Landscape-Scale Mining and Water Management in a Hyper-Arid Catchment: The Cuajone Mine, Moquegua, Southern Peru
Abstract
:1. Introduction

2. Materials and Methods
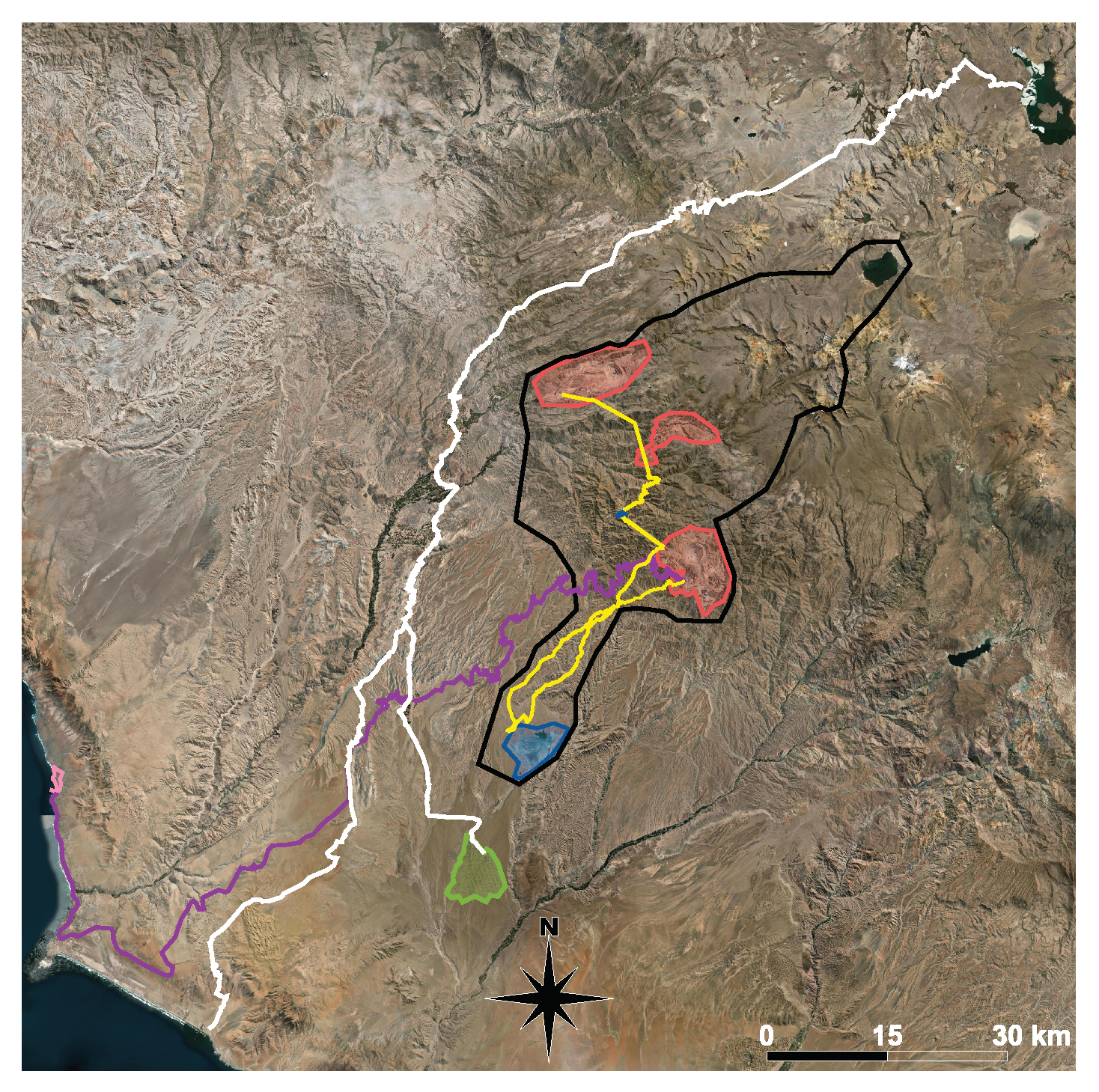
2.1. Study Area
2.2. Study Sites
2.3. Sample Seasons
2.3.1. Water Quality Parameters and River Data Collection
2.3.2. Water Sample Collection
2.4. Analysis of Water Samples
2.5. Statistical Analysis
2.6. Contextual Materials and Analyses
3. Results
3.1. Water Quality Parameters and Major Ion Composition
3.1.1. Headwater Sites above the Cuajone Mine (Sites 16, 17, 18, 19, 20)
3.1.2. Foothill Sites below the Cuajone Mine (Sites 5B, 5, 4, 3, 2, 1B, 1C, 1D, 1E)
3.1.3. Sites Downstream of Moquegua City (Sites 1 and 0A)
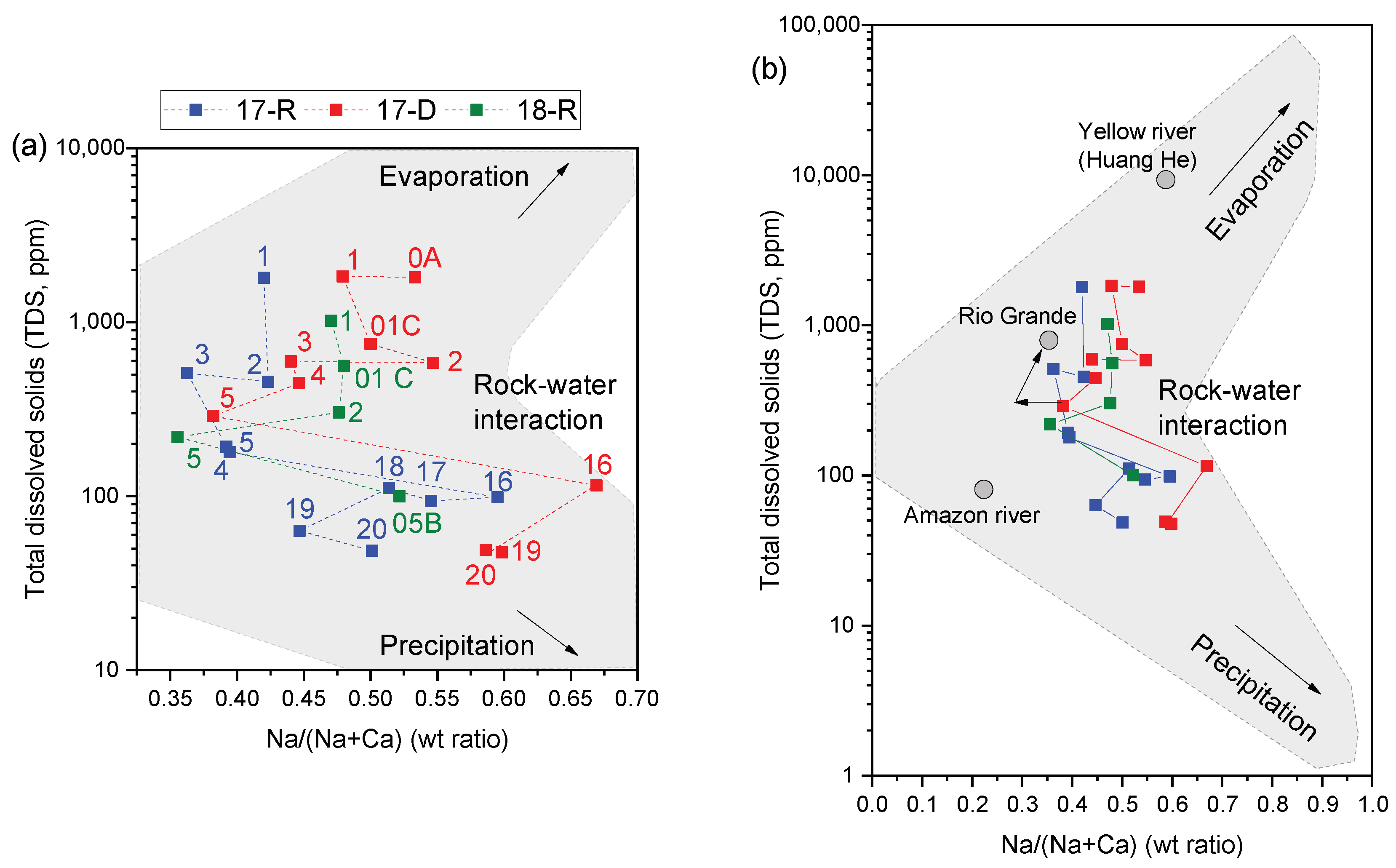
3.1.4. Endmember Controls on Water Chemistry in the Moquegua River Catchment
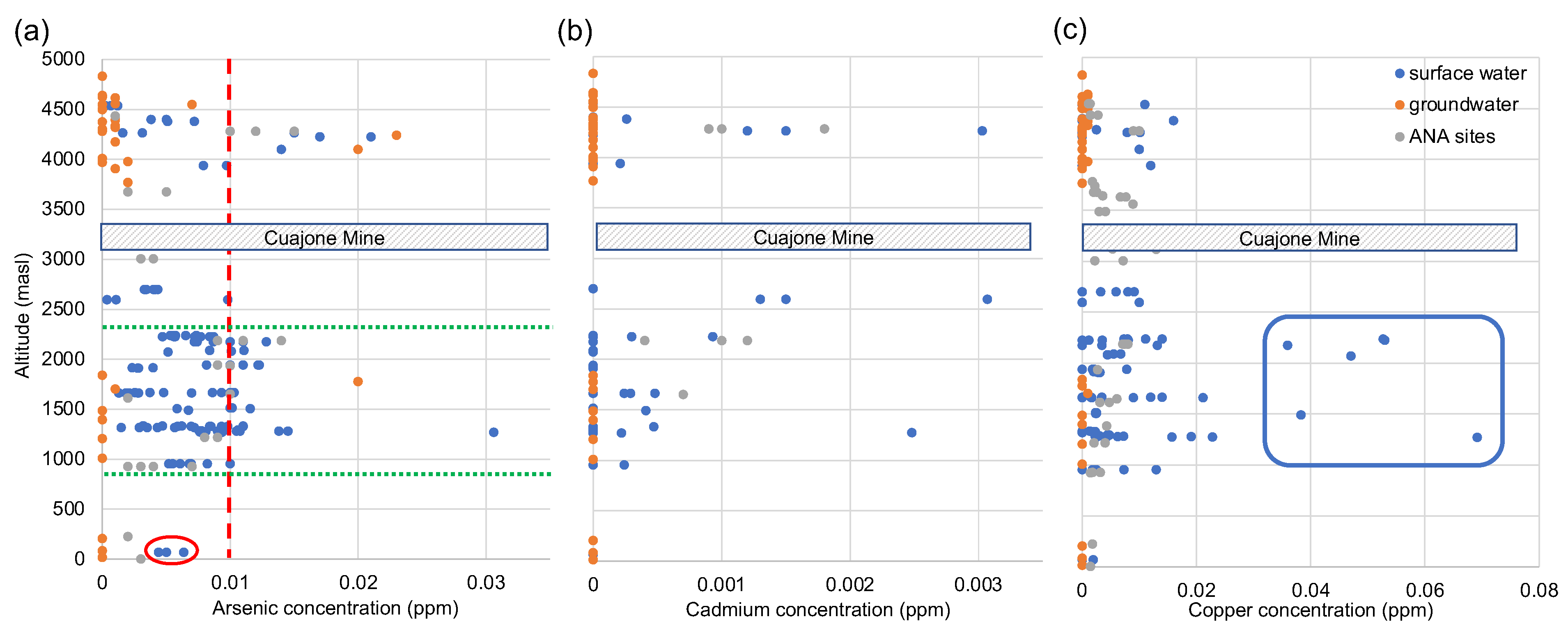
3.2. Trace Metals in the Moquegua River System
3.3. Arsenic in the Torata and Lower Moquegua River System
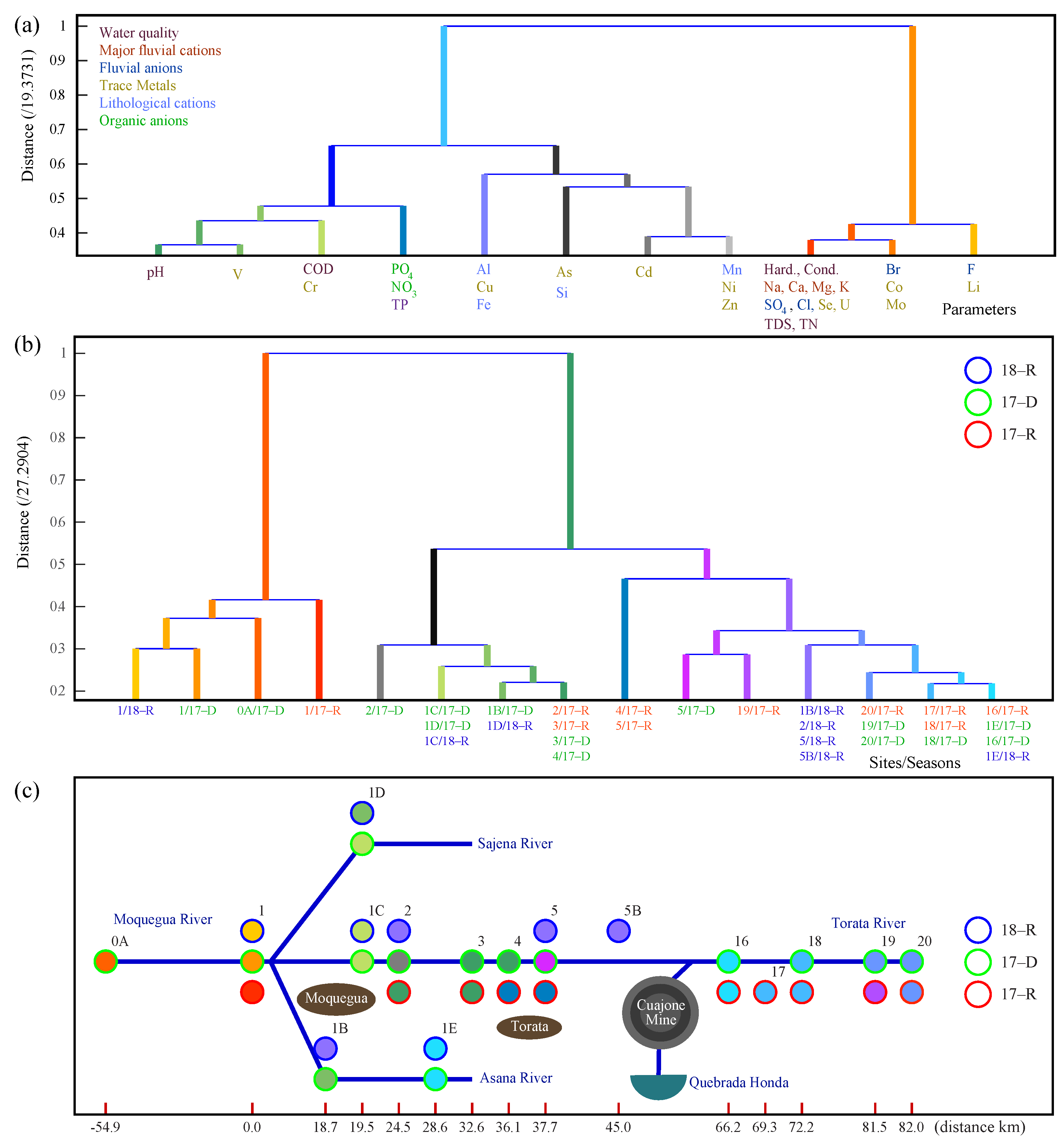
3.4. Multivariate Analysis of Water Parameters
3.4.1. Headwater Sites
3.4.2. Foothill Sites
3.4.3. Lowland Sites
3.5. Analysis of ANA and INGEMMET Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOAJ | Directory of Open Access Journals |
| ARD | Acid rock drainage |
| AMD | Acid mine drainage |
| EIS | Environmental impact statements |
