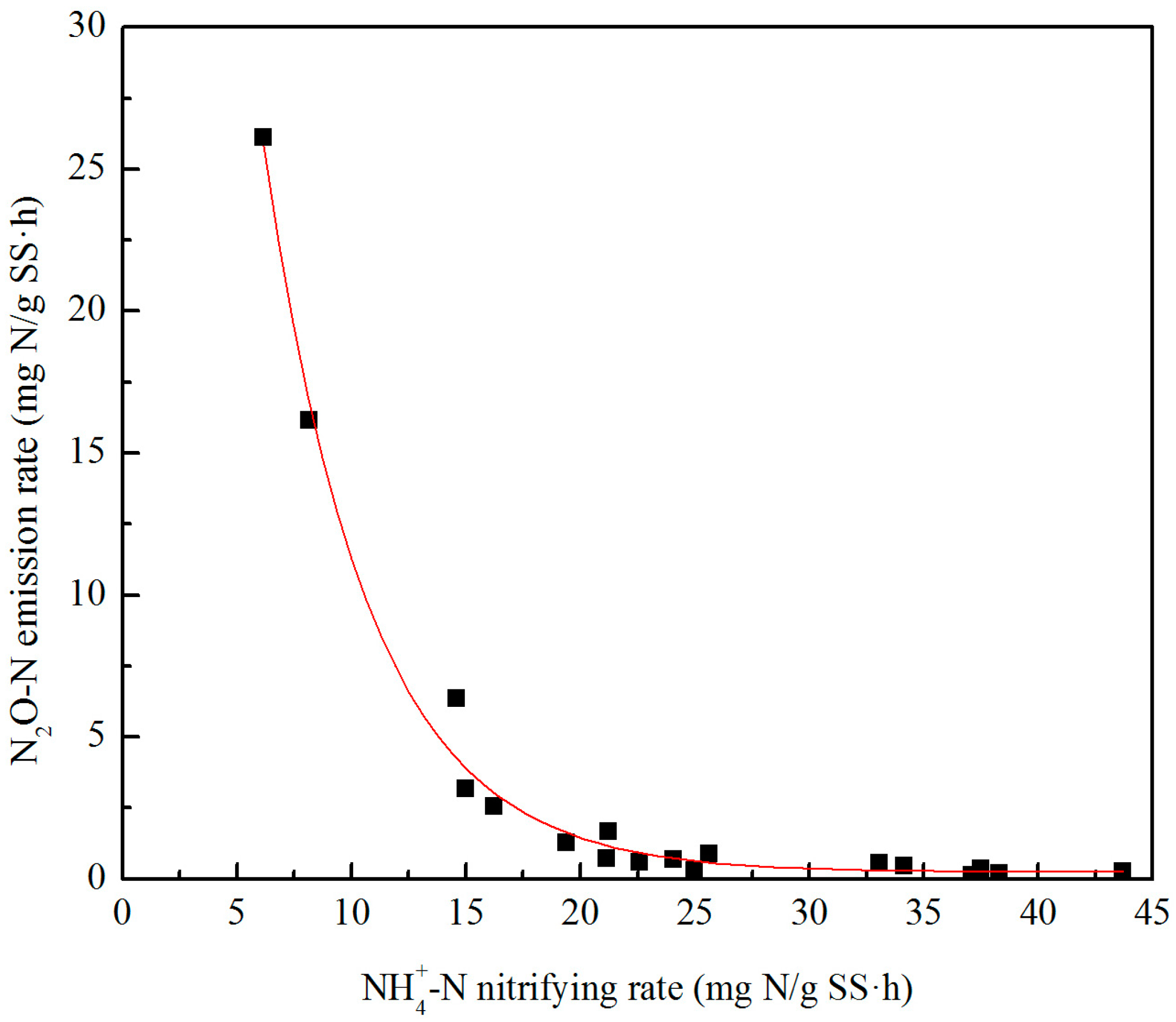
3.3. Effect of C/N Ratios on N2O Emissions during Denitrification
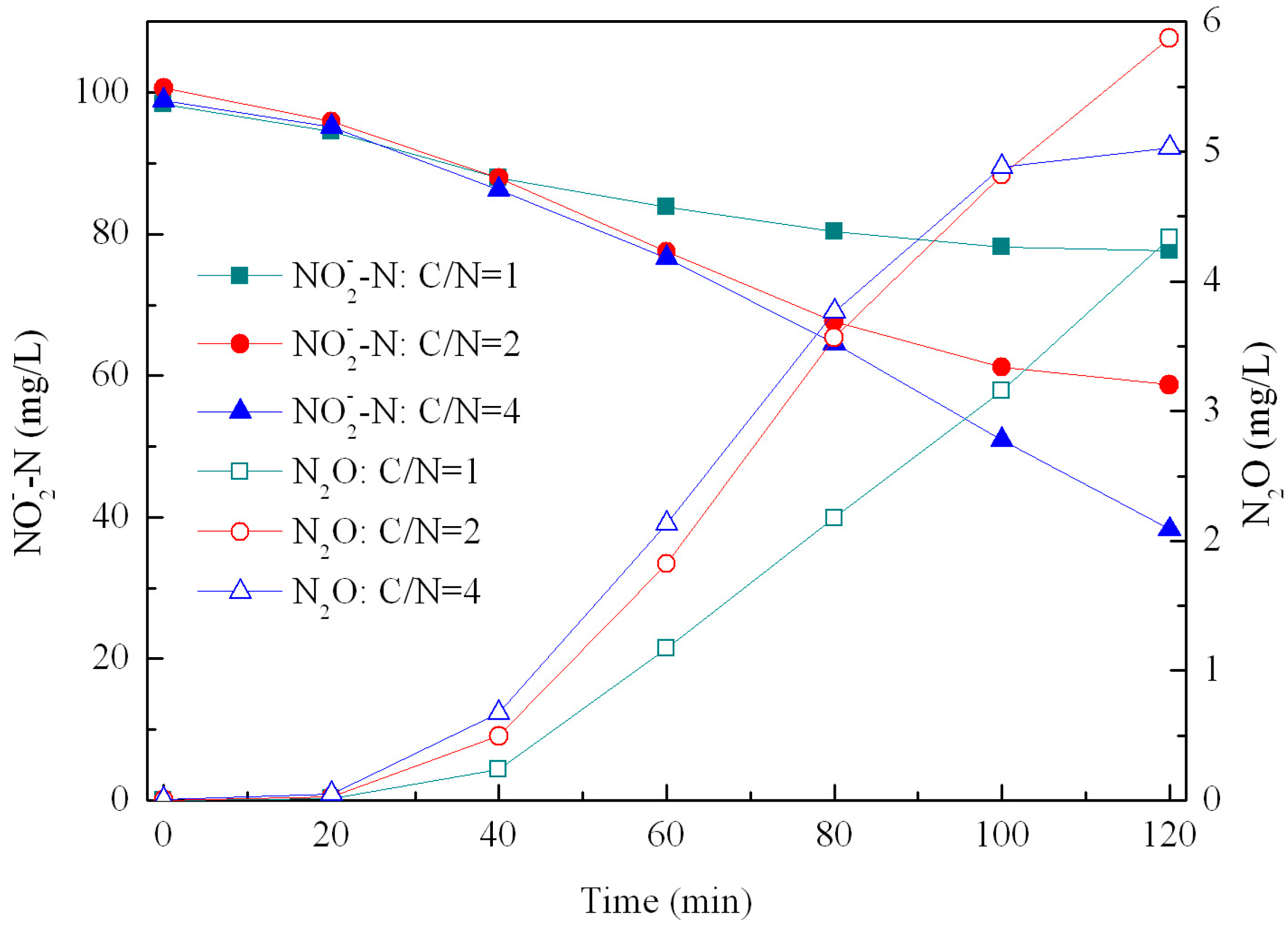
| C/N | rNO2-N (mg/g·h) | rN2O (mg/g·h) | rN2O-N/rNO2-N (%) |
|---|---|---|---|
| 1 | 6.52 | 1.71 | 26.63 |
| 2 | 12.96 | 2.27 | 17.55 |
| 4 | 18.35 | 2.17 | 11.98 |
4. Conclusions
- (1)
- For the treatment of high ammonium containing wastewater with a low C/N ratio, the two-sludge system achieved nitritation efficiently with a nitrite accumulation efficiency of 97.8%. As to the influent NH4-N of 800 mg/L, the nitrogen removal percentage was 43.4% and the NH4-N removal loading rate was 0.45 g/(L·d).
- (2)
- During nitrification, the N2O emission factor had a negatively exponential relationship with the NH4-N oxidation rate. A low NH4-N nitrifying rate caused high N2O emissions, and the N2O emission factor was in the range of 0.24%–0.78% when aeration rates were in the range of 0.6–1.2 L/min.
