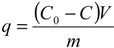3.2.1. Effect of Initial Copper Concentration
The rate of metal removal is an important factor in developing an adsorbent-based water treatment technology. The kinetics of the adsorption of Cu ions on MCS were studied using contact times ranging from 0 to 240 min.
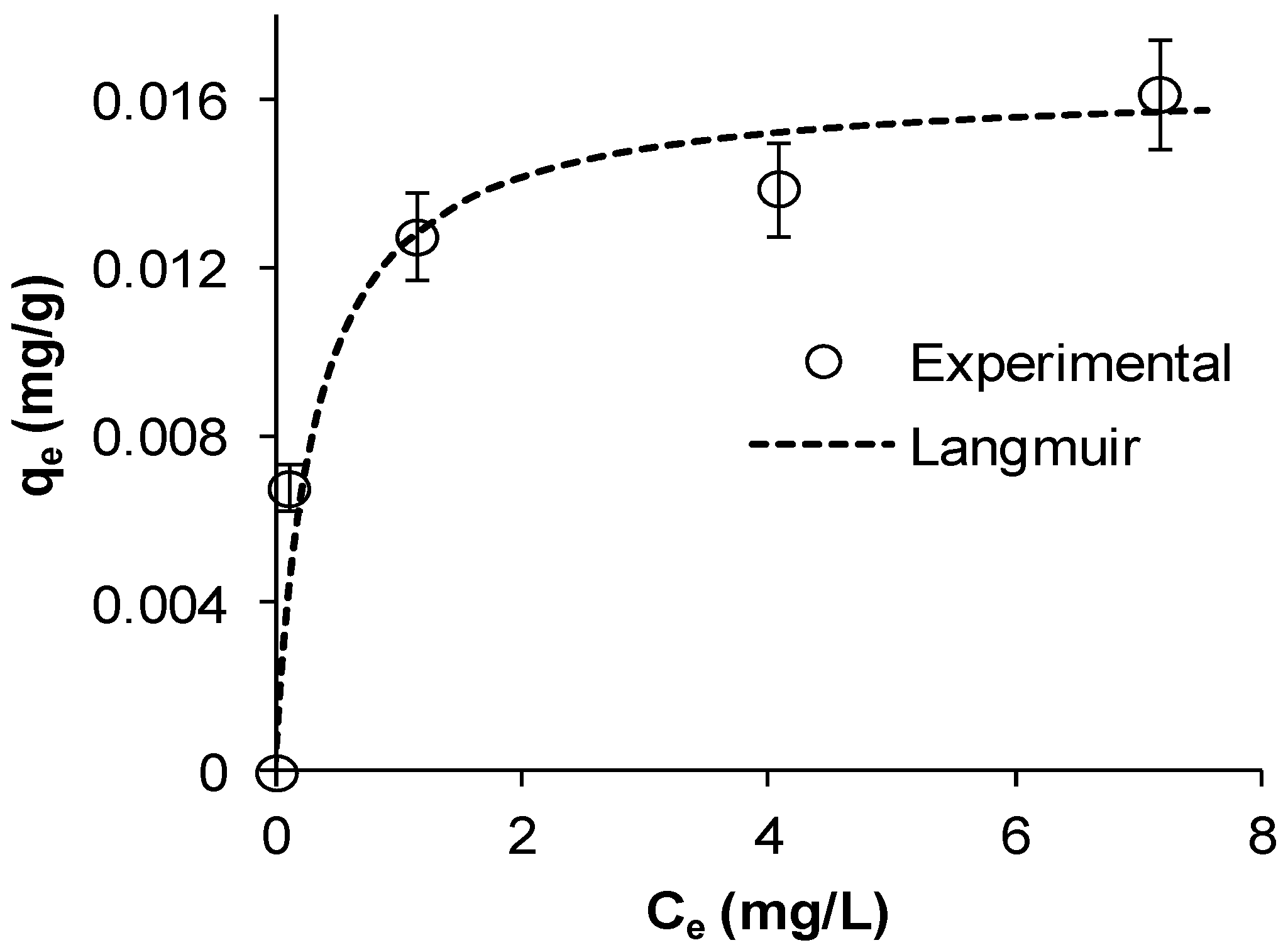
Figure 2a shows that Cu adsorption increased gradually with increasing contact times until reaching a plateau indicating equilibrium. The figure also shows that as
C0 increased, the uptake also increased, which is typical for adsorption processes. This is consistent with
Figure 1, which shows an increase in the amount of copper sorbed at equilibrium when
C0 was raised from 7 to 23 mg/L. Ghodbane
et al. [
14] observed similar trends in their study. Cu removal was fast in the initial 15 min followed by a slow decrease to almost a nil rate at the end of the experiment. Copper removals after 30 min were 52%, 63%, 65%, and 66% for initial concentrations of 23, 18, 14, and 7 mg/L, respectively. This indicates that the Mn coated sand is an effective adsorbent for the removal of Cu from water and it is likely that adsorption is the main mechanism by which the metal is removed from water. The results indicate that the adsorption equilibrium capacity of MCS increased from 0.0078 to 0.0177 mg/g as
C0 was raised from 7 to 23 mg/L (
Figure 2). The rate of Cu removal was highest at the beginning of the experiment, possibly due to the availability of a large number of empty surface sites during the early stage of adsorption. Afterwards, the remaining available sites were difficult to inhabit because of reduction in driving force and increased repulsive forces between the solute molecules on the bulk and solid phases [
18].