| masl | Meters above sea level |
| 17-R | January 2017 |
| 17-D | July 2017 |
| 18-R | January 2018 |
| UNAM | Universidad Nacional Autonoma de Moquegua |
| GPS | Global Positioning System |
| DO | Dissolved oxygen |
| TDS | Total dissolved solids |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| ICP-OES | Inductively coupled plasma - optical emission spectrometry |
| CN | Cyanide |
| TP | Total phosphorous |
| TN | Total nitrogen |
| COD | Chemical oxygen demand |
| ENSO | El Niño–Southern Oscillation |
| Ma | Millions of years |
| CA | Cluster analysis |
| PCA | Principal component analysis |
| PC | Principal component |
| EPA | Environmental Protection Agency |
| ISO | International Organization for Standardization |
| CCC | Cophenetic correlation coefficient |
| EC | Electrical conductivity |
| USEPA | United States Environmental Protection Agency |
| WHO | World Health Organization |
| FAO | Food and Agriculture Organization of the United Nations |
Appendix A. Waste Canal from the Cuajone Mine
References
- Ling, H.; Zhang, P.; Xu, H.; Zhang, G. Determining the ecological water allocation in a hyper-arid catchment with increasing competition for water resources. Glob. Planet. Chang. 2016, 145, 143–152. [Google Scholar] [CrossRef]
- Houston, J.; Hartley, A.J. The central Andean west-slope rainshadow and its potential contribution to the origin of hyper-aridity in the Atacama Desert. Int. J. Climatol. A J. R. Meteorol. Soc. 2003, 23, 1453–1464. [Google Scholar] [CrossRef]
