A Comprehensive Approach to Azo Dichlorotriazine Dye Treatment: Assessing the Impact of Physical, Chemical, and Biological Treatment Methods through Statistical Analysis of Experimental Data
Abstract
:1. Introduction
- This examination exhibits the integration of advanced oxidation processes (Fenton and photo-Fenton) with biological treatment for the effective removal of recalcitrant organic compounds from textile industry wastewater. The integrated schemes provide a novel, efficient, and economical solution for treating toxic textile effluents;
- A comprehensive optimization of critical process parameters like catalyst dose, H2O2 amount, irradiation time etc. was carried out for both Fenton and photo-Fenton processes to achieve maximum COD and color removal;
- The development of statistical regression models correlating the process factors to treatment efficiency, providing important design guidelines;
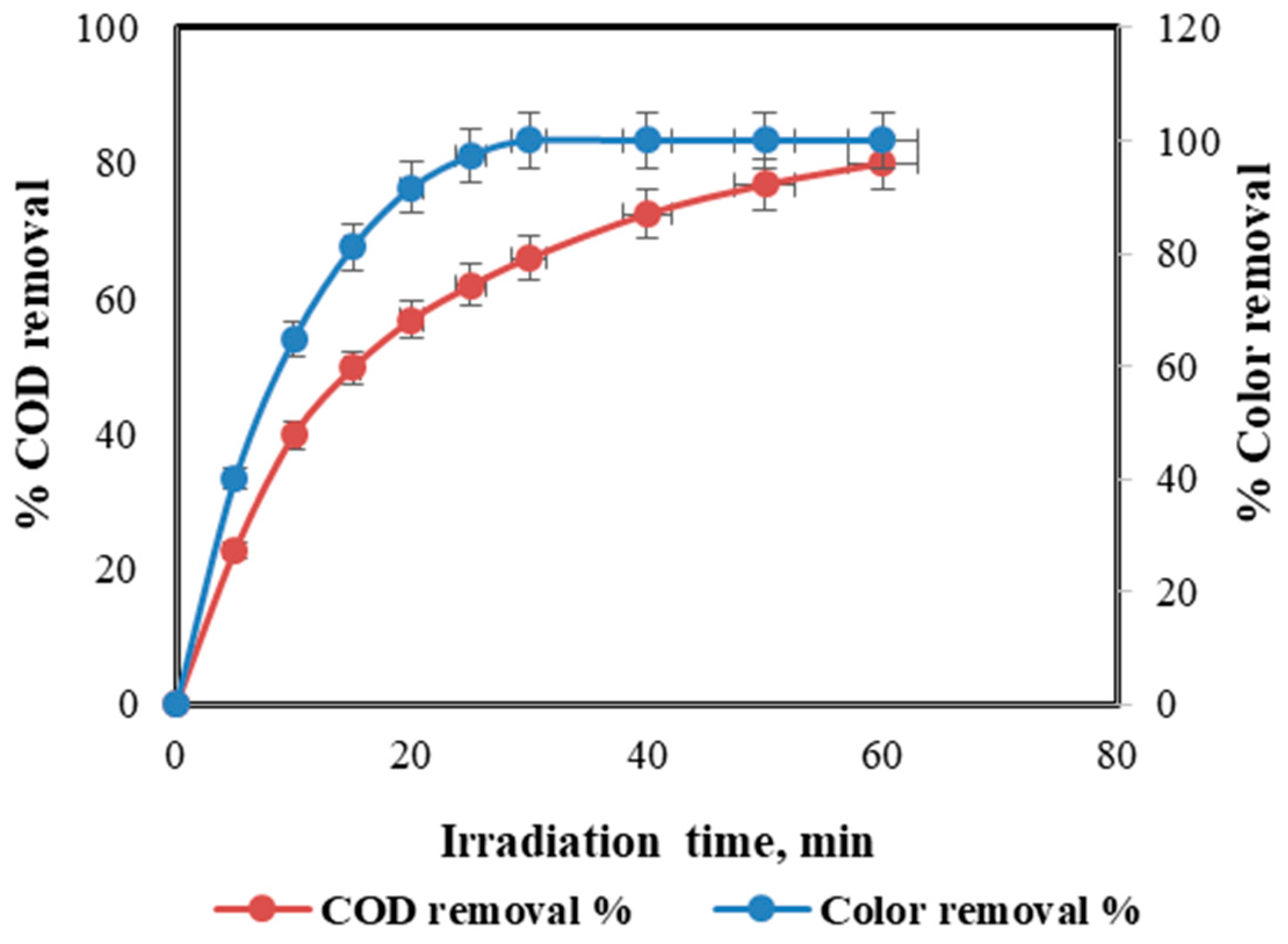
