Spatiotemporal Absorption Features of Yellow Willow Water Usage on the Southern Edge of the Semi-Arid Hunshandak Sandland in China
Abstract
:1. Introduction
2. Materials and Methods
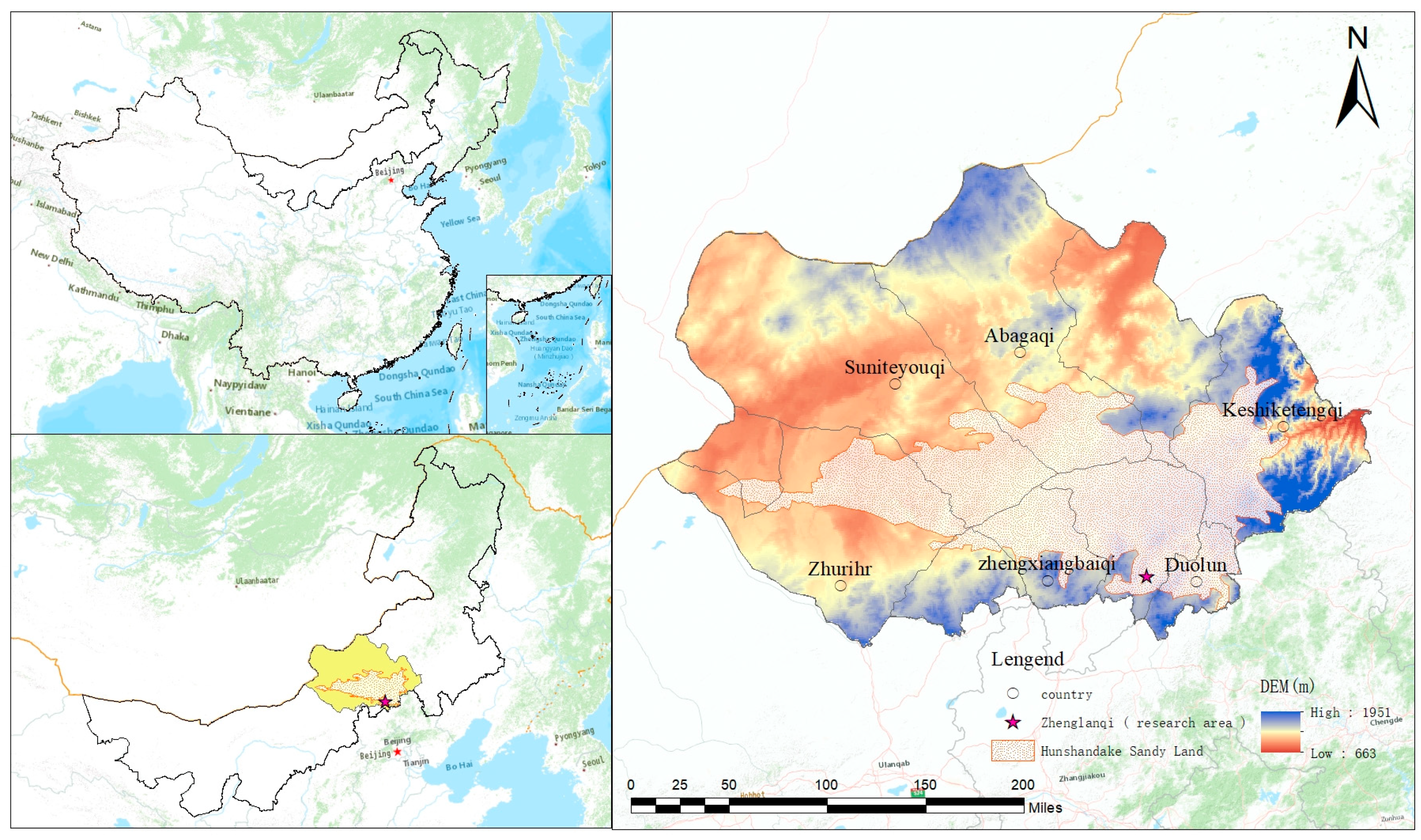
2.1. Study Area
2.2. Sample Collection and Treatment
2.3. Statistical Analysis
3. Results
3.1. Isotopic Characteristics of Different Water Sources
3.2. Local Atmospheric Precipitation Characteristics
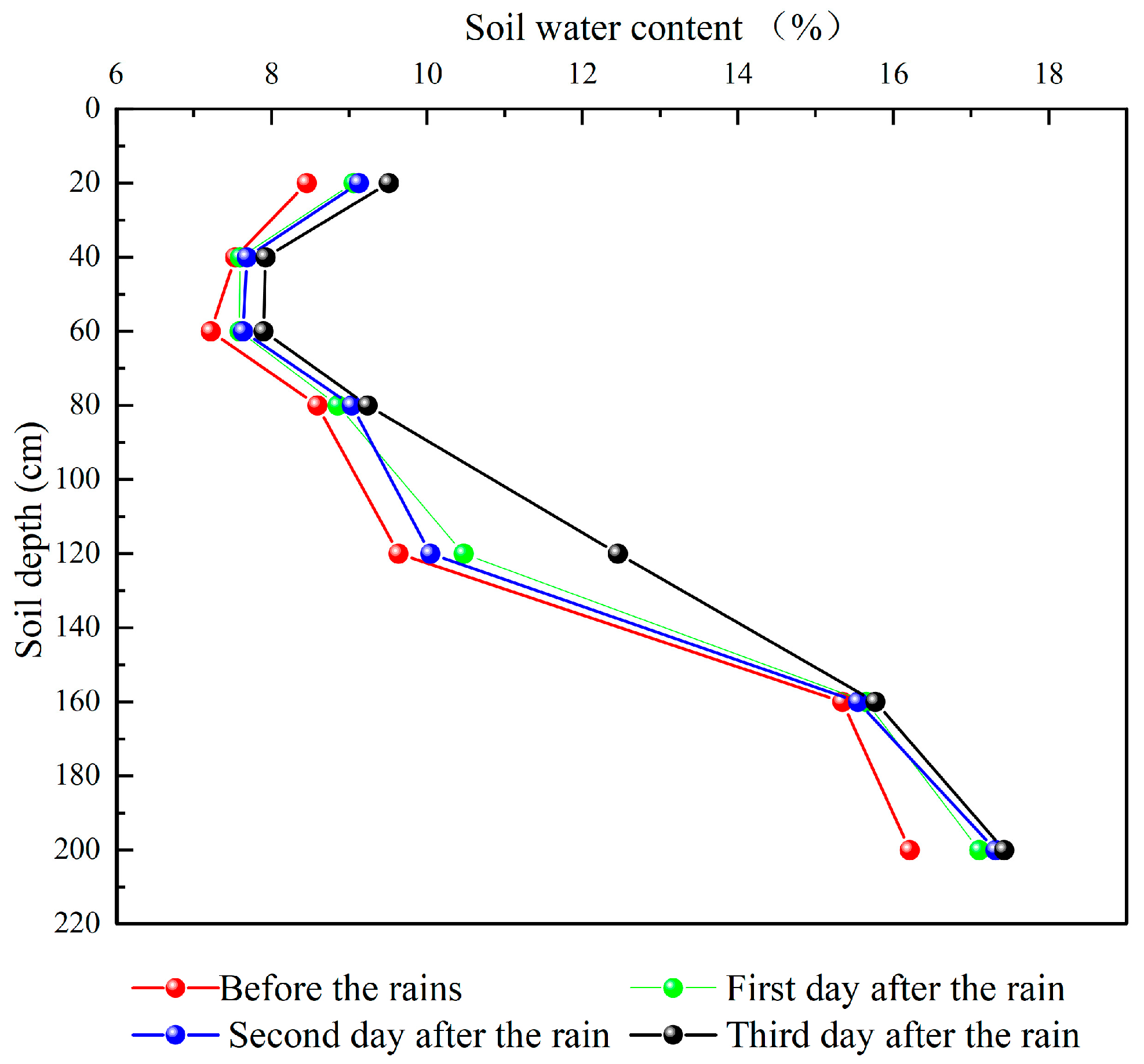
3.3. Variation in Soil Water Content between Seasons
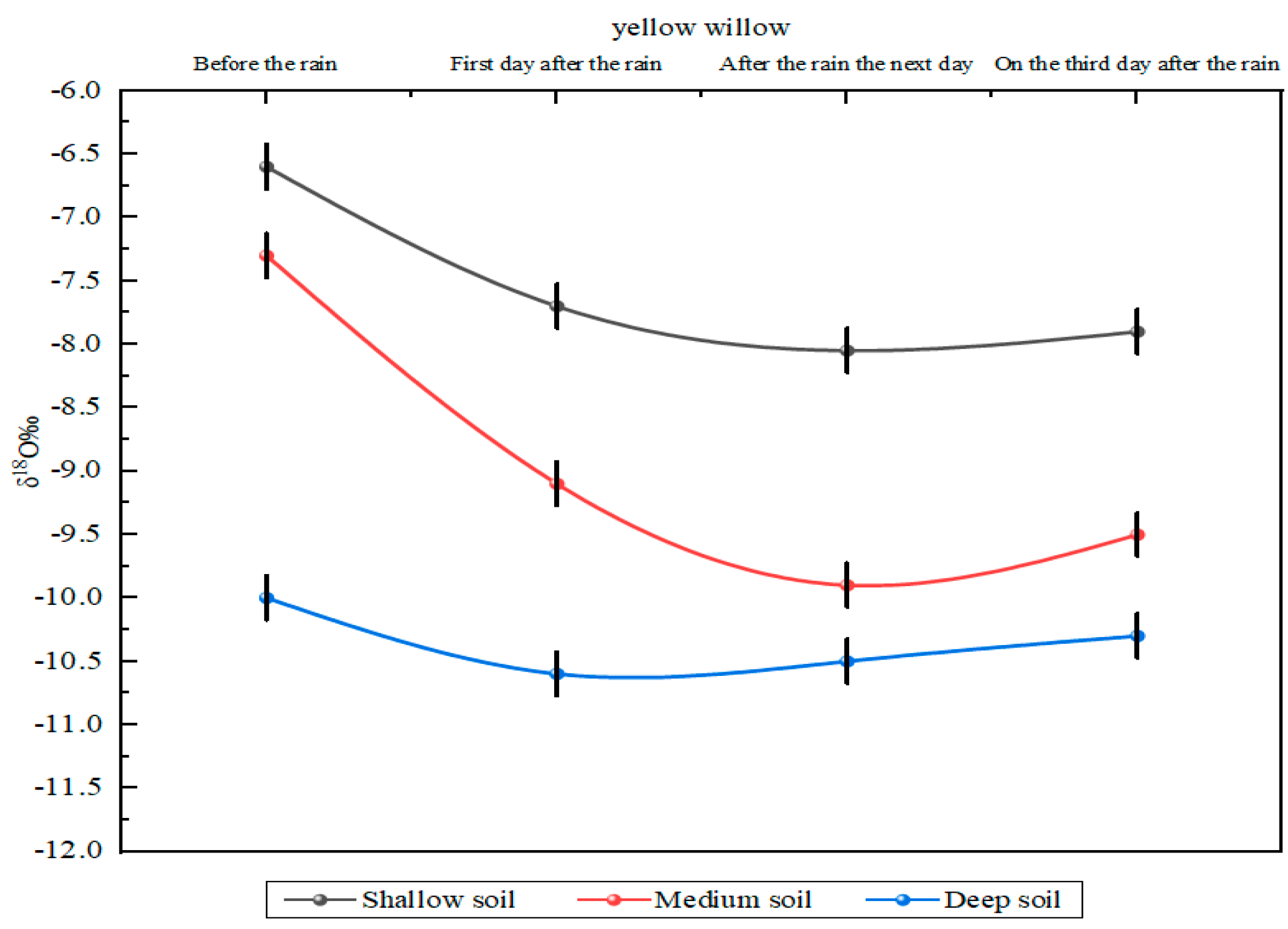
3.4. Effects of Precipitation on Soil Hydrogen and Oxygen Isotopes
3.5. Determining the Main Water Sources in Artificially Planted Yellow Willow
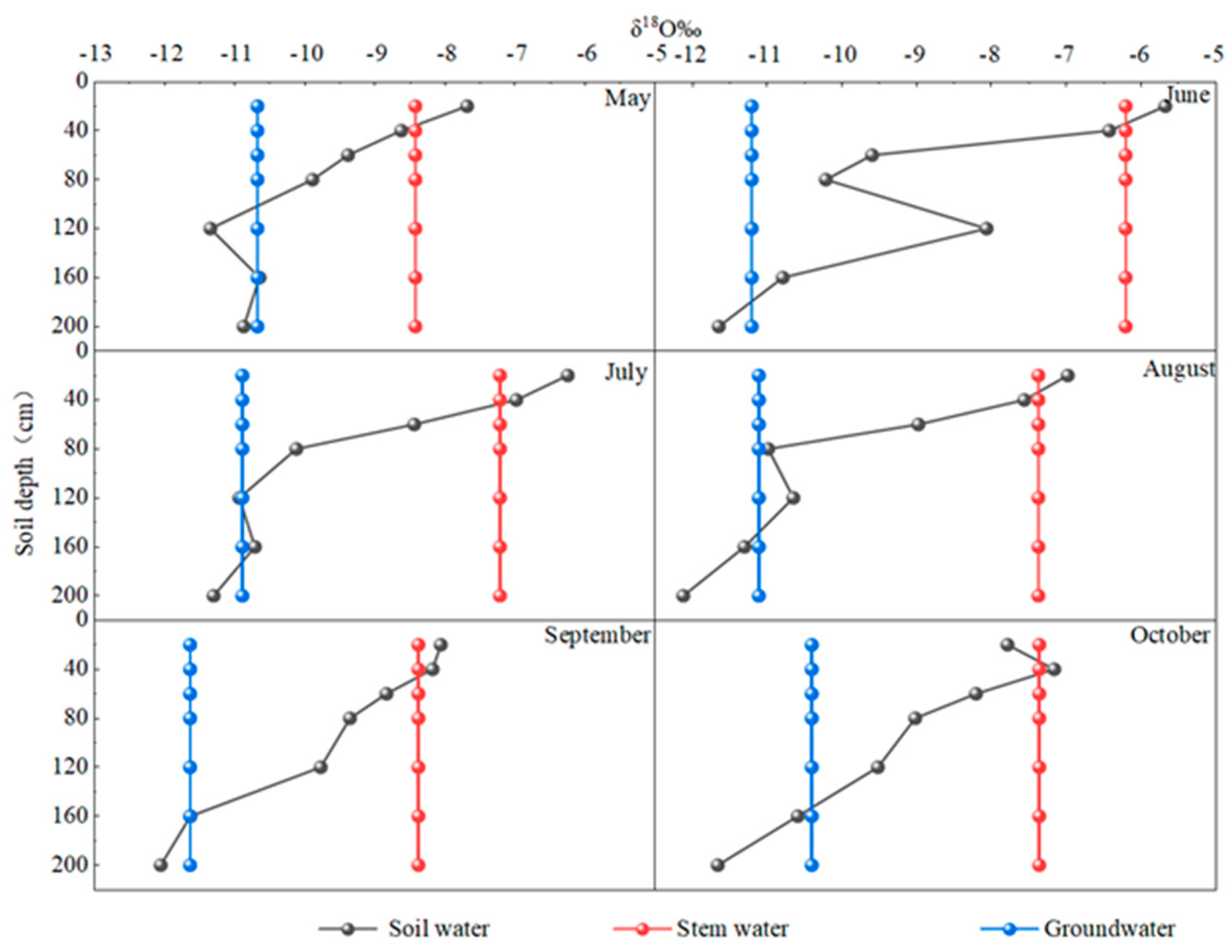
3.5.1. Vertical Variation in Soil Water Isotope
3.5.2. Lateral Variation in Soil Water Isotope
3.6. Quantitative Analysis of Yellow Willow Moisture Sources
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thomas, D.S. (Ed.) Arid Zone Geomorphology: Process, Form, and Change in Drylands; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Huang, J.; Li, Y.; Fu, C.; Chen, F.; Fu, Q.; Dai, A.; Shinoda, M.; Ma, Z.; Guo, W.; Li, Z.; et al. Dryland climate change: Recent progress and challenges. Rev. Geophys. 2017, 55, 719–778. [Google Scholar] [CrossRef]
- Ungar, E.D.; Rotenberg, E.; Raz-Yaseef, N.; Cohen, S.; Yakir, D.; Schiller, G. Transpiration and annual water balance of Aleppo pine in a semiarid region: Implications for forest management. For. Ecol. Manag. 2013, 298, 39–51. [Google Scholar] [CrossRef]
- Dymond, S.F.; Bradford, J.B.; Bolstad, P.V.; Kolka, R.K.; Sebestyen, S.D.; De Sutter, T.M. Topographic, edaphic, and vegetative controls on plant-available water. Ecohydrology 2017, 10, e1897. [Google Scholar] [CrossRef]
- Haregeweyn, N.; Tsunekawa, A.; Nyssen, J.; Poesen, J.; Tsubo, M.; Tsegaye Meshesha, D.; Tegegne, F. Soil erosion and conservation in Ethiopia: A review. Prog. Phys. Geogr. 2015, 39, 750–774. [Google Scholar] [CrossRef]