- Villalobos-Puma, E.; Flores-Rojas, J.L.; Martinez-Castro, D.; Morales, A.; Lavado-Casimiro, W.; Mosquera-Vásquez, K.; Silva, Y. Summertime precipitation extremes and the influence of atmospheric flows on the western slopes of the southern Andes of Perú. Int. J. Climatol. 2022, 42, 9909–9930. [Google Scholar] [CrossRef]
- Zarch, M.A.A.; Sivakumar, B.; Malekinezhad, H.; Sharma, A. Future aridity under conditions of global climate change. J. Hydrol. 2017, 554, 451–469. [Google Scholar] [CrossRef]
- Ng Cutipa, W.L.; Peña Laureano, F.; Acosta Pereira, H. Hidrogeología de la Cuenca del río Ilo-Moquegua (13172), Región Moquegua [Boletín–H 6]; Repositorio Ingemmet: Lima, Peru, 2019. [Google Scholar]
- Brêda, J.P.L.; de Paiva, R.C.D.; Siqueira, V.A.; Collischonn, W. Assessing climate change impact on flood discharge in South America and the influence of its main drivers. J. Hydrol. 2023, 619, 129284. [Google Scholar] [CrossRef]
- Clark, A.H.; Farrar, E.; Kontak, D.J.; Langridge, R.J.; Arenas F, M.J.; France, L.J.; McBride, S.L.; Woodman, P.L.; Wasteneys, H.A.; Sandeman, H.A.; et al. Geologic and geochronologic constraints on the metallogenic evolution of the Andes of southeastern Peru. Econ. Geol. 1990, 85, 1520–1583. [Google Scholar] [CrossRef]