The adsorption of Cu ions onto MCS may proceed through three steps including adsorption of Cu ions to the external surface of MCS by diffusion through a film located in the fluid phase followed by intra-particle diffusion in the interior of the coating and adsorption at the surface of MCS that may involve chemical binding of the adsorbate. To discern which mechanism controls the overall adsorption process of Cu
2+ on MCS, kinetic models for film diffusion [
19], intra-particle diffusion [
20], and adsorption reaction models were used to correlate the experimental data. The adsorption reaction models used here were Lagergren’s pseudo first-order [
21], pseudo second-order [
22], and Elovich’s equation [
23].
Table 3 summarizes the linear form of each model used in this study and
Figure 2b plots the linearized form for the pseudo second-order model at the different initial concentrations used.
Figure 2.
Kinetics of Cu ions adsorption on MCS at different initial copper concentrations (a) experimental data, dashed lines represent the pseudo second-order model; (b) linearized pseudo second-order model (γMn = 0.0709 mg Mn/g sand, msand = 50 g, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
Figure 2.
Kinetics of Cu ions adsorption on MCS at different initial copper concentrations (a) experimental data, dashed lines represent the pseudo second-order model; (b) linearized pseudo second-order model (γMn = 0.0709 mg Mn/g sand, msand = 50 g, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
Whilst the adsorption reaction models cannot identify the diffusion mechanisms during the adsorption process, they describe well the experimental data if the adsorption process involves chemisorption [
24]. As high-speed mixing of the solution, which reduces the boundary layer resistance to mass transfer, was used in this study, the film diffusion was assumed to not be a controlling step as also evidenced by the poor fitting of the experimental data with a liquid film model (results are not shown here). The correlation coefficient R
2 was used to measure how good the fitting of the experimental data to each model was by comparing its closeness to one. The parameters obtained by fitting the experimental data at different
C0 to the models are shown in
Table 3. The Table shows that, overall, the values of the equilibrium capacity,
qe, obtained by the pseudo second-order and the pseudo first-order models were close to the experimental values (
Figure 1). The Table also shows that the pseudo second-order kinetic model has the greatest
R2 with an average value of 0.995 (a minimum of 0.993) for all the
C0 used in the study followed by Elovich then the pseudo first-order models. As the pseudo second-order kinetic model is based on the assumption that the rate-limiting step is that of chemisorption [
12,
25], and considering the good correlation between the model and the experimental data, it can be assumed that the adsorption of Cu ions on MCS occurred through chemisorption involving valence forces through sharing or exchange of electrons between copper and MCS [
25]. Similar findings have also been reported in the literature [
14,
15,
25,
26].
Table 3 shows that the rate constant
K2 obtained by the pseudo second-order kinetic model, increased as
C0 decreased and the initial adsorption rate,
h, and the theoretical amount of Cu adsorbed at equilibrium,
qe, decreased with a decrease in the initial concentration
C0, which is confirmed by the experimental data. Sen and Sarzali [
25] have also found that
h decreased with a decrease in
C0 for cadmium adsorption on aluminium oxide (Al
2O
3) and Demirbas
et al. [
27] have also found
K2 to increase as the initial concentration decreased.