- Huang, L.M.; Shao, M.A. Advances and perspectives on soil water research in China’s loess plateau. Earth-Sci. Rev. 2019, 199, 102962. [Google Scholar] [CrossRef]
- Harrison, J.L.; Blagden, M.; Green, M.B.; Salvucci, G.D.; Templer, P.H. Water sources for red maple trees in a northern hardwood forest under a changing climate. Ecohydrology 2020, 13, e2248. [Google Scholar] [CrossRef]
- Gow, L.J.; Barrett, D.J.; O’grady, A.P.; Renzullo, L.J.; Phinn, S.R. Subsurface water use strategies and physiological responses of subtropical eucalypt woodland vegetation under changing water availability conditions. Agric. For. Meteorol. 2018, 248, 348–360. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.J.; Lu, N.; Zhang, L. Seasonal variation in water uptake patterns of three plant species based on stable isotopes in the semi-arid loess plateau. Sci. Total Environ. 2017, 609, 27–37. [Google Scholar] [CrossRef]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Yang, J. Water source utilization by woody plants growing on dolomite outcrops and nearby soils during dry seasons in the Karst Region of Southwest China. J. Hydrol. 2012, 420, 264–274. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L. Insights into the isotopic mismatch between bulk soil water and Salix matsudana Koidz trunk water from root water stable isotope measurements. Hydrol. Earth Syst. Sci. 2021, 25, 3975–3989. [Google Scholar] [CrossRef]
- Huo, G.P.; Zhao, X.N.; Gao, X.D.; Wang, S.F.; Pan, Y.H. Seasonal water use patterns of rainfed jujube trees in stands of different ages under semiarid Plantations in China. Agric. Ecosyst. Environ. 2018, 265, 392–401. [Google Scholar] [CrossRef]
- Lanning, M.; Wang, L.; Benson, M.; Zhang, Q.; Novick, K.A. Canopy isotopic investigation reveals different water uptake dynamics of maples and oaks. Phytochemistry 2020, 175, 112389. [Google Scholar] [CrossRef]