- Fraser, B. Water wars come to the Andes. Sci. Am. 2009, 19, 1–3. [Google Scholar]
- Reich, M.; Palacios, C.; Vargas, G.; Luo, S.; Cameron, E.M.; Leybourne, M.I.; Parada, M.A.; Zúñiga, A.; You, C.F. Supergene enrichment of copper deposits since the onset of modern hyperaridity in the Atacama Desert, Chile. Miner. Depos. 2009, 44, 497–504. [Google Scholar] [CrossRef]
- Lepage, H.V.; Barnes, E.; Kor, E.; Hunter, M.; Barnes, C.H. Greening and Browning Trends on the Pacific Slope of Peru and Northern Chile. Remote Sens. 2023, 15, 3628. [Google Scholar] [CrossRef]
- Bebbington, A.; Williams, M. Water and mining conflicts in Peru. Mt. Res. Dev. 2008, 28, 190–195. [Google Scholar] [CrossRef]
- Saenz, C. Stakeholders, social and environmental impact management as key factors of the corporate social management in the mining industry: A Peruvian case study. Corp. Soc. Responsib. Environ. Manag. 2024, 31, 697–708. [Google Scholar] [CrossRef]
- Saenz, C. Enhancing community development management and the management of social and environmental impacts to get social license to operate in the mining industry: A Peruvian case study. Bus. Strategy Dev. 2023, 6, 873–884. [Google Scholar] [CrossRef]
- Wood Group USA Inc. Cuajone Operations Peru Technical Report Summary. 2023. Available online: https://minedocs.com/24/Cuajone-TR-12312022.pdf (accessed on 12 February 2024).