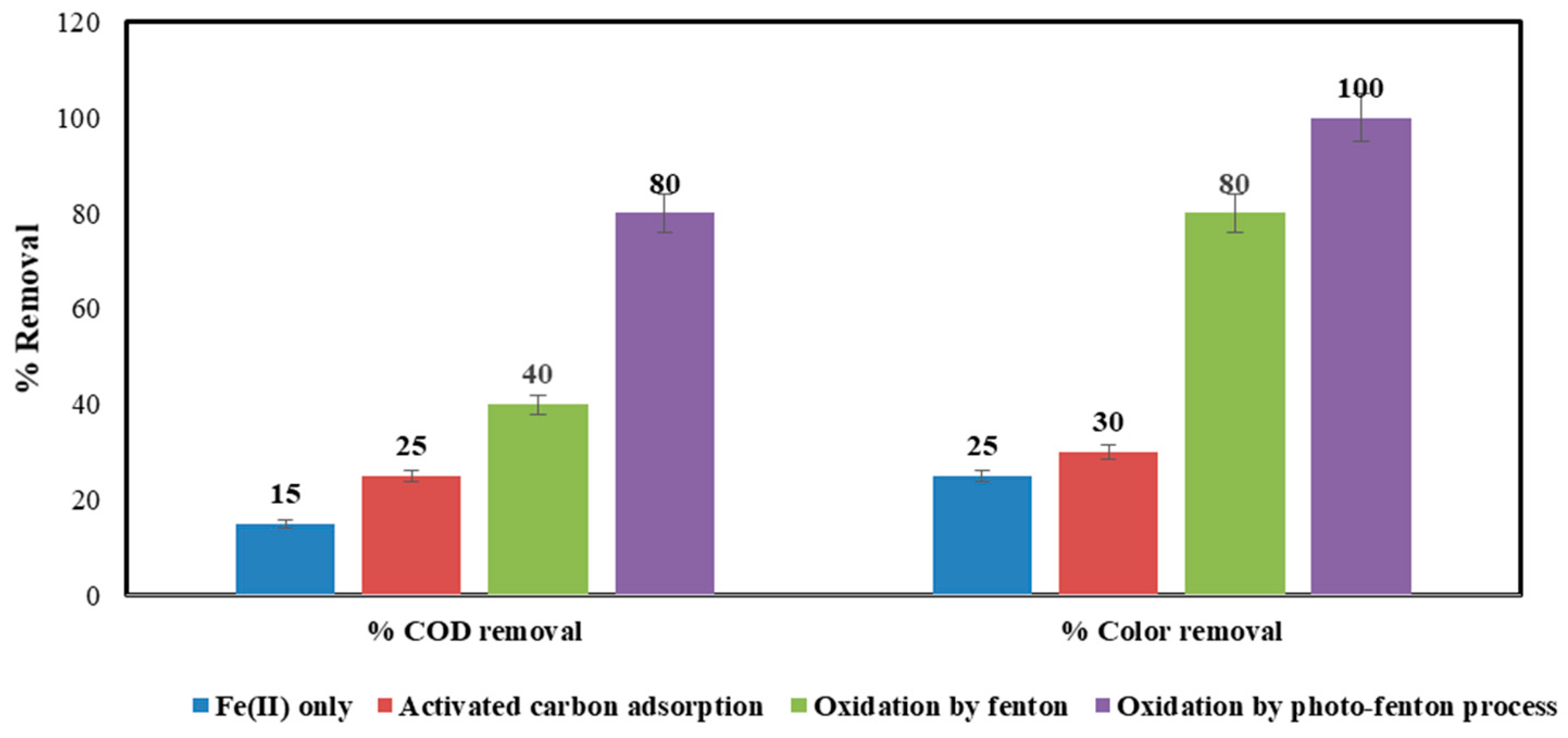
- Photo-Fenton realized 80% COD removal and complete decolorization of the dye, enabling direct discharge without needing an additional biological treatment step. This makes it an economical and sustainable solution;
- For Fenton, the effluent COD exceeded discharge limits, so a post-biological treatment was required to finally comply with regulations. The Fenton–biological integrated scheme provides a novel treatment approach exploiting synergism between chemical and biological processes;
- A techno-economic analysis revealed photo-Fenton to be more economical than UV-only and UV/H2O2 treatment in terms of energy consumption and operating costs;
- Overall, the study successfully demonstrates integrated AOP–biological schemes as a superior and sustainable alternative to conventional methods for removing recalcitrant compounds from textile wastewater (simulated azo dye) in an economical manner.
