Constructing Z-Scheme 3D WO3@Co2SnO4 Heterojunction as Dual-Photocathode for Production of H2O2 and In-Situ Degradation of Organic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of WO3 Nanobricks
2.2. Synthesis of WO3@Co2SnO4 Composites
2.3. Preparation of Photocathode
2.4. Characterization
2.5. PEC H2O2 Production
2.6. PEC Degradation of Contaminations
3. Results
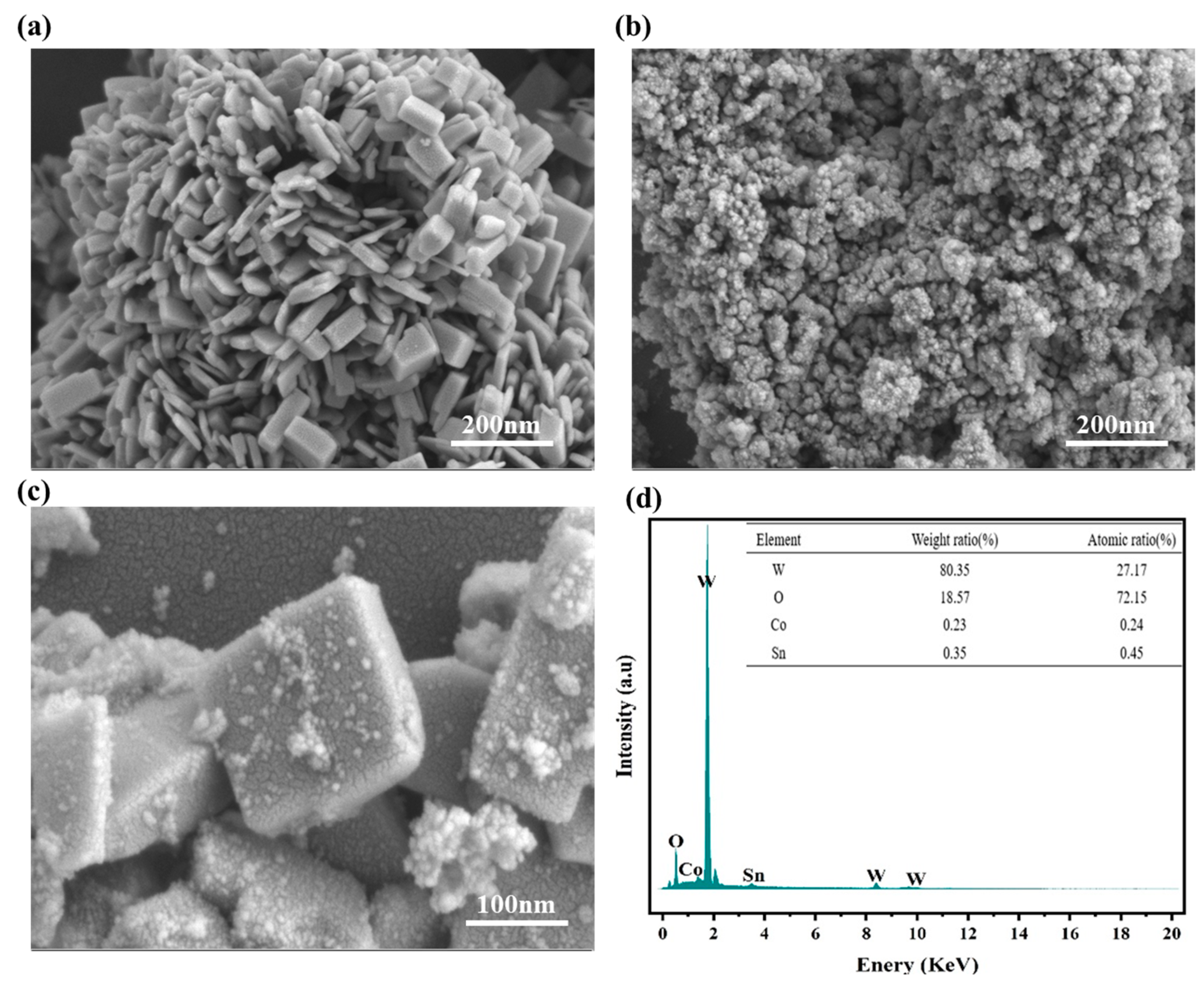
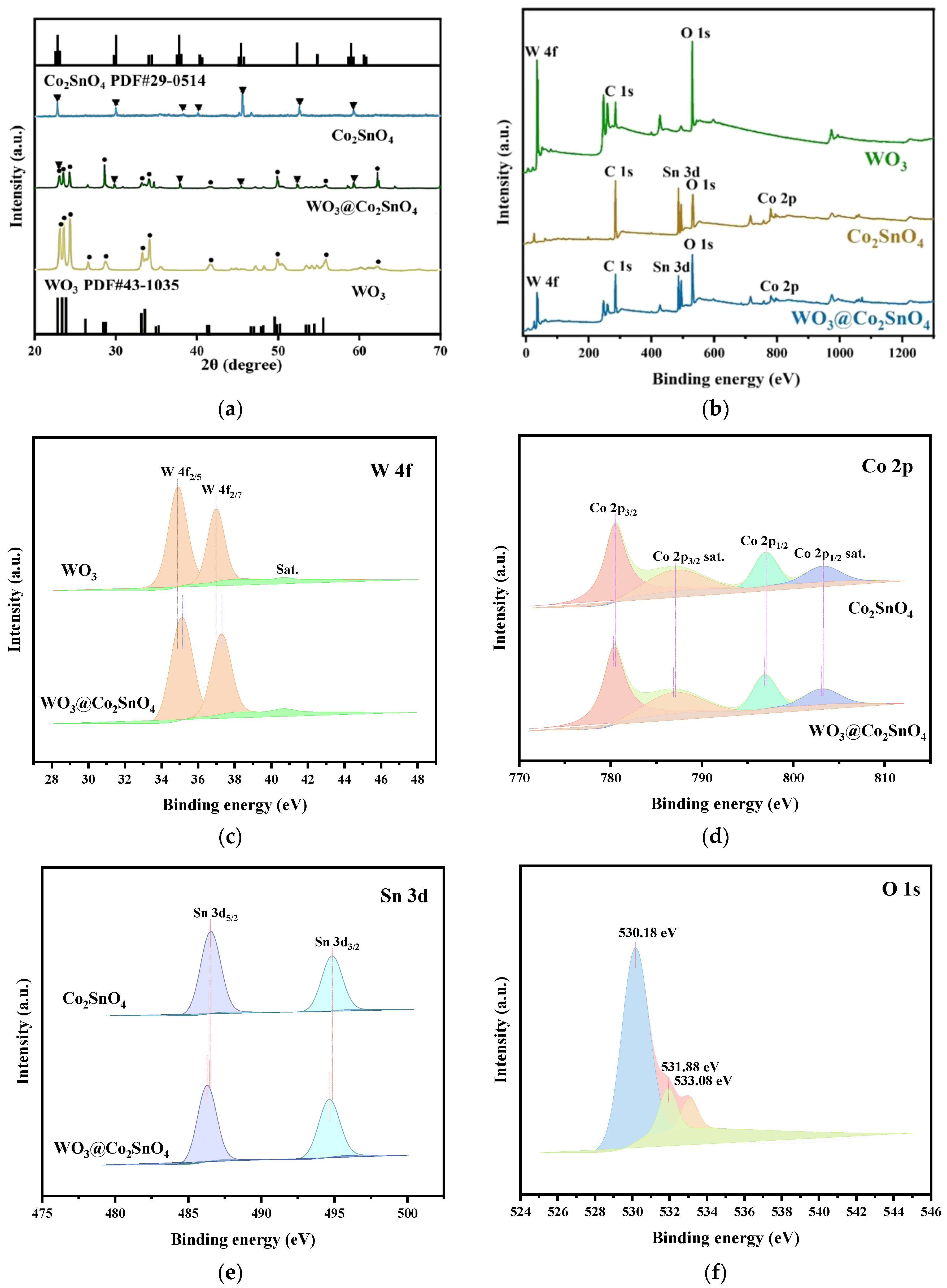
3.1. Characteristic of WO3@Co2SnO4 Composite
3.2. PEC Performances