- Ali, M.M.; Ali, M.L.; Islam, M.S.; Rahman, M.Z. Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2016, 5, 27–35. [Google Scholar] [CrossRef]
- Varol, M.; Şen, B. Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. Catena 2012, 92, 1–10. [Google Scholar] [CrossRef]
- Budds, J.; Hinojosa, L. Restructuring and rescaling water governance in mining contexts: The co-production of waterscapes in Peru. Water Altern. 2012, 5, 119. [Google Scholar]
- Han, Y.S.; Youm, S.J.; Oh, C.; Cho, Y.C.; Ahn, J.S. Geochemical and eco-toxicological characteristics of stream water and its sediments affected by acid mine drainage. Catena 2017, 148, 52–59. [Google Scholar] [CrossRef]
- Tume, P.; González, E.; Reyes, F.; Fuentes, J.P.; Roca, N.; Bech, J.; Medina, G. Sources analysis and health risk assessment of trace elements in urban soils of Hualpen, Chile. Catena 2019, 175, 304–316. [Google Scholar] [CrossRef]
- Trudgill, S.T.; Goudie, A.S.; Viles, H.A. Weathering Processes and Forms; Geological Society: London, UK, 2022. [Google Scholar]
- Visitación-Bustamante, K.; Ramos-Fernandez, L.; Visitación-Figueroa, L. Characterization of the hydrochemistry in a high Andean sub-basin in the region of Moquegua, Peru. Tecnol. Y Cienc. Agua 2023, 14, 257–289. [Google Scholar] [CrossRef]
- Olías, M.; Nieto, J.M.; Pérez-López, R.; Cánovas, C.R.; Macías, F.; Sarmiento, A.M.; Galván, L. Controls on acid mine water composition from the Iberian Pyrite Belt (SW Spain). Catena 2016, 137, 12–23. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Rey, J.; Martínez, J.; Hidalgo, M.; Rojas, D. Heavy metal pollution in the Quaternary Garza basin: A multidisciplinary study of the environmental risks posed by mining (Linares, Southern Spain). Catena 2013, 110, 234–242. [Google Scholar] [CrossRef]
- Martin, C.W. Trace metal concentrations along tributary streams of historically mined areas, Lower Lahn and Dill River basins, central Germany. Catena 2019, 174, 174–183. [Google Scholar] [CrossRef]
- Punia, A. Role of temperature, wind, and precipitation in heavy metal contamination at copper mines: A review. Environ. Sci. Pollut. Res. 2021, 28, 4056–4072. [Google Scholar] [CrossRef]
- Sun, T.; Bao, H.; Reich, M.; Hemming, S.R. More than ten million years of hyper-aridity recorded in the Atacama Gravels. Geochim. Cosmochim. Acta 2018, 227, 123–132. [Google Scholar] [CrossRef]
- Colica, A.; Benvenuti, M.; Chiarantini, L.; Costagliola, P.; Lattanzi, P.; Rimondi, V.; Rinaldi, M. From point source to diffuse source of contaminants: The example of mercury dispersion in the Paglia River (Central Italy). Catena 2019, 172, 488–500. [Google Scholar] [CrossRef]
- Shaban, N.S.; Abdou, K.A.; Hassan, N.E.H.Y. Impact of toxic heavy metals and pesticide residues in herbal products. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 102–106. [Google Scholar] [CrossRef]
- Davies, B.R.; Thoms, M.; Meador, M. An assessment of the ecological impacts of inter-basin water transfers, and their threats to river basin integrity and conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 1992, 2, 325–349. [Google Scholar] [CrossRef]
- Li, F. Documenting accountability: Environmental impact assessment in a Peruvian mining project. PoLAR Political Leg. Anthropol. Rev. 2009, 32, 218–236. [Google Scholar] [CrossRef]
- Bebbington, A.J.; Bury, J.T. Institutional challenges for mining and sustainability in Peru. Proc. Natl. Acad. Sci. USA 2009, 106, 17296–17301. [Google Scholar] [CrossRef]
- Samimi Namin, F.; Shahriar, K.; Bascetin, A. Environmental impact assessment of mining activities. A new approach for mining methods selection. Gospod. Surowcami Miner. 2011, 27, 113–143. [Google Scholar]
- Gwimbi, P.; Nhamo, G. Benchmarking the effectiveness of mitigation measures to the quality of environmental impact statements: Lessons and insights from mines along the Great Dyke of Zimbabwe. Environ. Dev. Sustain. 2016, 18, 527–546. [Google Scholar] [CrossRef]
- Brodny, J.; Tutak, M. The use of artificial neural networks to analyze greenhouse gas and air pollutant emissions from the mining and quarrying sector in the European Union. Energies 2020, 13, 1925. [Google Scholar] [CrossRef]
- Magilligan, F.J.; Goldstein, P.S.; Fisher, G.B.; Bostick, B.C.; Manners, R.B. Late Quaternary hydroclimatology of a hyper-arid Andean watershed: Climate change, floods, and hydrologic responses to the El Niño-Southern Oscillation in the Atacama Desert. Geomorphology 2008, 101, 14–32. [Google Scholar] [CrossRef]
- Santoso, A.; Mcphaden, M.J.; Cai, W. The defining characteristics of ENSO extremes and the strong 2015/2016 El Niño. Rev. Geophys. 2017, 55, 1079–1129. [Google Scholar] [CrossRef]
- Decou, A.; Von Eynatten, H.; Mamani, M.; Sempere, T.; Wörner, G. Cenozoic forearc basin sediments in Southern Peru (15–18 S): Stratigraphic and heavy mineral constraints for Eocene to Miocene evolution of the Central Andes. Sediment. Geol. 2011, 237, 55–72. [Google Scholar] [CrossRef]
- Oerter, E.; Amundson, R.; Heimsath, A.; Jungers, M.; Chong, G.; Renne, P. Early to middle Miocene climate in the Atacama Desert of northern Chile. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 441, 890–900. [Google Scholar] [CrossRef]
- Poveda, G.; Espinoza, J.C.; Zuluaga, M.D.; Solman, S.A.; Garreaud, R.; Van Oevelen, P.J. High impact weather events in the Andes. Front. Earth Sci. 2020, 8, 162. [Google Scholar] [CrossRef]
- Clark, A.H.; Tosdal, R.M.; Farrar, E.; Plazolles V, A. Geomorphologic environment and age of supergene enrichment of the Cuajone, Quellaveco, and Toquepala porphyry copper deposits, southeastern Peru. Econ. Geol. 1990, 85, 1604–1628. [Google Scholar] [CrossRef]