- Pei, Y.W.; Huang, L.M.; Shao, M.A.; Zhang, Y.L.; Pan, Y.H. Water use pattern and transpiration of Mongolian pine plantations about stand age on northern Loess Plateau of China. Agric. For. Meteorol. 2023, 330, 109320. [Google Scholar] [CrossRef]
- McCole, A.A.; Stern, L.A. Seasonal water use patterns of Juniperus ashei on the Edwards Plateau, Texas, based on stable isotopes in water. J. Hydrol. 2007, 342, 238–248. [Google Scholar] [CrossRef]
- Chang, E.H.; Li, P.; Li, Z.B.; Xiao, L.; Feng, Z.H. Using water isotopes to analyze water uptake during vegetation succession on abandoned cropland on the Loess Plateau, China. Catena 2019, 181, 104095. [Google Scholar] [CrossRef]
- Wang, S.F.; An, J.; Zhao, X.N.; Gao, X.D.; Wu, P.T.; Huo, G.P.; Robinson, B.H. Age and climate-related water use patterns of apple trees on China’s Loess Plateau. J. Hydrol. 2020, 582, 124462. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.J.; Jiao, L.; Lu, N.; Li, J.Y.; Chen, W.L.; Wang, L.X. Age-related water use characteristics of Robinia pseudoacacia on the Loess Plateau. Agric. For. Meteorol. 2021, 301, 108344. [Google Scholar] [CrossRef]
- Jia, H.X.; Yang, H.F.; Sun, P.; Li, J.B.; Zhang, J.; Guo, Y.H.; Han, X.J.; Zhang, G.S.; Lu, M.Z.; Hu, J.J. De novo transcriptome assembly, development of esters markers, and population genetic analyses for the desert biomass willow, Salix psammophila. Sci. Rep. 2016, 6, 3. [Google Scholar]
- Wang, Y.; Meng, H.; Zhang, W.; Shao, W.; Zhang, G.; Ning, M.; Ge, G. Morphological characters and inflorescence distribution habits of Salix psammophila in Mu Us Sand Land. Acta Bot. Bor-Occid. Sin. 2009, 29, 775–780. (In Chinese) [Google Scholar]
- Hayat, M.; Zha, T.; Jia, X.; Iqbal, S.; Qian, D.; Bourque, C.P.A.; Khan, A.; Tian, Y.; Bai, Y.J.; Liu, P.; et al. A multiple-temporal scale analysis of biophysical control of sap flow in Salix psammophila growing in a semiarid shrubland ecosystem of northwest China. Agric. For. Meteorol. 2020, 288–289, 107985. [Google Scholar] [CrossRef]