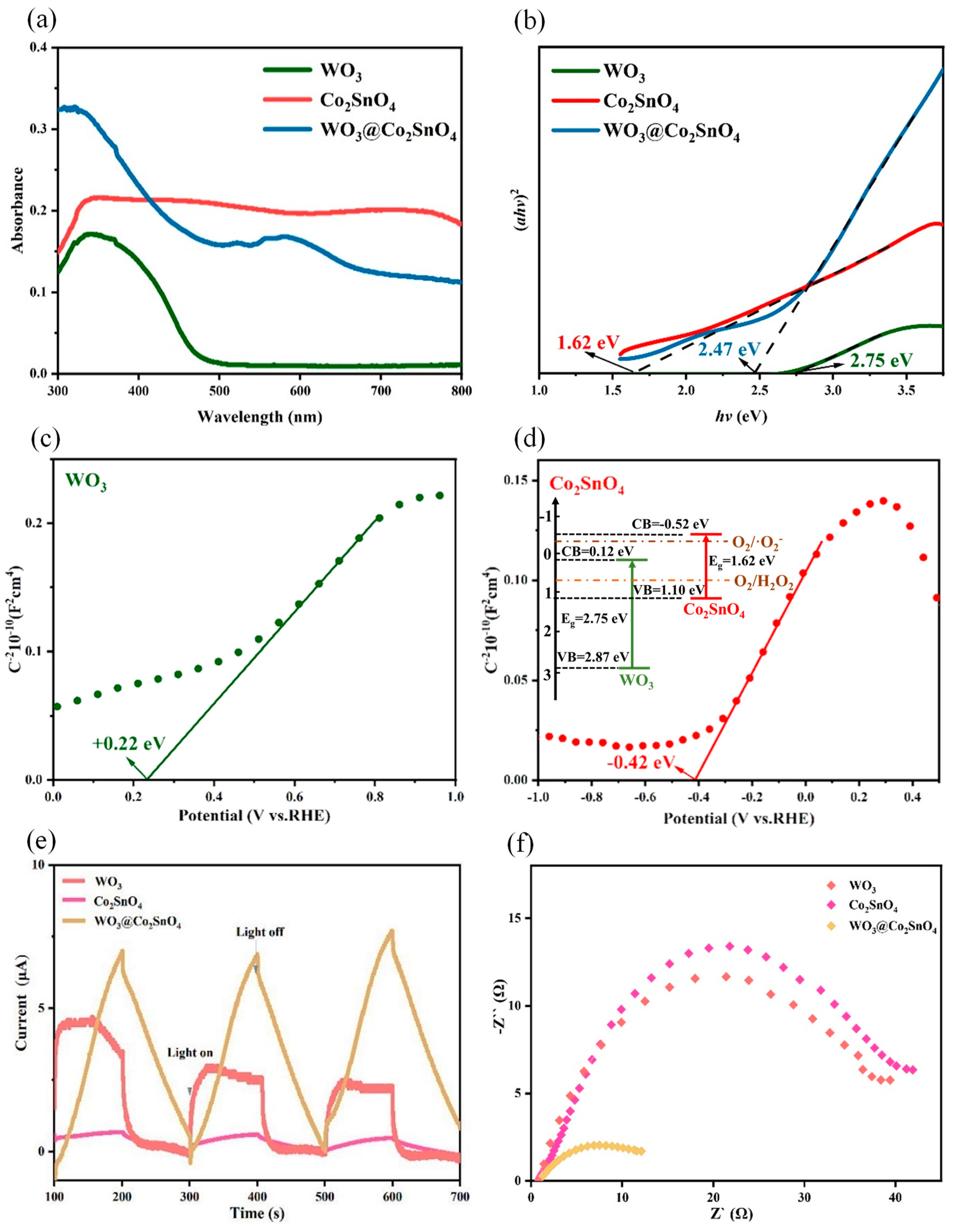
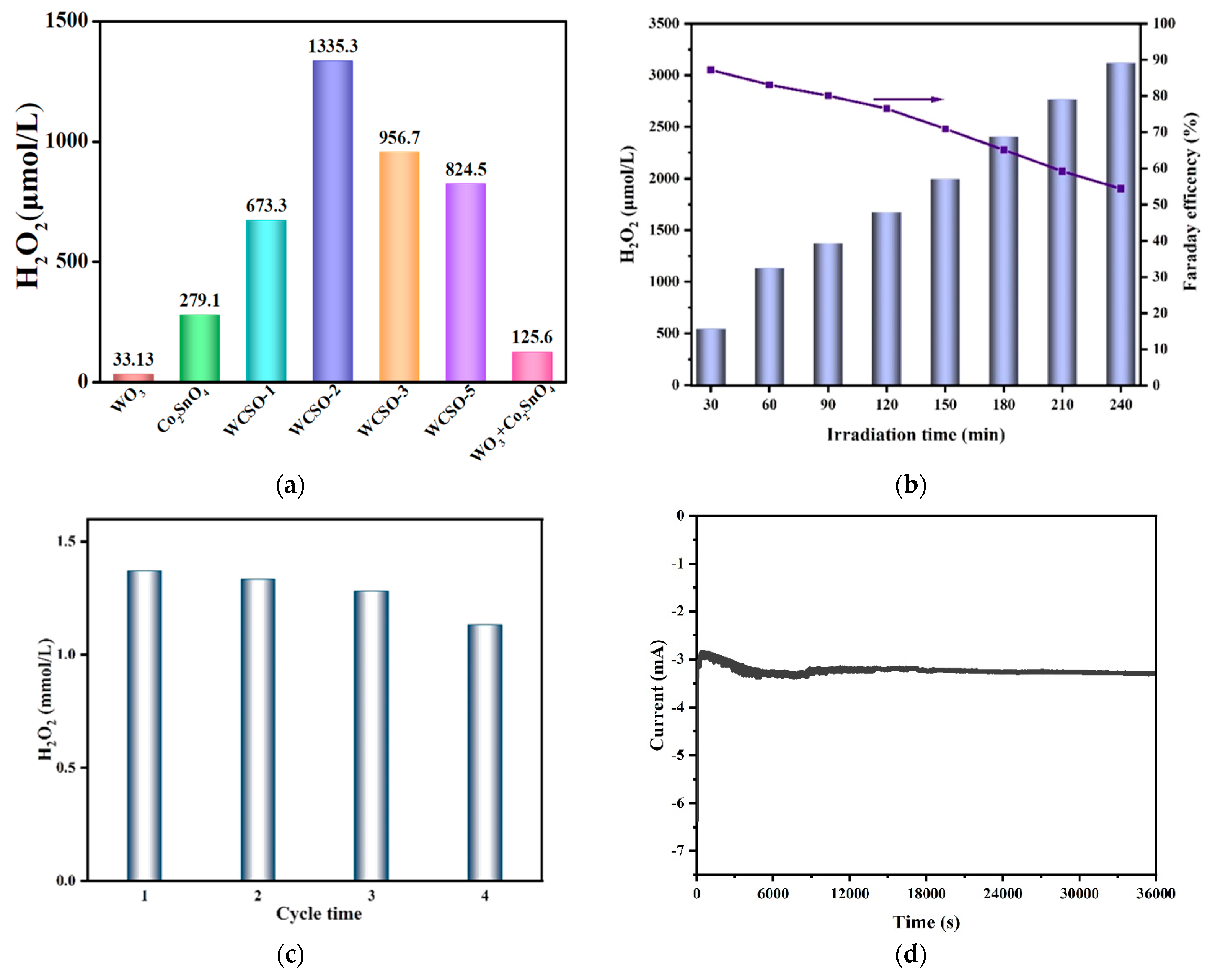
3.2.1. In-Situ Generation of H2O2 on the WO3@Co2SnO4 Photocathode
3.2.2. PEC Degradation of Organic Pollutants on Double Photocathode
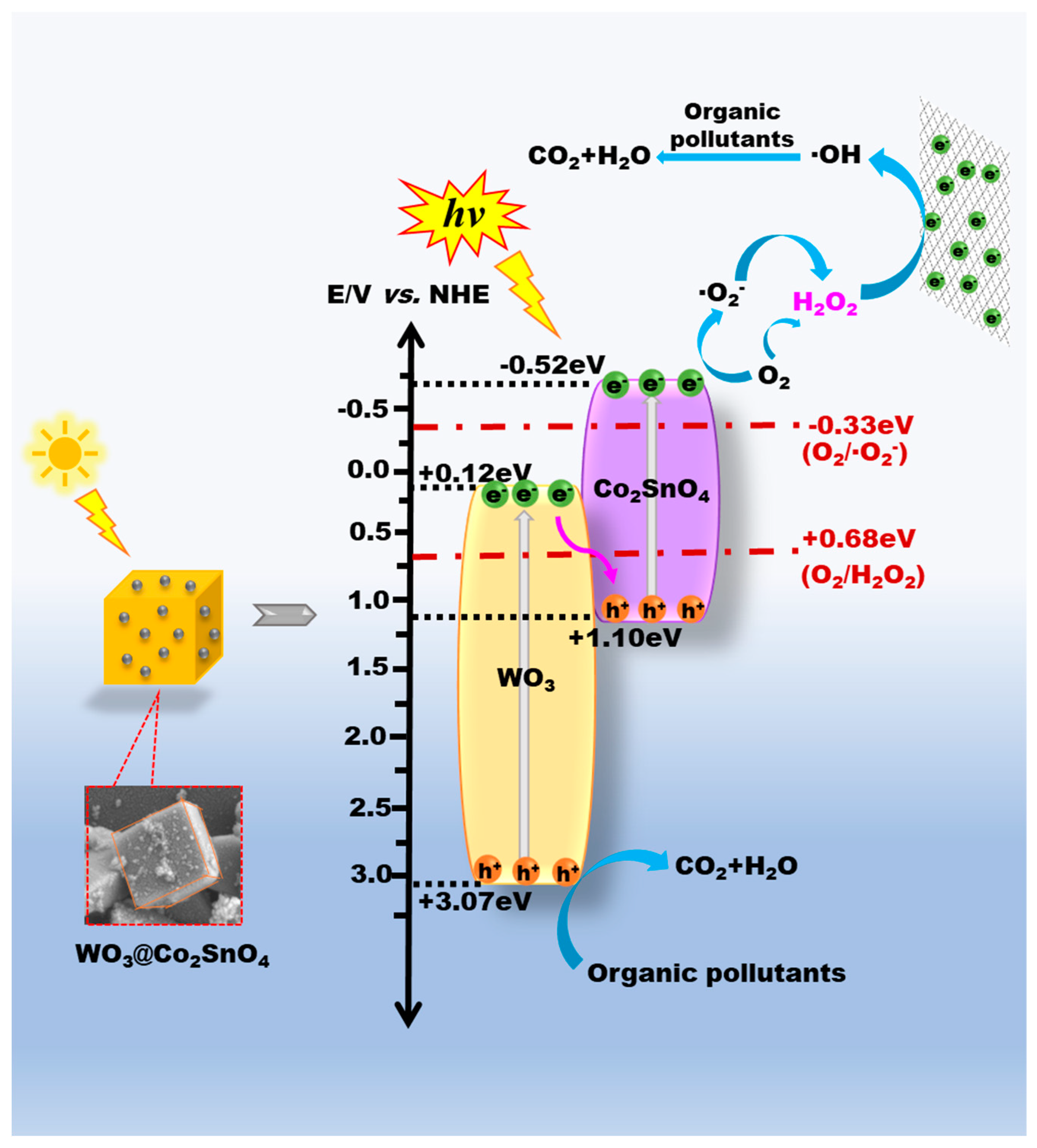
3.3. Deduced PEC Reaction Mechanisms