Table 3.
Kinetic parameters for different initial Cu2+ concentrations (γMn = 0.0709 mg Mn/g sand, pH = 5, T = 25.6 °C).
3.2.2. Effect of Sand Coating
Coated sand (0.0709 mg Mn/g sand) and uncoated sand were used to study the effect of sand coating on the kinetics of Cu2+ adsorption.msand, C0, Vsolution, pH, T, and shaking speed were kept constant at 50 g, 23 mg/L, 50 mL, 5, 25.6 °C, and 150 rpm respectively.
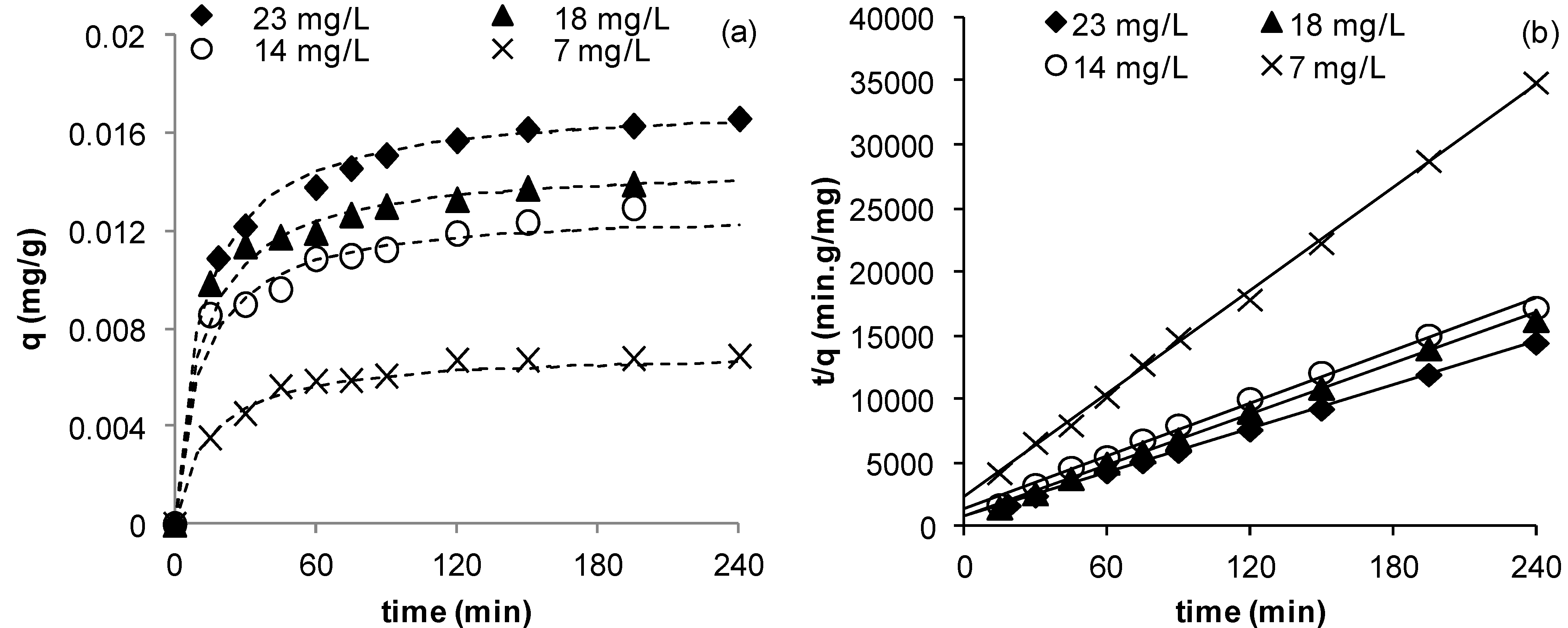
Figure 3 illustrates the change in copper uptake as function of time by the coated and uncoated sands. Although similar profiles for both sands are obtained (
Figure 3), clearly the maximum uptake by the coated sand was higher than that of the uncoated sand with values of 0.0172 and 0.0102 mg Cu
2+/g sand for coated and uncoated sands respectively (
Table 4). This indicates that the coating has improved copper removal by about 70%. This may be explained by the increased surface area of the Mn-coated sand as compared to uncoated sand [
13], which results in more active sites. The experimental kinetic data was well fitted to the pseudo second-order kinetic model with R
2 values >0.999 for both sands indicating that chemisorption may control the adsorption of Cu
2+ on uncoated sand. The initial adsorption rate,
h, was found to be slightly higher for coated sand (coated: 0.0015 mg/g·min; uncoated: 0.0013 mg/g·min) whereas values of
K2 decreased with coating the sand (coated: 5.01 g/mg·min; uncoated: 12.64 g/mg·min).