- Yang, Z.P.; Li, X.Y.; Liu, L.Y.; Wu, J.J.; Hasi, E.; Sun, Y.L. Characteristics of streamflow for sand-fixed shrubs in the Mu Us sandy land, Northwest China. Chin. Sci. Bull. 2008, 53, 2214–2221. (In Chinese) [Google Scholar]
- Zhang, Z.Y.; Wang, W.K.; Gong, C.C.; Zhao, M.; Franssen, H.J.H.; Brunner, P. Salix psammophila afforestation can cause a decline in the water table, prevent groundwater recharge, and reduce effective infiltration. Sci. Total Environ. 2021, 780, 146336. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.P.; Yu, H.P.; Dai, A.G.; Wei, Y.; Kang, L.T. Drylands face potential threat under global warming target. Nat. Clim. Chang. 2017, 7, 417–422. [Google Scholar] [CrossRef]
- Jing, J. The Coupling of Water Source and Consumption Based on the Hydrogen and Oxyger Stable Isotope of Natural Salix gordejevii in Otinday Sandland. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2016; pp. 14–18. [Google Scholar]
- Lang, Y.I.; Ying, S.U.N.; ShaoHua, Y.I.N.; Xiao, W.E.I. Construction of Ecological Security Pattern: Concept, framework, and prospect. Ecol. Environ. 2022, 31, 845. [Google Scholar]
- Zimmermann, U.; Münnich, K.O.; Roether, W.; Kreutz, W.; Schubach, K.; Siegel, O. Tracers determine the movement of soil moisture and evapotranspiration. Science 1966, 152, 346–347. [Google Scholar] [CrossRef]
- Yang, L.; Jia, D.; Gao, R.; Su, W.; Lu, F.; Wendu, R. Isotope characteristics of hydrogen and oxygen in different water bodies in the southern margin of the Hunshandak Sandy Land. Earth Environ. 2022, 50, 630–638. [Google Scholar] [CrossRef]
- Schmugge, T.J.; Jackson, T.J.; McKim, H.L. Survey of methods for soil moisture determination. Water Resour. Res. 1980, 16, 961–979. [Google Scholar] [CrossRef]