3.3.1. PEC H2O2 Production Mechanism
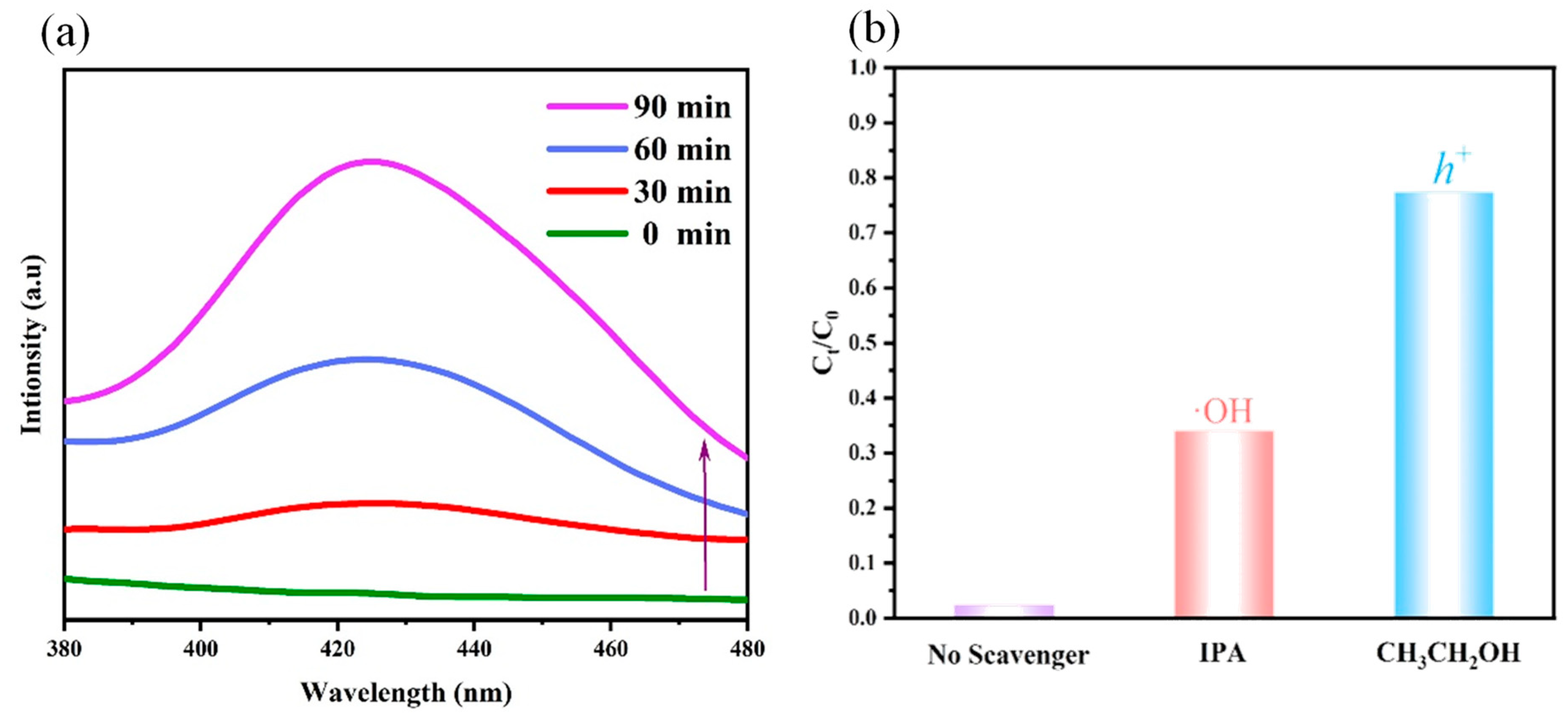
3.3.2. PEC Degradation of Organic Pollutants Mechanism
4. Conclusions
- (1)
- Z-scheme 3D WO3@Co2SnO4 heterojunction composites were prepared through a hydrothermal and then calcining technique. By employing the characterization methods of DRS, Mott-Schottky plots, Transient photocurrent, and electrochemical impedance spectroscopy, the bandgap structure of WO3@Co2SnO4 and its separation capability of photogenerated carriers were detected.