Figure 3.
Effect of sand coating on the adsorption of Cu2+ (msand = 50 g, C0 = 23 mg/L, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
Figure 3.
Effect of sand coating on the adsorption of Cu2+ (msand = 50 g, C0 = 23 mg/L, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
Table 4.
Kinetic parameters obtained using pseudo-second-order kinetic model for different coating conditions (msand = 50 g, C0 = 23 mg/L, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
Table 4.
Kinetic parameters obtained using pseudo-second-order kinetic model for different coating conditions (msand = 50 g, C0 = 23 mg/L, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
| Parameters | Coated (=0.0709 mg Mn/g sand) | Uncoated (= 0 mg Mn/g sand) |
|---|
| K2 (g/mg·min) | 5.01 | 12.64 |
| qe (mg/g) | 0.0172 | 0.0102 |
| h (mg/g·min) | 0.0015 | 0.0013 |
| R2 | 0.9973 | 0.9959 |
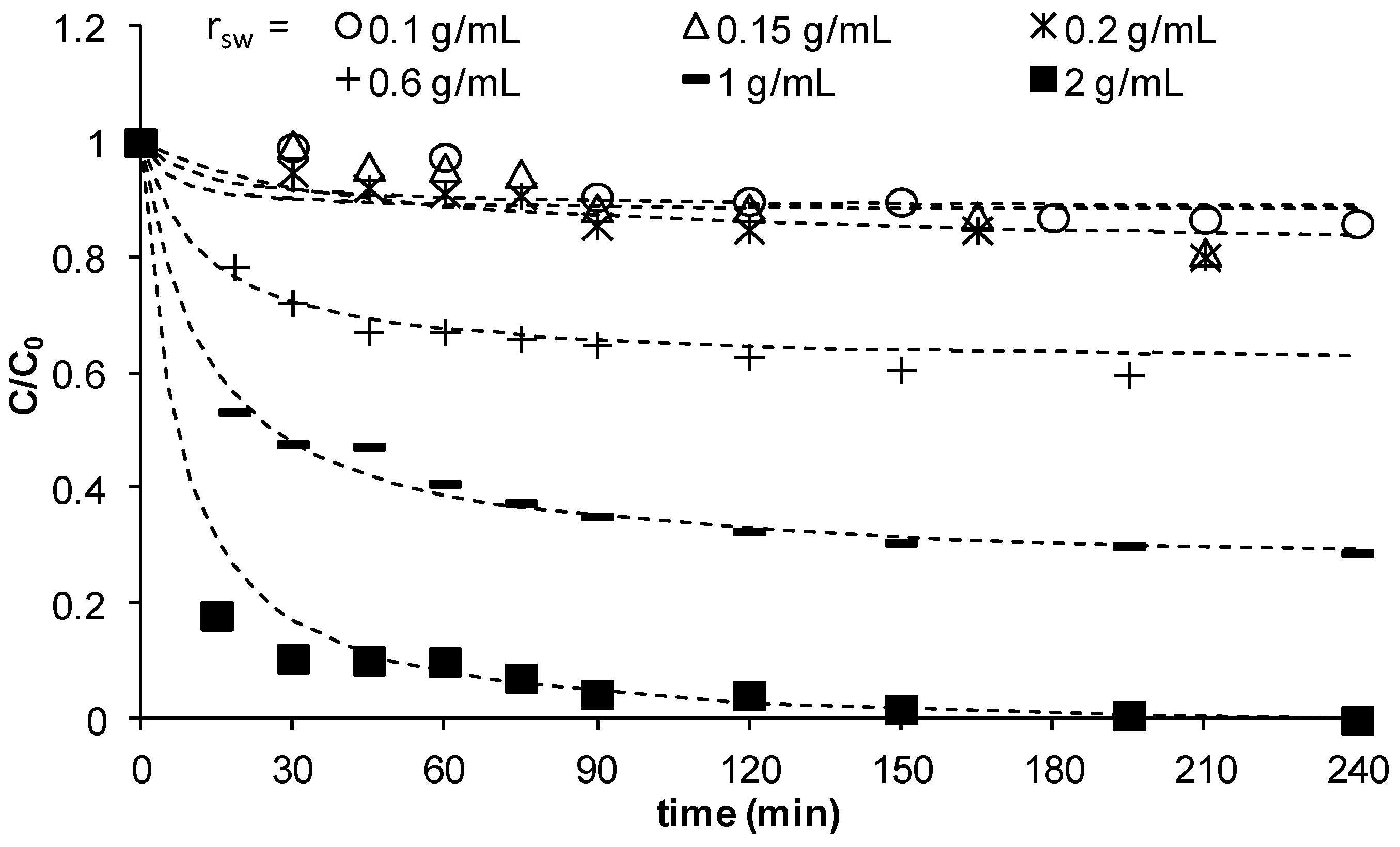
3.2.3. Effect of the Ratio Mass of Sand to Solution Volume, rsw
The effect of the ratio of mass of sand to the solution volume,
rsw, on the adsorption of Cu
2+ on MCS was studied using different sand masses 5, 7.5, 10, 30, 50, and 100 g at a constant solution volume of 50 mL.
Figure 4 illustrates the changes of
C/
C0 as function of time at different
rsw values and shows that the change of
C/C0 at low
rsw <0.2 g/mL is significantly small due to the small mass of sand available in solution to remove copper. As
rsw increased to 0.6, and further to 1, then to 2 g/mL, a clear reduction in concentration is observed meaning significant removal of copper. At all
rsw values, the concentration reduces with time until reaching a plateau indicating saturation of the sand with lowest reduced concentrations obtained as
rsw increased. However, the mass of copper removed at equilibrium per unit mass of MCS decreased with an increase in