- He, S.; Li, P.; Su, F.; Wang, D.; Ren, X. Identification and apportionment of shallow groundwater nitrate pollution in Weining Plain, northwest China, using hydrochemical indices, nitrate stable isotopes, and the new Bayesian stable isotope mixing model (MixSIAR). Environ. Pollut. 2022, 298, 118852. [Google Scholar] [CrossRef]
- Davidson Egbi, C.; Anornu, G.; Ganyaglo, S.F.; Appiah-Adjei, E.K.; Dampare, S.B.; Li, S.-L. Nitrate contamination of groundwater in the Lower Volta River Basin of Ghana: Sources and related human health risks. EGU General Assembly Conference Abstracts. Ecotoxicol. Environ. Saf. 2019, 191, 110227. [Google Scholar]
- Pei, Y.; Huang, L.; Jia, X.; Tang, X.; Zhang, Y.; Pan, Y. Water sources used by artificial Salix psammophila in stands of different ages based on stable isotope analysis in northeastern Mu Us Sandy Land. Catena 2023, 226, 107087. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Sternberg, L.S.L. Utilization of surface water by red mangrove (Rhizophora mangle L.): An isotopic study. Bull. Mar. Sci. 1994, 54, 94–102. [Google Scholar]
- Wang, H.; Li, G.; Xu, J.; Wang, W.; Jin, L. Advances in Research on Salt Tolerance Mechanism of Plants. Crops 2022, 38, 1–12. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, B.; Deng, Y.; Li, Y.; Shu, L.; Wang, W. Simulation of surface water-groundwater interaction in the plain area of the Poyang Lake considering the change of water body area. Hydrogeol. Eng. Geol. 2023, 50, 95–104. [Google Scholar]
- Wu, Y.M.; Han, L.; Liu, K.Y.; Hu, X.; Fu, Z.Q.; Chen, L.X. Water source of Robinia pseudoacacia and Platycladus orientalis plantations under different soil moisture conditions in the Loess Plateau of Western Shanxi, China. Yingyong Shengtai Xuebao 2023, 34, 588–596. [Google Scholar] [PubMed]
- Sui, M.Z.; Gao, Q.; Xu, Q.; He, D.M.; Wang, L.; Wang, T. Characteristics of hydrogen and oxygen isotopes in precipitation and moisture sources in Gaoyou, Jiangsu Province, China. Yingyong Shengtai Xuebao 2019, 30, 1823–1832. [Google Scholar]