- Masuno, R.K.; Barahona, H.P.A.; Bazán, E.F.; Zenteno, E.G.; Lizárraga, A.M.; Paucara, J.T. Evaluacion de Peligros de la Ciudad de Moquegua; INDECI: Arequipa, Peru, 2001. [Google Scholar]
- Clesceri, L.S. Standard Methods for Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998; Volume 9. [Google Scholar]
- Otto, M. Multivariate methods. In Analytical Chemistry; Kellner, R., Mermet, J.M., Otto, M., Widmer, H.M., Eds.; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Kazi, T.; Arain, M.; Jamali, M.K.; Jalbani, N.; Afridi, H.; Sarfraz, R.; Baig, J.; Shah, A.Q. Assessment of water quality of polluted lake using multivariate statistical techniques: A case study. Ecotoxicol. Environ. Saf. 2009, 72, 301–309. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.; Islam, M.; Dampare, S.B.; Parvez, L.; Suzuki, S. Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J. Hazard. Mater. 2010, 179, 1065–1077. [Google Scholar] [CrossRef]
- Jolliffe, I.; Lovric, M. International encyclopedia of statistical science. In Principa l Component Analysis; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1094–1096. [Google Scholar]
- Wurts, W.A.; Durborow, R.M. Interactions of pH, Carbon Dioxide, Alkalinity and Hardness in Fish Ponds; SRAC Publication: Stoneville, MS, USA, 1992. [Google Scholar]
- Talling, J. The depletion of carbon dioxide from lake water by phytoplankton. J. Ecol. 1976, 64, 79–121. [Google Scholar] [CrossRef]
- Möller, D. The Na/Cl ratio in rainwater and the seasalt chloride cycle. Tellus B 1990, 42, 254–262. [Google Scholar] [CrossRef]
- Magaritz, M.; Nadler, A.; Koyumdjisky, H.; Dan, J. The use of Na/Cl ratios to trace solute sources in a semiarid zone. Water Resour. Res. 1981, 17, 602–608. [Google Scholar] [CrossRef]
- Gorchev, H.G.; Ozolins, G. WHO Guidelines for Drinking-Water Quality; WHO Chronicle: Washington, DC, USA, 1984; Volume 38. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Kalin, M.; Fyson, A.; Wheeler, W.N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Sci. Total Environ. 2006, 366, 395–408. [Google Scholar] [CrossRef]
- Sheoran, A.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Rösner, U. Effects of historical mining activities on surface water and groundwater-an example from northwest Arizona. Environ. Geol. 1998, 33, 224–230. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.A.; Schell, C.; Macklin, M.G. Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Appl. Geochem. 1999, 14, 1015–1030. [Google Scholar] [CrossRef]
- Miller, J.; Hudson-Edwards, K.; Lechler, P.; Preston, D.; Macklin, M. Heavy metal contamination of water, soil and produce within riverine communities of the Rıo Pilcomayo basin, Bolivia. Sci. Total Environ. 2004, 320, 189–209. [Google Scholar] [CrossRef]
- Pan, Y.; Koopmans, G.F.; Bonten, L.T.; Song, J.; Luo, Y.; Temminghoff, E.J.; Comans, R.N. Influence of pH on the redox chemistry of metal (hydr) oxides and organic matter in paddy soils. J. Soils Sediments 2014, 14, 1713–1726. [Google Scholar] [CrossRef]
- Canfield, D.E. The geochemistry of river particulates from the continental USA: Major elements. Geochim. Cosmochim. Acta 1997, 61, 3349–3365. [Google Scholar] [CrossRef]
- Tipping, E.; Rey-Castro, C.; Bryan, S.E.; Hamilton-Taylor, J. Al (III) and Fe (III) binding by humic substances in freshwaters, and implications for trace metal speciation. Geochim. Cosmochim. Acta 2002, 66, 3211–3224. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Sci. Total Environ. 2009, 407, 853–868. [Google Scholar] [CrossRef]
- McPhaden, M.J.; Santoso, A.; Cai, W. Introduction to El Niño Southern Oscillation in a changing climate. In El Niño Southern Oscillation in a Changing Climate; American Geophysical Union: Washington, DC, USA, 2020; pp. 1–19. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations Rome: Rome, Italy, 1985; Volume 29. [Google Scholar]
- ANA. Modifican los Estándares Nacionales de Calidad Ambiental para Agua y establecen disposiciones complementarias para su aplicación. In Decreto Supremo, 015–2015–MINAM; Ministerio del Ambiente: Lima, Peru, 2015. [Google Scholar]
- Poshtegal, M.K.; Mirbagheri, S.A. The heavy metals pollution index and water quality monitoring of the Zarrineh river, Iran. Environ. Eng. Geosci. 2019, 25, 179–188. [Google Scholar] [CrossRef]
- Harrison, R.M.; De Mora, S.J. Introductory Chemistry for the Environmental Sciences; Cambridge University Press: Cambridge, UK, 1996; Volume 7. [Google Scholar]
- Xiao, J.; Jin, Z.D.; Zhang, F.; Wang, J. Solute geochemistry and its sources of the groundwaters in the Qinghai Lake catchment, NW China. J. Asian Earth Sci. 2012, 52, 21–30. [Google Scholar] [CrossRef]
- MINAGRI Peru. Boletín Estadístico de Medios de Producción Agropecuarios. Available online: https://www.midagri.gob.pe/portal/boletin-estadistico-de-medios-de-produccion-agropecuarios (accessed on 20 January 2024).
- Borrok, D.M.; Engle, M.A. The role of climate in increasing salt loads in dryland rivers. J. Arid Environ. 2014, 111, 7–13. [Google Scholar] [CrossRef]
- LaZerte, B.D.; Burling, K. Manganese speciation in dilute waters of the Precambrian Shield, Canada. Water Res. 1990, 24, 1097–1101. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rammohan, V.; Sahayam, J.D.; Jeevanandam, M. Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ. Monit. Assess. 2009, 159, 341–351. [Google Scholar] [CrossRef]
- US EPA. National Primary Drinking Water Regulations; Technical Fact; 2019. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 20 January 2024).