rsw (data not shown here). Other studies have also obtained similar effect [
14,
25], which can be explained by particle-particle interactions [
25] at high solid content. These interactions result in blockage of some active sites, hence a decrease in adsorption capacity. It may also lead to electrostatic interferences such that the electrical surface charges on the closely packed particles reduce attraction between the adsorbate and surfaces of individual sand particles. The experimental kinetic data was found to fit well to the pseudo-second-order kinetic model (dashed lines in
Figure 4). The data showed that
h decreased as
rsw increased, whilst no clear trend was observed for
K2. Ghodbane
et al. [
14] have also observed that
h decreased with increasing the adsorbent mass (
m), though they found that increasing
m enhanced
K2 but reduced
qe.
Figure 4.
C/C0 vs time for different ratios of mass of sand to solution volume (γMn = 0.0709 mg Mn/g sand, C0 = 23 mg/L, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
Figure 4.
C/C0 vs time for different ratios of mass of sand to solution volume (γMn = 0.0709 mg Mn/g sand, C0 = 23 mg/L, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
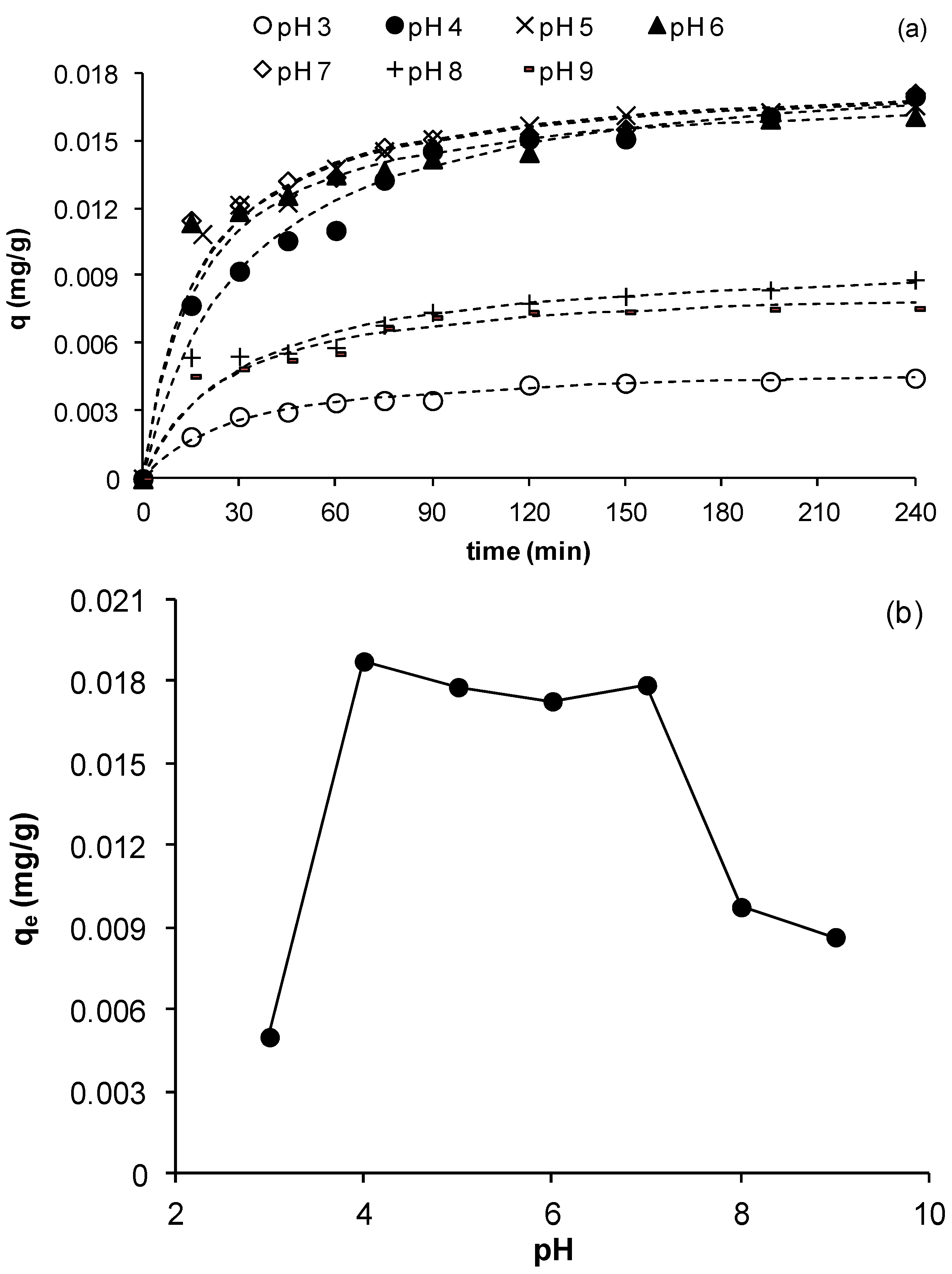
3.2.4. Effect of Initial pH
As pH is an important parameter controlling the removal of metals in solution, its effect on the kinetics of copper adsorption on MCS was studied using initial values in the range 3 to 9. The other experimental parameters were as in previous experiments.
Figure 5a shows the change of copper uptake as a function of time for the different pH values studied.