- Wang, Y.J.; Song, X.F.; Ma, Y.; Zhang, Y.H.; Zheng, F.D.; Yang, L.H.; Bu, H.M. Characterizing the hydrogen and oxygen isotopic compositions of different waters at reclaimed water irrigated district in the southeast suburb of Beijing. Geogr. Res. 2017, 36, 361–371. [Google Scholar]
- Chen, Y.; Jiang, L.; Liang, L.; Zhou, Z. Monitoring permafrost deformation in the upstream Heihe River, Qilian Mountain by using multi-temporal Sentinel-1 InSAR dataset. Chin. J. Geophys. 2019, 62, 2441–2454. [Google Scholar] [CrossRef]
- Bögelein, R.; Thomas, F.M.; Kahmen, A. Leaf water 18O and 2H enrichment along vertical canopy profiles in a broadleaved and a conifer forest tree. Plant Cell Environ. 2017, 40, 1086–1103. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, M.; Wang, S.; Argiriou, A.A.; Chen, R.; Meng, H.; Guo, R. Water stable isotopes in an alpine setting of the Northeastern Tibetan Plateau. Water 2019, 11, 770. [Google Scholar] [CrossRef]
- Che, C.; Zhang, M.; Argiriou, A.A.; Wang, S.; Du, Q.; Zhao, P.; Ma, Z. The stable isotopic composition of different water bodies at the soil–plant–atmosphere continuum (SPAC) of the western Loess Plateau, China. Water 2019, 11, 1742. [Google Scholar] [CrossRef]
- Benstead, J.P.; March, J.G.; Fry, B.; Ewel, K.C.; Pringle, C.M. Testing IsoSource: Stable isotope analysis of a tropical fishery with diverse organic matter sources. Ecology 2006, 87, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.S.; Zhang, X.N.; Jiang, H.Y.; Bai, L.Y. Pan evaporation change and its impact on the water cycle over the upper reach of the Yangtze River. Chin. J. Geophys. 2012, 55, 2889–2897. [Google Scholar]
- Tian, F.; Miao, Q.; Shi, H.; Li, R.; Dou, X.; Duan, J.; Feng, W. SimulatingWater and Salt Migration through Soils with a Clay Layer and Subsurface Pipe Drainage System at Different Depths Using the DRAINMOD-S Model. Agronomy 2024, 14, 17. [Google Scholar] [CrossRef]