- Vera Delgado, J. The socio-cultural, institutional and gender aspects of the water transfer-agribusiness model for food and water security. Lessons learned from Peru. Food Secur. 2015, 7, 1187–1197. [Google Scholar] [CrossRef]
- Tessier, A.; Fortin, D.; Belzile, N.; DeVitre, R.; Leppard, G. Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: Narrowing the gap between field and laboratory measurements. Geochim. Cosmochim. Acta 1996, 60, 387–404. [Google Scholar] [CrossRef]
- Pagenkopf, G.K. Gill surface interaction model for trace-metal toxicity to fishes: Role of complexation, pH, and water hardness. Environ. Sci. Technol. 1983, 17, 342–347. [Google Scholar] [CrossRef]
- Pascoe, D.; Evans, S.A.; Woodworth, J. Heavy metal toxicity to fish and the influence of water hardness. Arch. Environ. Contam. Toxicol. 1986, 15, 481–487. [Google Scholar] [CrossRef]
- Zitko, V.; Carson, W. Mechanism of the effects of water hardness on the lethality of heavy metals to fish. Chemosphere 1976, 5, 299–303. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.I.; Parvez, F.; Román-Ross, G.; Nicolli, H.B.; Jean, J.S.; Liu, C.W.; López, D.; Armienta, M.A.; Guilherme, L.R.; et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci. Total Environ. 2012, 429, 2–35. [Google Scholar] [CrossRef]
- Schwartz, M.O. Arsenic in porphyry copper deposits: Economic geology of a polluting element. Int. Geol. Rev. 1995, 37, 9–25. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Cameron, E.M. Source, transport, and fate of rhenium, selenium, molybdenum, arsenic, and copper in groundwater associated with porphyry–Cu deposits, Atacama Desert, Chile. Chem. Geol. 2008, 247, 208–228. [Google Scholar] [CrossRef]
- Byrne, P.; Reid, I.; Wood, P.J. Sediment geochemistry of streams draining abandoned lead/zinc mines in central Wales: The Afon Twymyn. J. Soils Sediments 2010, 10, 683–697. [Google Scholar] [CrossRef]
- Byrne, P.; Taylor, K.G.; Hudson-Edwards, K.A.; Barrett, J.E. Speciation and potential long-term behaviour of chromium in urban sediment particulates. J. Soils Sediments 2017, 17, 2666–2676. [Google Scholar] [CrossRef]
- de agua Moquegua, A.L. Informe del Tercer 1. Monitoreo Participativo de Calidad de Agua Superficial de la Cuenca Moquegua-Ilo. 2013. Available online: https://hdl.handle.net/20.500.12543/2771 (accessed on 20 January 2024).
- de agua Moquegua, A.L. Informe del Cuarto Monitoreo Participativo de Calidad de Agua Superficial de la Cuenca MOQUEGUA-Ilo. 2014. Available online: https://hdl.handle.net/20.500.12543/2830 (accessed on 20 January 2024).
- de agua Moquegua, A.L. Quinto Monitoreo Participativo de Calidad de Agua Superficial de la Cuenca Moquegua-Ilo. 2014. Available online: https://hdl.handle.net/20.500.12543/2834 (accessed on 20 January 2024).
- Pincetti-Zúniga, G.; Richards, L.; Daniele, L.; Boyce, A.; Polya, D. Hydrochemical characterization, spatial distribution, and geochemical controls on arsenic and boron in waters from arid Arica and Parinacota, northern Chile. Sci. Total Environ. 2022, 806, 150206. [Google Scholar] [CrossRef]
- Yin, S.; Yang, L.; Wen, Q.; Wei, B. Temporal variation and mechanism of the geogenic arsenic concentrations in global groundwater. Appl. Geochem. 2022, 146, 105475. [Google Scholar] [CrossRef]
- Forbes, W.F.; Hill, G.B. Is exposure to aluminum a risk factor for the development of Alzheimer disease?—Yes. Arch. Neurol. 1998, 55, 740–741. [Google Scholar] [CrossRef]
- Flaten, T.P. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res. Bull. 2001, 55, 187–196. [Google Scholar] [CrossRef]
- Smith, A.H.; Hopenhayn-Rich, C.; Bates, M.N.; Goeden, H.M.; Hertz-Picciotto, I.; Duggan, H.M.; Wood, R.; Kosnett, M.J.; Smith, M.T. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992, 97, 259–267. [Google Scholar] [CrossRef]
- Davis, M.A.; Signes-Pastor, A.J.; Argos, M.; Slaughter, F.; Pendergrast, C.; Punshon, T.; Gossai, A.; Ahsan, H.; Karagas, M.R. Assessment of human dietary exposure to arsenic through rice. Sci. Total Environ. 2017, 586, 1237–1244. [Google Scholar] [CrossRef]