Figure 5a clearly shows that copper removal is strongly pH dependent and that at all pHs, the uptake evolved until reaching a plateau, indicating exhaustion of the adsorbent as saturation was reached. The figure also shows that the uptake of copper,
q, increased as pH increased from 3 to 4 and remained almost constant until pH 7 after which, a further increase to pHs 8 and 9 reduced
q significantly. This is clearly depicted in
Figure 5b, which shows the equilibrium adsorption uptake as a function of pH. A similar effect of pH on adsorption of metals was also observed by Ghodbane
et al. [
14], and Sen and Sarzali [
25]. The reduction in the equilibrium adsorption uptake at pHs 8 and 9 may be due to a blockage of the exterior surface of MCS as a result of precipitation of copper hydroxide, which is expected to take place strongly at these pHs (note: at
C0 = 23 mg/L, precipitation occurs at pH 5.91 [
13]). On the other hand, the low adsorption observed at low pH 3 may be attributed to excessive protonation of the adsorbent surface and to high mobility of H
+ ions, which are more preferentially adsorbed than Cu ions [
17,
25]. In addition, it was observed as discussed below that at pH 3, the leaching of Mn
2+ was high (17% and 5% of initial Mn
2+ leached out at pHs 3 and 5 respectively). The leaching of Mn
2+ into solution should result in reduction of adsorption sites on MCS hence contributing to the observed reduction in equilibrium capacity at pH 3. As pH increases, the surface charge becomes less positive favoring attraction of Cu cations to MCS surface. In addition, as the proportion of hydrated Cu ions increases with pH, these ions may become more strongly attracted to MCS surface than unhydrated ions. Both of these synergistically increase the adsorption of Cu ions as pH increases to below precipitation pHs, which explains the observed increase in removal for pHs >3.