- (2)
- As a photocathode, 3D WO3@Co2SnO4 was one of the satisfied materials for production of H2O2. Under the optimal reaction conditions, the yield of H2O2 reached 1335 μmol·L−1·h−1. The reaction path of H2O2 was investigated by capturing experiments and the rotating disk electrode test, which was mainly an indirect two-step one-electron ORR process.
- (3)
- As the other cathode was designed to conduct a two-cathode photoelectron-Fenton system, 3D WO3@Co2SnO4 as photocathode and stainless-steel mess was carried out in-situ PEC degradation of different organic environmental pollutants. The PEC degradation mechanism was explored, finding that it obeyed a new type of Z-scheme mode through fluorescence spectrophotometry and trapping agents experiments.
- (4)
- This work provided a new idea for preparation of multifunction materials, gave an economic and environmentally friendly way for photoelectrocatalysis production of H2O2, and established a photoelectron-Fenton system without additional additives.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, T.; An, X.; Han, H.; Chen, J.Q.; Li, C. Photoelectrocatalytic Materials for Solar Water Splitting. Adv. Energy Mater. 2018, 8, 1800210. [Google Scholar] [CrossRef]
- Huang, H.; Steiniger, K.A.; Lambert, T.H. Electrophotocatalysis: Combining Light and Electricity to Catalyze Reactions. J. Am. Chem. Soc. 2022, 144, 12567–12583. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Wang, W.; Zhang, H. Photoelectrocatalytic Principles for Meaningfully Studying Photocatalyst Properties and Photocatalysis Processes: From Fundamental Theory to Environmental Applications. J. Energy Chem. 2023, 86, 84–117. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Photoelectrocatalytic Technologies for Environmental Applications. J. Photochem. Photobiol. A 2012, 238, 41–52. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Hansora, D.; Yoo, J.W.; Mehrotra, R.; Byun, W.J.; Lim, D.; Kim, Y.K.; Noh, E.; Lim, H.; Jang, J.-W.; Seok, S.I.; et al. All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production. Nat. Energy 2024. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Muzzillo, C.P.; Klein, W.E.; Li, Z.; DeAngelis, A.D.; Horsley, K.; Zhu, K.; Gaillard, N. Low-Cost, Efficient, and Durable H2 Production by Photoelectrochemical Water Splitting with CuGa3Se5 Photocathodes. ACS Appl. Mater. Inter. 2018, 10, 19573–19579. [Google Scholar] [CrossRef]
- Lu, H.; Li, X.; Monny, S.A.; Wang, Z.; Wang, L. Photoelectrocatalytic Hydrogen Peroxide Production Based on Transition-Metal-Oxide Semiconductors. Chin. J. Catal. 2022, 43, 1204–1215. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Vertova, A.; Minguzzi, A.; Rondinini, S. Photoelectrochemical and Photocatalytic Systems Based on Titanates for Hydrogen Peroxide Formation. Electroanal. Chem. 2018, 808, 395–402. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y.; Pan, Z.; Sayama, K. Electrochemical and Photoelectrochemical Water Oxidation for Hydrogen Peroxide Production. Angew. Chem. Int. Edit. 2021, 60, 10469–10480. [Google Scholar] [CrossRef]
- Chao, C.; Yasugi, M.; Yu, L.; Teng, Z.; Ohno, T. Visible Light-Driven H2O2 Synthesis by a Cu3BiS3 Photocathode Via a Photoelectrochemical Indirect Two-Electron Oxygen Reduction Reaction. Appl. Catal. B-Environ. 2022, 307, 121152. [Google Scholar]