- Hou, C.; Miao, Q.; Shi, H.; Hu, Z.; Zhao, Y.; Yu, C.; Yan, Y.; Feng, W. Water and Salinity Variation along the Soil Profile and Groundwater Dynamics of a Fallow Cropland System in the Hetao Irrigation District, China. Water 2023, 15, 4098. [Google Scholar] [CrossRef]
- Feng, W.; Wang, T.; Yang, F.; Cen, R.; Liao, H.; Qu, Z. Effects of biochar on soil evaporation and moisture content and the associated mechanisms. Environ. Sci. Eur. 2023, 35, 66. [Google Scholar] [CrossRef]
- Cheng, S.; Manru, G.; Huairui, S. Advances in Research on Flavonoids in Ginkgo Biloba Leaf; Scientia Silvae: Beijing, China, 2000. [Google Scholar]
- Tian, F.; Miao, Q.; Shi, H.; Li, R.; Dou, X.; Duan, J.; Liu, J.; Feng, W. Study on Water and Salt Transport under Different Subsurface Pipe Arrangement Conditions in Severe Saline–Alkali Land in Hetao Irrigation District with DRAINMOD Model. Water 2023, 15, 3001. [Google Scholar] [CrossRef]
- Tian, F.; Shi, H.; Miao, Q.; Li, R.; Duan, J.; Dou, X.; Feng, W. Soil Water and Salt Transport in Severe Saline–Alkali Soil after Ditching under Subsurface Pipe Drainage Conditions. Agriculture 2023, 13, 2196. [Google Scholar] [CrossRef]
- Feng, Z.; Miao, Q.; Shi, H.; Feng, W.; Li, X.; Yan, J.; Liu, M.; Sun, W.; Dai, L.; Liu, J. Simulation of water balance and irrigation strategy of typical sand-layered farmland in the Hetao Irrigation District, China. Agric. Water Manag. 2023, 280, 108236. [Google Scholar] [CrossRef]
- Cen, R.; Feng, W.; Yang, F.; Wu, W.; Liao, H.; Qu, Z. Effect mechanism of biochar application on soil structure and organic matter in semi-arid areas. J. Environ. Manag. 2021, 286, 112198. [Google Scholar] [CrossRef]
- Feng, W.; Wang, T.; Zhu, Y.; Sun, F.; Giesy, J.P.; Wu, F. Chemical composition, sources, and ecological effect of organic phosphorus in water ecosystems: A review. Carbon Res. 2023, 2, 12. [Google Scholar] [CrossRef]