Figure 5b shows that for 4 < pH < 7,
qe does not change significantly hence an average value within this pH range was calculated;
qe,4 < pH < 7 = 0.0179 mg/g ± 4%. From an operating point of view, this result is important since the process may be operated within this large pH range without the need for significant pH adjustment.
Figure 5.
Effect of initial pH on the adsorption of Cu ions by MCS (a) change of q as function of time at different pHs; (b) effect of pH on equilibrium adsorption uptake, qe (γMn = 0.0709 mg Mn/g sand, msand = 50 g, C0= 23 mg/L, Vsolution = 50 mL, shaker speed = 150 rpm, T = 25.6 °C).
Figure 5.
Effect of initial pH on the adsorption of Cu ions by MCS (a) change of q as function of time at different pHs; (b) effect of pH on equilibrium adsorption uptake, qe (γMn = 0.0709 mg Mn/g sand, msand = 50 g, C0= 23 mg/L, Vsolution = 50 mL, shaker speed = 150 rpm, T = 25.6 °C).
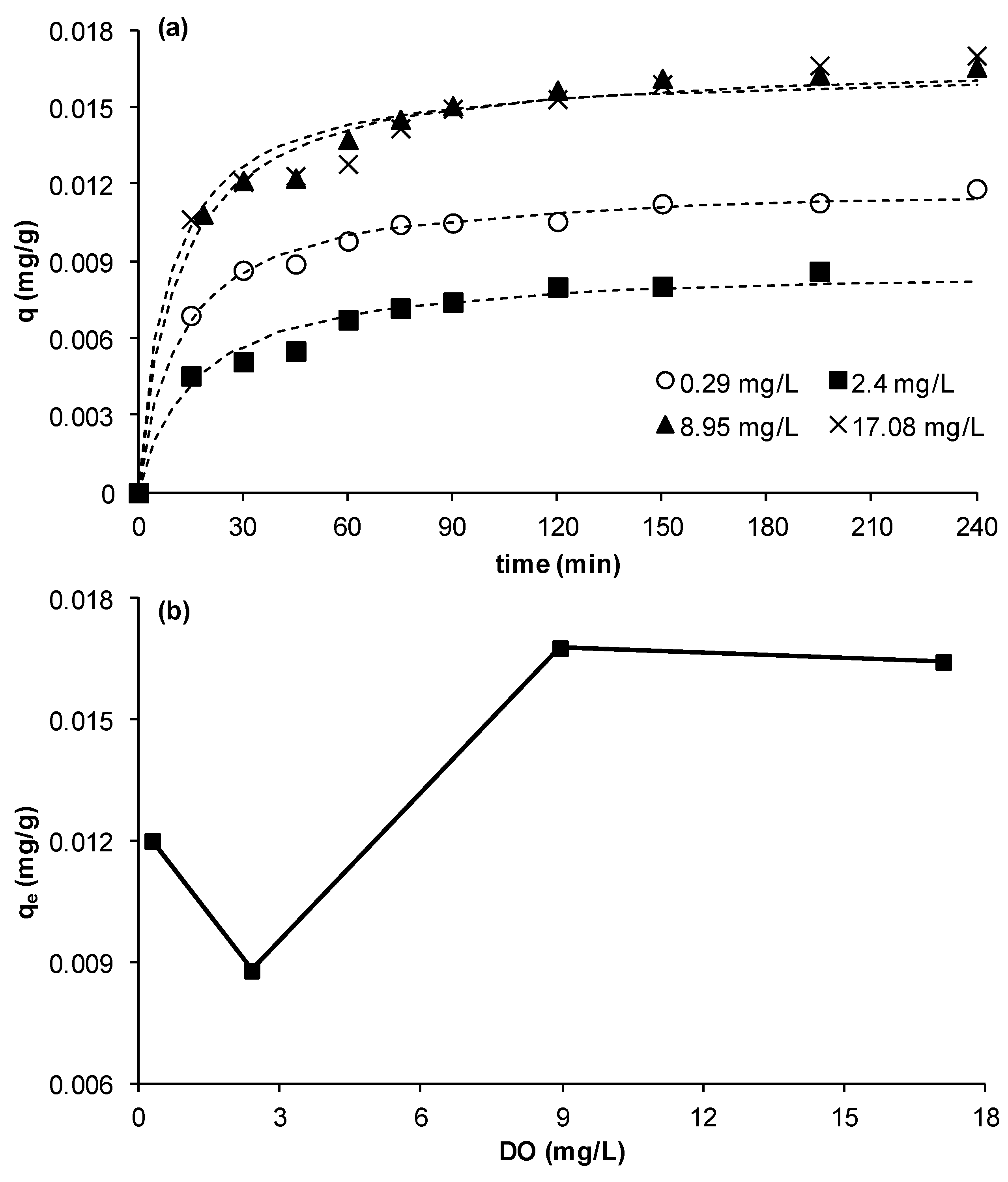
3.2.5. Effect of Dissolved Oxygen Concentration (DO)
The effect of DO on the adsorption kinetics of Cu on MCS was studied using DO values of 0.29, 2.40, 8.95, and 17.08 mg/L. The other experimental parameters were as in previous experiments. It was observed that the removal of Cu reduced by about 27% when DO increased from 0.29 to 2.4 mg/L however, when DO was raised to 8.95 mg/L a significant increase in removal by about 91% occurred. A further increase of DO to 17.08 mg/L did not result in any further change in copper ion removal (
Figure 6a,b).
Figure 6.
Effect of dissolved oxygen on copper ion removal by MCS (a) change of q as function of time at different DO concentrations; (b) effect of DO concentration on equilibrium adsorption uptake, qe (γMn= 0.0709 mg Mn/g sand, msand = 50 g, C0= 23 mg/L, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
Figure 6.
Effect of dissolved oxygen on copper ion removal by MCS (a) change of q as function of time at different DO concentrations; (b) effect of DO concentration on equilibrium adsorption uptake, qe (γMn= 0.0709 mg Mn/g sand, msand = 50 g, C0= 23 mg/L, Vsolution = 50 mL, pH = 5, shaker speed = 150 rpm, T = 25.6 °C).