- Zhiyuan, P.; Su, Y.; Siaj, M. Encapsulation of Tin Oxide Layers on Gold Nanoparticles Decorated One-Dimensional CdS Nanoarrays for Pure Z-Scheme Photoanodes Towards Solar Hydrogen Evolution. Appl. Catal. B-Environ. 2023, 330, 122614. [Google Scholar]
- Sotirios, M.; Göltz, M.; Perry, S.C.; Bogdan, F.; Leung, P.K.; Rosiwal, S.; Wang, L.; de León, C.P. Effective Hydrogen Peroxide Production from Electrochemical Water Oxidation. ACS Energy Lett. 2021, 6, 2369. [Google Scholar]
- Sivula, K.; van de Krol, R. Semiconducting Materials for Photoelectrochemical Energy Conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Shan, Y.; Xiujuan, T.; Dongsheng, S.; Zhiruo, Z.; Meizhen, W. A Review of Tungsten Trioxide (WO3)-Based Materials for Antibiotics Removal Via Photocatalysis. Ecotoxicol. Environ. Saf. 2023, 259, 114988. [Google Scholar]
- Qingyi, Z.; Li, J.; Li, L.; Bai, J.; Xia, L.; Zhou, B. Synthesis of WO3/BiVO4 Photoanode Using a Reaction of Bismuth Nitrate with Peroxovanadate on Wo3 Film for Efficient Photoelectrocatalytic Water Splitting and Organic Pollutant Degradation. Appl. Catal. B-Environ. 2017, 217, 21–29. [Google Scholar]
- Asma, M.A.; Taha, T.A.M.; Abdullah, M.; Abid, A.G.; Manzoor, S.; Khosa, R.Y.; Farid, H.M.T.; Trukhanov, S.; Sayyed, M.I.; Tishkevich, D.; et al. Highly Performed Tungsten Trioxide-Polyaniline Composite Thin Film and Their Accelerated Oxygen Evolution Electrocatalyst Activity. Electroanal. Chem. 2023, 941, 117550. [Google Scholar]
- Razali, M.; Aqilah, N.; Salleh, W.N.W.; Aziz, F.; Jye, L.W.; Yusof, N.; Ismail, A.F. Review on Tungsten Trioxide as a Photocatalysts for Degradation of Recalcitrant Pollutants. J. Clean. Prod. 2021, 309, 127438. [Google Scholar] [CrossRef]
- Sheng, W.; Shi, X.; Shao, G.; Duan, X.; Yang, H.; Wang, T. Preparation, Characterization and Photocatalytic Activity of Multi-Walled Carbon Nanotube-Supported Tungsten Trioxide Composites. J. Phys. Chem. Solids 2008, 69, 2396–2400. [Google Scholar]
- Ştefan, N.; Neaţu, F.; Diculescu, V.C.; Trandafir, M.M.; Petrea, N.; Somacescu, S.; Krumeich, F.; Wennmacher, J.T.C.; Knorpp, A.J.; van Bokhoven, J.A.; et al. Undoped SnO2 as a Support for Ni Species to Boost Oxygen Generation through Alkaline Water Electrolysis. ACS Appl. Mater. Interfaces 2020, 12, 18407–18420. [Google Scholar]
- Shunyan, Z.; Li, C.; Liu, J.; Liu, N.; Qiao, S.; Han, Y.; Huang, H.; Liu, Y.; Kang, Z. Carbon Quantum Dots/SnO2–Co3O4 Composite for Highly Efficient Electrochemical Water Oxidation. Carbon 2015, 92, 64–73. [Google Scholar]
- Raj, G.S.; Bhuvaneshwari, S.; Wu, J.J.; Asiri, A.M.; Anandan, S. Sonochemical Synthesis of Co2SnO4 Nanocubes for Supercapacitor Applications. Ultrason. Sonochem. 2018, 41, 435. [Google Scholar]
- Hwa, K.Y.; Santhan, A.; Sharma, T.S.K. One-Dimensional Self-Assembled Co2SnO4 Nanosphere to Nanocubes Intertwined in Two-Dimensional Reduced Graphene Oxide: An Intriguing Electrocatalytic Sensor Toward mesalamine Detection. Mater. Today Chem. 2022, 23, 100739. [Google Scholar] [CrossRef]
- Chang, C.; Ru, Q.; Hu, S.; An, B.; Song, X.; Hou, X. Co2SnO4 Nanocrystals Anchored on Graphene Sheets as High-Performance Electrodes for Lithium-Ion Batteries. Electrochim. Acta 2015, 151, 203. [Google Scholar]
- Lei, Z.; Hu, Y.; Zheng, J. Fabrication of 3D Hierarchical CoSnO3@CoO Pine Needle-Like Array Photoelectrode for Enhanced Photoelectrochemical Properties. J. Mater. Chem. A 2017, 5, 18664–18673. [Google Scholar]