- (3)
- Denitrifying activities with NO2-N as the electron acceptor increased with increasing C/N ratios, but the N2O emission factor decreased with values in the range of 12.0%–26.6%.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Kempen, R.; Mulder, J.W.; Uijterlinde, C.A.; van Loosdrecht, M.C.M. Overview: Full scale experience of the SHARON® process for treatment of rejection water of digested sludge dewatering. Water Sci. Technol. 2001, 44, 145–152. [Google Scholar]
- Wunderlin, P. Mechanisms of N2O Production in Biological Wastewater Treatment: From Pathway Identification to Process Control. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2013. [Google Scholar]
- Desloover, J.; de Clippeleir, H.; Boeckx, P.; du Laing, G.; Colsen, J.; Verstraete, W.; Vlaeminck, S.E. Floc based sequential partial nitritation and anammox at full scale with contrasting N2O emissions. Water Res. 2011, 45, 2811–2821. [Google Scholar] [CrossRef]
- Rathnayake, R.M.L.D.; Song, Y.; Tumendelger, A.; Oshiki, M.; Ishii, S.; Satoh, H.; Toyada, S.; Yoshida, N.; Okabe, S. Source identification of nitrous oxide on autotrophic partial nitrification in a granular sludge reactor. Water Res. 2013, 47, 7078–7086. [Google Scholar] [PubMed]
- Shen, L.; Guan, Y.; Wu, G.; Zhan, X. Nitrous oxide emission from a sequencing batch reactor for biological nitrogen and phosphorus removal. Front. Environ. Sci. Eng. 2014, 8, 776–783. [Google Scholar] [CrossRef]
- Andrews, J.H.; Harris, R.F. r- and K-selection and microbial ecology. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer Science and Business Media: New York, NY, USA, 1986. [Google Scholar]
- Li, J.P.; Healy, M.G.; Zhan, X.M.; Rodgers, M. Nutrient removal from slaughterhouse wastewater in an intermittently aerated sequencing batch reactor. Bioresour. Technol. 2008, 99, 7644–7650. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, N. Partial nitrification under limited dissolved oxygen conditions. Process Biochem. 2004, 39, 1223–1229. [Google Scholar] [CrossRef]
- Zekker, I.; Rikmann, E.; Tenno, T.; Menert, A.; Lemmiksoo, V.; Saluste, A.; Tenno, T.; Tomingas, M. Modification of nitrifying biofilm into nitritating one by combination of increased free ammonia concentrations, lowered HRT and dissolved oxygen concentration. J. Environ. Sci. 2011, 23, 1113–1121. [Google Scholar] [CrossRef]
- Zeng, R.J.; Lemaire, R.; Yuan, Z.; Keller, J. Simultaneous nitrification, denitrification, and phosphorus removal in a lab-scale sequencing batch reactor. Biotechnol. Bioeng. 2003, 84, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Kampschreur, M.J.; Tan, N.C.; Kleerebezem, R.; Picioreanu, C.; Jetten, M.S.; van Loosdrecht, M.C.M. Effect of dynamic process conditions on nitrogen oxides emission from a nitrifying culture. Environ. Sci. Technol. 2007, 42, 429–435. [Google Scholar] [CrossRef]
- Blackburne, R.; Yuan, Z.; Keller, J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 2008, 19, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Abeling, U.; Seyfried, C.F. Anaerobic-aerobic treatment of high-strength ammonium wastewater-nitrogen removal via nitrite. Water Sci. Technol. 1992, 26, 1007–1015. [Google Scholar]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.; van Loosdrecht, M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Beline, F.; Martinez, J. Nitrogen transformations during biological aerobic treatment of pig slurry: Effect of intermittent aeration on nitrous oxide emissions. Bioresour. Technol. 2002, 83, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Caballero, A.; Pijuan, M. N2O and NO emissions from a partial nitrification sequencing batch reactor: Exploring dynamics, sources and minimization mechanisms. Water Res. 2013, 47, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Caballero, A.; Ribera, A.; Balcázar, J.L.; Pijuan, M. Nitritation versus full nitrification of ammonium-rich wastewater: Comparison in terms of nitrous and nitric oxides emissions. Bioresour. Technol. 2013, 139, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Adouani, N.; Lendormi, T.; Limousy, L.; Sire, O. Effect of the carbon source on N2O emissions during biological denitrification. Resour. Conserv. Recycl. 2010, 54, 299–302. [Google Scholar] [CrossRef]
- Pijuan, M.; Torà, J.; Rodríguez-Caballero, A.; César, E.; Carrera, J.; Pérez, J. Effect of process parameters and operational mode on nitrous oxide emissions from a nitritation reactor treating reject wastewater. Water Res. 2014, 49, 23–33. [Google Scholar] [CrossRef]
- Tallec, G.; Garnier, J.; Billen, G.; Gousailles, M. Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: Effect of oxygenation level. Water Res. 2006, 40, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Stenström, F.; Tjus, K.; la Cour Jansen, J. Oxygen-induced dynamics of nitrous oxide in water and off-gas during the treatment of digester supernatant. Water Sci. Technol. 2014, 69, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Zhang, M.; Lawlor, P.G.; Yang, Z.; Zhan, X. Nitrous oxide emission and nutrient removal in aerobic granular sludge sequencing batch reactors. Water Res. 2012, 46, 4981–4990. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Chung, M.S. Determination of optimal COD/nitrate ratio for biological denitrification. Int. Biodeterior. Biodegrad. 2003, 51, 43–49. [Google Scholar] [CrossRef]
- Li, Q.; Li, P.; Zhu, P.; Wu, J.; Liang, S. Effects of exogenous organic carbon substrates on nitrous oxide emissions during the denitrification process of sequence batch reactors. Environ. Eng. Sci. 2008, 25, 1221–1228. [Google Scholar] [CrossRef]
- Smolders, G.J.F.; van der Meij, J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Model of the anaerobic metabolism of the biological phosphorus removal process: Stoichiometry and pH influence. Biotechnol. Bioeng. 1994, 43, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Clescerl, L.S.; Greenberg, A.E.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1999.