- Insel, N.; Poulsen, C.J.; Ehlers, T.A. Influence of the Andes Mountains on South American moisture transport, convection, and precipitation. Clim. Dyn. 2010, 35, 1477–1492. [Google Scholar] [CrossRef]
- Taylor, M.P.; Kesterton, R.G. Heavy metal contamination of an arid river environment: Gruben River, Namibia. Geomorphology 2002, 42, 311–327. [Google Scholar] [CrossRef]
- Arakaki, M.; Cano, A. Floral composition of the Ilo-Moquegua and Lomas de Ilo river basin, Moquegua, Peru. Peruv. J. Biol. 2003, 10, 5–19. [Google Scholar] [CrossRef]






| Site | Site Name | GPS Coordinates | Altitude (m asl) | Distance from Site 1 (km) | |
|---|---|---|---|---|---|
| Sites on Moquegua river (Osmore) | |||||
| 0A | Lower Moquegua valley—El Algarrobal | 17 S | 71 W | 68 | 45.8 |
| 1 | Middle Moquegua valley—El Conde | 17 S | 70 W | 955 | 0 |
| Sites on Torata river | |||||
| 1C | Above Sajena–Torata river confluence | 17 S | 70 W | 1330 | 19.5 |
| 2 | Puente Estuquina—beginning of agriculture area | 17 S | 70 W | 1495 | 24.5 |
| 3 | Puente Coplay—end of agriculture area | 17 S | 70 W | 1941 | 32.6 |
| 4 | Puente Canilay—downstream of Torata settlement | 17 S | 70 W | 2175 | 36.1 |
| 5 | Fire station—beginning of agriculture area and upstream of Torata settlement | 17 S | 70 W | 2225 | 37.7 |
| 5B | Middle Torata valley—the highest downstream of Cuajone mine | 17 S | 70 W | 2700 | 45 |
| 16 | Upper Torata valley—Lowest upstream of Cuajone mine | 16 S | 70 W | 3939 | 66.2 |
| 17 | Upper Torata valley—Middle | 16 S | 70 W | 4100 | 69.3 |
| 18 | Upper Torata valley—Highest | 16 S | 70 W | 4225 | 72.2 |
| 19 | Bofedale.at Torata river | 16 S | 70 W | 4380 | 81.5 |
| 20 | Torata river source | 16 S | 70 W | 4400 | 82 |
| Sites on Moquegua river (Asana river) | |||||
| 1B | Below Moquegua city. | 17 S | 70 W | 1318 | 18.7 |
| 1E | Above Moquegua and Samegua. | 17 S | 70 W | 1662 | 28.6 |
| Sites on Sajena/Otora river (Huaracane river) | |||||
| 1D | Before Sajena–Torata confluence | 17 S | 70 W | 1440 | 19.5 |
| Parameter | Min–Max | Parameter | Min–Max | Parameter | Min–Max |
|---|---|---|---|---|---|
| (mg/L) | 11–490 | (mg/L) | 13.4–350 | K (mg/L) | 2.1–11 |
| (mg/L) | 0–0.486 | Hard. (mg/L ) | 17.3–764.3 | Li (μg/L) | 0–170 |
| (mg/L) | 0–19.65 | Al (mg/L) | 0–2.0 | Mg (mg/L) | 1.8–36 |
| (mg/L) | 0–0.57 | As (μg/L) | 2.1–21.0 | Mn (mg/L) | 0–0.65 |
| (mg/L) | 0–0 | Be (μg/L) | 0–0.4 | Mo (μg/L) | 0.56–8.3 |
| CN Total (mg/L) | 0–0 | Bi (mg/L) | 0–0 | Na (mg/L) | 4.9–240 |
| TP (mg/L as P) | 0–0.41 | Br (mg/L) | 0–0.82 | Ni (μg/L) | 0–26 |
| TN (mg/L) | 0–8.1 | Ca (mg/L) | 4.5–251.8 | Pb (μg/L) | 0–3.9 |
| pH | 7.63–9.45 | Cd (μg/L) | 0–0.93 | Se (μg/L) | 0–4.8 |
| Cond. (mS/cm) | 95.4–2711.4 | Cl (mg/L) | 0–360.3 | Si (mg/L) | 2.5–25 |
| TDS (mg/L) | 0–1833 | Cr (μg/L) | 0–3.4 | Te (mg/L) | 0–0 |
| Temp. (°C) | 3.8–29.2 | Co (μg/L) | 0–4 | Th (μg/L) | 0–0.029 |
| DO (mg/L) | 0–12.4 | Cu (μg/L) | 0–53.0 | U (μg/L) | 0–13 |
| COD | 0–27.85 | F (mg/L) | 0–0.49 | V (μg/L) | 1.9–11 |
| Fe (mg/L) | 0–1.5 | Zn (μg/L) | 0–55.0 | ||
| Hg (mg/L) | 0–0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunter, M.; Perera, D.H.N.; Barnes, E.P.G.; Lepage, H.V.; Escobedo-Pacheco, E.; Idros, N.; Arvidsson-Shukur, D.; Newton, P.J.; de los Santos Valladares, L.; Byrne, P.A.; et al. Landscape-Scale Mining and Water Management in a Hyper-Arid Catchment: The Cuajone Mine, Moquegua, Southern Peru. Water 2024, 16, 769. https://doi.org/10.3390/w16050769
Hunter M, Perera DHN, Barnes EPG, Lepage HV, Escobedo-Pacheco E, Idros N, Arvidsson-Shukur D, Newton PJ, de los Santos Valladares L, Byrne PA, et al. Landscape-Scale Mining and Water Management in a Hyper-Arid Catchment: The Cuajone Mine, Moquegua, Southern Peru. Water. 2024; 16(5):769. https://doi.org/10.3390/w16050769
Chicago/Turabian StyleHunter, Morag, D. H. Nimalika Perera, Eustace P. G. Barnes, Hugo V. Lepage, Elias Escobedo-Pacheco, Noorhayati Idros, David Arvidsson-Shukur, Peter J. Newton, Luis de los Santos Valladares, Patrick A. Byrne, and et al. 2024. "Landscape-Scale Mining and Water Management in a Hyper-Arid Catchment: The Cuajone Mine, Moquegua, Southern Peru" Water 16, no. 5: 769. https://doi.org/10.3390/w16050769