- Cheng, Z.; Zheng, X.; Ning, Y.; Li, Z.; Wu, Z.; Feng, X.; Li, G.; Huang, Z.; Hu, Z. Enhancing Long-Term Stability of Bio-Photoelectrochemical Cell by Defect Engineering of a WO3- Photoanode. J. Energy Chem. 2023, 80, 584. [Google Scholar]
- Karuppaiya, P.; Raman, N. Electrochemical Detection of 2-Nitroaniline at a Novel Sphere-Like Co2SnO4 Modified Glassy Carbon Electrode. New J. Chem. 2020, 44, 8454. [Google Scholar]
- Cao, G.; Li, X.; Yu, H.; Mao, L.; Wong, L.H.; Yan, Q.; Wang, J. A Novel Hollowed CoO-in-CoSnO3 Nanostructure with Enhanced Lithium Storage Capabilities. Nanoscale 2014, 6, 13824. [Google Scholar]
- Yile, W.; Yu, D.; Wang, W.; Gao, P.; Zhang, L.; Zhong, S.; Liu, B. The Controllable Synthesis of Novel Heterojunction CoO/BiVO4 Composite Catalysts for Enhancing Visible-Light Photocatalytic Property. Colloid. Surfaces 2019, 578, 123608. [Google Scholar]
- Danfeng, Z.; Zhang, G.; Wang, Q.; Zhang, L. Dual-Functional Catalytic Materials: Magnetically Hollow Prous Ni-Manganese Oxides Microspheres/Cotton Cellulose Fiber. J. Taiwan Inst. Chem. E 2017, 77, 311. [Google Scholar]
- Kadarkarai, G.; Chandran, H.T.; Mohan, R.; Maheswari, S.U.; Murali, R. Electron Scavenger-Assisted Photocatalytic Degradation of Amido Black 10B Dye with Mn3O4 Nanotubes: A Response Surface Methodology Study with Central Composite Design. J. Photochem. Photobiol. A Chem. 2017, 341, 146. [Google Scholar]
- Doan, N.T.T.; Nguyen, D.; Vo, P.P.; Doan, H.N.; Pham, H.T.N.; Hoang, V.H.; Le, K.T.; Kinashi, K.; Huynh, V.T.; Nguyen, P.T. The Roles of Ethanol and Isopropanol as Hole Scavengers in the Photoreduction Reaction of Graphene Oxide by TiO2: A Competition of Oxygenated Groups Removal and Carbon Defects Invasion. J. Mol. Liq. 2023, 381, 121831. [Google Scholar]
- Mateusz, M.; Szlachcikowska, D.; Stępień, K.; Kielar, P.; Galiniak, S. Two Faces of Tempo (2,2,6,6-Tetramethylpiperidinyl-1-Oxyl)—An Antioxidant or a Toxin? BBA—Mol. Cell Res. 2023, 1870, 119412. [Google Scholar]
- Mengyu, L.; Han, R.; Si, C.; Han, X.; Lu, Q.; Pang, Y. NiO/Bi2MoxW1−XO6 (0 ≤ X ≤ 1) Nanofibers as a Bifunctional Platform Boosting Photocatalytic Tetracycline Conversion and Electrocatalytic Oxygen Reduction. J. Alloys Compd. 2023, 947, 169607. [Google Scholar]
- Shicheng, X.; Kim, Y.; Higgins, D.; Yusuf, M.; Jaramillo, T.F.; Prinz, F.B. Building Upon the Koutecky-Levich Equation for Evaluation of Next-Generation Oxygen Reduction Reaction Catalysts. Electrochim. Acta 2017, 255, 99. [Google Scholar]
- Yu, D.; Yu, Z.-Y.; Yang, L.; Zheng, L.-R.; Zhang, C.-T.; Yang, X.-T.; Gao, F.-Y.; Zhang, X.-L.; Yu, X.; Liu, R.; et al. Bimetallic Nickel-Molybdenum/Tungsten Nanoalloys for High-Efficiency Hydrogen Oxidation Catalysis in Alkaline Electrolytes. Nat. Commun. 2020, 11, 4789. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Zhang, L.; An, C.; Wang, M. Constructing Z-Scheme 3D WO3@Co2SnO4 Heterojunction as Dual-Photocathode for Production of H2O2 and In-Situ Degradation of Organic Pollutants. Water 2024, 16, 406. https://doi.org/10.3390/w16030406
Zhang D, Zhang L, An C, Wang M. Constructing Z-Scheme 3D WO3@Co2SnO4 Heterojunction as Dual-Photocathode for Production of H2O2 and In-Situ Degradation of Organic Pollutants. Water. 2024; 16(3):406. https://doi.org/10.3390/w16030406
Chicago/Turabian StyleZhang, Danfeng, Lei Zhang, Changwei An, and Min Wang. 2024. "Constructing Z-Scheme 3D WO3@Co2SnO4 Heterojunction as Dual-Photocathode for Production of H2O2 and In-Situ Degradation of Organic Pollutants" Water 16, no. 3: 406. https://doi.org/10.3390/w16030406