| Isotope | Eigenvalue | Precipitation | Soil Water | Stem Water | Groundwater |
|---|---|---|---|---|---|
| δ D (‰) | MAX | −12.16 | −65.4 | −57.12 | −77.98 |
| MIN | −134.26 | −91.33 | −78.02 | −83.43 | |
| AVG | −65.06 | −76.35 | −66.86 | −80.21 | |
| SD | 27 | 9.35 | 8.75 | 1.83 | |
| δ18O (‰) | MAX | −0.93 | 2.51 | 8.42 | −10.68 |
| MIN | −17.86 | −11.84 | −7.55 | −11.64 | |
| AVG | −9.31 | −8.59 | −1.4 | −10.98 | |
| SD | 3.45 | 2.14 | 3.05 | 0.28 |
| Soil Depth (cm) | May | June | July | August | September | October |
|---|---|---|---|---|---|---|
| 0–20 | 5.41 | 7.52 | 10.70 | 9.04 | 8.86 | 7.70 |
| 20–40 | 5.34 | 5.50 | 7.85 | 7.68 | 7.37 | 6.98 |
| 40–60 | 5.26 | 6.03 | 6.16 | 7.58 | 7.12 | 6.19 |
| 60–80 | 9.43 | 10.19 | 8.48 | 8.93 | 9.21 | 9.57 |
| 80–120 | 9.64 | 10.30 | 9.07 | 10.65 | 13.43 | 10.93 |
| 120–160 | 14.79 | 16.04 | 13.12 | 15.45 | 16.17 | 15.78 |
| 160–200 | 13.82 | 14.31 | 15.30 | 16.69 | 14.15 | 11.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, M.; Jia, D.; Miao, Q.; Hao, Y.; Chen, S.; Liu, T.; Yang, L.; Li, X.; Feng, W. Spatiotemporal Absorption Features of Yellow Willow Water Usage on the Southern Edge of the Semi-Arid Hunshandak Sandland in China. Water 2024, 16, 827. https://doi.org/10.3390/w16060827
Ji M, Jia D, Miao Q, Hao Y, Chen S, Liu T, Yang L, Li X, Feng W. Spatiotemporal Absorption Features of Yellow Willow Water Usage on the Southern Edge of the Semi-Arid Hunshandak Sandland in China. Water. 2024; 16(6):827. https://doi.org/10.3390/w16060827
Chicago/Turabian StyleJi, Mingyu, Debin Jia, Qingfeng Miao, Yusheng Hao, Shuling Chen, Ting Liu, Lina Yang, Xiaoyan Li, and Weiying Feng. 2024. "Spatiotemporal Absorption Features of Yellow Willow Water Usage on the Southern Edge of the Semi-Arid Hunshandak Sandland in China" Water 16, no. 6: 827. https://doi.org/10.3390/w16060827