Although it is not clear why there was a dip at 2.4 mg/L or an increase at 0.29 mg/L, the results clearly show that copper removal was DO dependent. The overall trend is that the removal is enhanced with increasing DO up to a maximum obtained at ambient DO concentrations. This trend is in agreement with that obtained in our previous work which showed that aeration enhanced removal of copper in a bed of MCS [
13]. A plausible explanation is that DO increases the formation of manganese oxide, which results from the reaction between oxygen and manganese (Mn
2+ + 0.5O
2 + H
2O → MnO
2(s) + 2H
+), promoting the removal of copper ions under the studied pH [
13]. It was also observed in this study that the final pH of the solution was lower for higher DO concentrations by about 0.5 pH units, which reflects that more copper was removed by the coated sand at high DO. Indeed, lower pH (
i.e., increased concentration of H
+) may result from the above reaction but also based on ion exchange principle, the more copper ions adsorbed by the coated sand, the more hydrogen ions are released. Moreover, it was observed in this study and as discussed in the next section, that leaching of Mn
2+ into solution was high at low DO (about 9% of initial Mn
2+ leached out at DO = 0.29 mg/L as compared to 5% at DO = 8.95 mg/L), which should result in reduction of adsorption sites on the surface of MCS hence contributing to the observed reduction in equilibrium capacity at low DO. The experimental kinetic data were well fitted to the pseudo-second-order kinetic model (
Figure 6a). It was found that the value of the rate constant
K2 was not significantly affected by DO concentrations; an average value for
K2 of 6.45 ° 12% (g/mg·min) was calculated, whilst
h was almost doubled when DO increased from 0.29 to 17.08 mg/L. Although there are no previous studies on DO effect to compare to the results obtained in this study, clearly the effect of DO should be studied when removing metal ions by adsorption. This is important as, in real practical cases, DO may change as a result of for example biological processes or changes in temperature.