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar] [PubMed]
- Law, Y.; Lant, P.; Yuan, Z. The effect of pH on N2O production under aerobic conditions in a partial nitritation system. Water Res. 2011, 45, 5934–5944. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.; Jeison, D.; Chamy, R. Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration. Water Res. 2003, 37, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Bernet, N.; Sanchez, O.; Cesbron, D.; Steyer, J.P.; Delgenès, J.P. Modeling and control of nitrite accumulation in a nitrifying biofilm reactor. Biochem. Eng. J. 2005, 24, 173–183. [Google Scholar] [CrossRef]
- Schneider, Y.; Beier, M.; Rosenwinkel, K.H. Nitrous oxide formation during nitritation and nitrification of high-strength wastewater. Water Sci. Technol. 2013, 67, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.; Ni, B.J.; Lant, P.; Yuan, Z. N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res. 2012, 46, 3409–3419. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.-J.; Peng, L.; Law, Y.; Guo, J.; Yuan, Z. Modeling of nitrous oxide production by autotrophic ammonia-oxidizing bacteria with multiple production pathways. Environ. Sci. Technol. 2014, 48, 3916–924. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.-J.; Ye, L.; Law, Y.; Byers, C.; Yuan, Z. Mathematical modeling of nitrous oxide (N2O) emissions from full-scale wastewater treatment plants. Environ. Sci. Technol. 2013, 47, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Kishida, N.; Kim, J.; Kimochi, Y.; Nishimura, O.; Sasaki, H.; Sudo, R. Effect of C/N ratio on nitrous oxide emission from swine wastewater treatment process. Water Sci. Technol. 2004, 49, 359–371. [Google Scholar] [PubMed]
- Alinsafi, A.; Adouani, N.; Béline, F.; Lendormi, T.; Limousy, L.; Sire, O. Nitrite effect on nitrous oxide emission from denitrifying activated sludge. Process Biochem. 2008, 43, 683–689. [Google Scholar] [CrossRef]
- Itokawa, H.; Hanaki, K.; Matsuo, T. Nitrous oxide production in high-loading biological nitrogen removal process under low COD/N ratio condition. Water Res. 2001, 35, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Goretski, J.; Hollocher, T.C. Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J. Biol. Chem. 1988, 263, 2316–2323. [Google Scholar] [PubMed]
- Von Schulthess, R.; Wild, D.; Gujer, W. Nitric and nitrous oxides from denitrifying activated sludge at low oxygen concentration. Water Sci. Technol. 1994, 30, 123–132. [Google Scholar]
- Lemaire, R.; Meyer, R.; Taske, A.; Crocetti, G.R.; Keller, J.; Yuan, Z.G. Identifying causes for N2O accumulation in a lab-scale sequencing batch reactor performing simultaneous nitrification, denitrification and phosphorus removal. J. Biotechnol. 2006, 122, 62–72. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Zheng, D.; Xing, L. Nitritation and N2O Emission in a Denitrification and Nitrification Two-Sludge System Treating High Ammonium Containing Wastewater. Water 2014, 6, 2978-2992. https://doi.org/10.3390/w6102978
Wu G, Zheng D, Xing L. Nitritation and N2O Emission in a Denitrification and Nitrification Two-Sludge System Treating High Ammonium Containing Wastewater. Water. 2014; 6(10):2978-2992. https://doi.org/10.3390/w6102978
Chicago/Turabian StyleWu, Guangxue, Derui Zheng, and Lizhen Xing. 2014. "Nitritation and N2O Emission in a Denitrification and Nitrification Two-Sludge System Treating High Ammonium Containing Wastewater" Water 6, no. 10: 2978-2992. https://doi.org/10.3390/w6102978