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Systems
2.3. Experimental Run
2.4. Physical Treatment
2.5. Chemical Treatment
2.5.1. Fenton and Photo-Fenton Process
2.5.2. Biological Treatment
Pre-Biological Preparations
Biological Treatment Procedure
3. Result and Discussion
3.1. Fenton Process
Effect of Irradiation Time and Catalyst Dose on COD and Color Removal Percent
3.2. Effect of Hydrogen Peroxide Amount on COD and Color Removal Percent
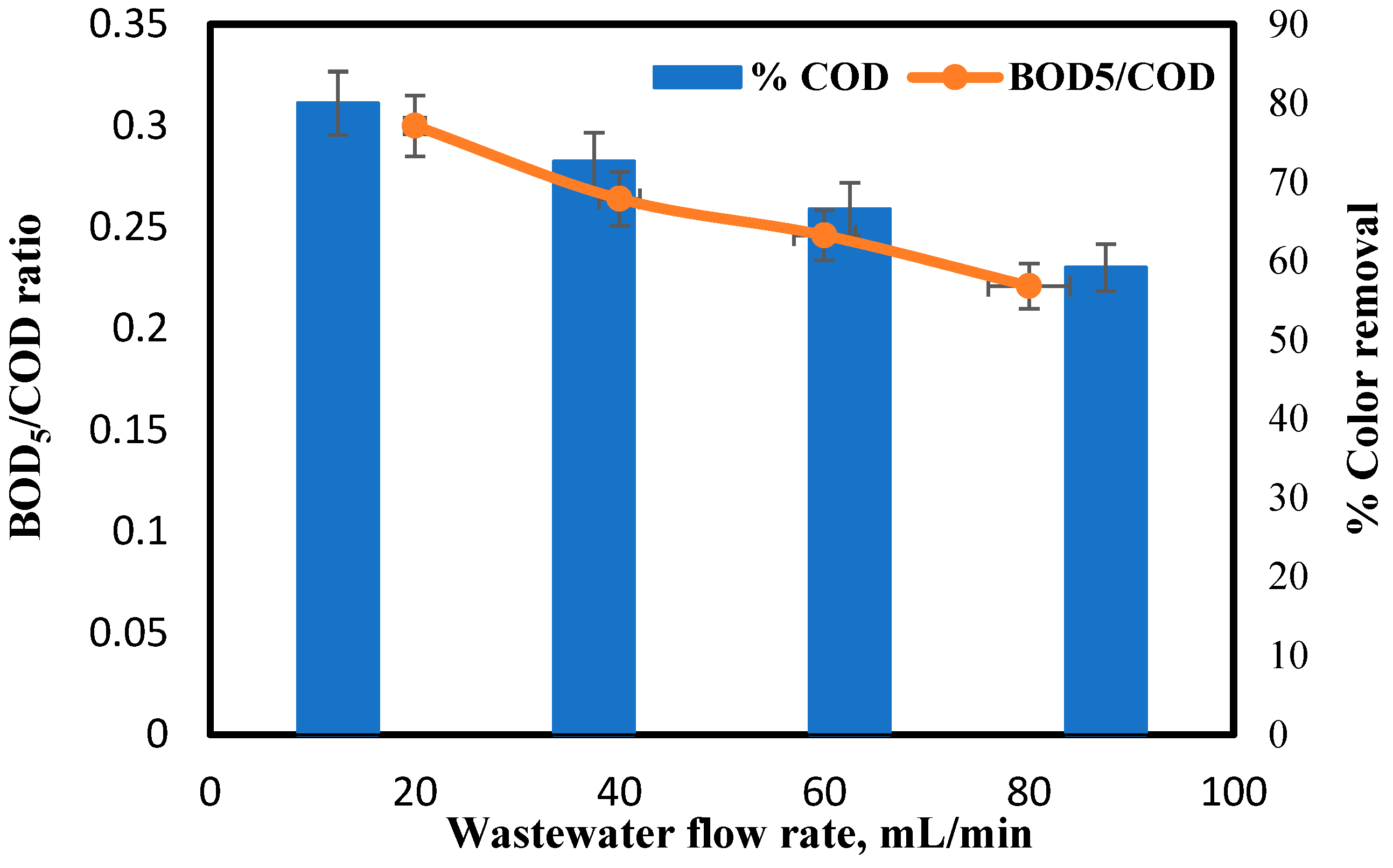
3.3. Effect of Flow Rate of Wastewater on COD and Color Removal Percent
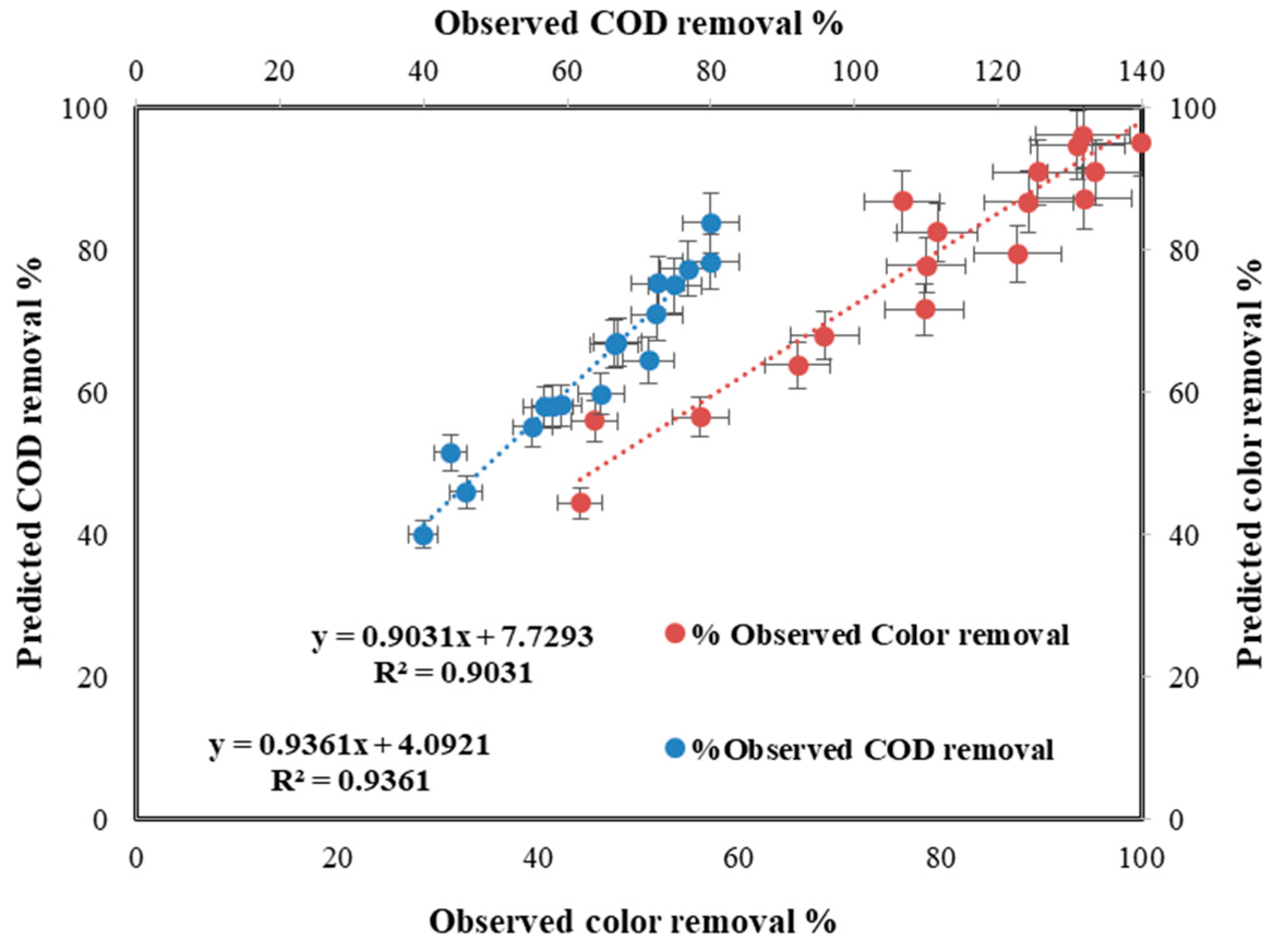
3.4. Statistic Study for Fenton the Process
3.5. Fenton Followed by Biological Treatment
4. Photo-Fenton Process
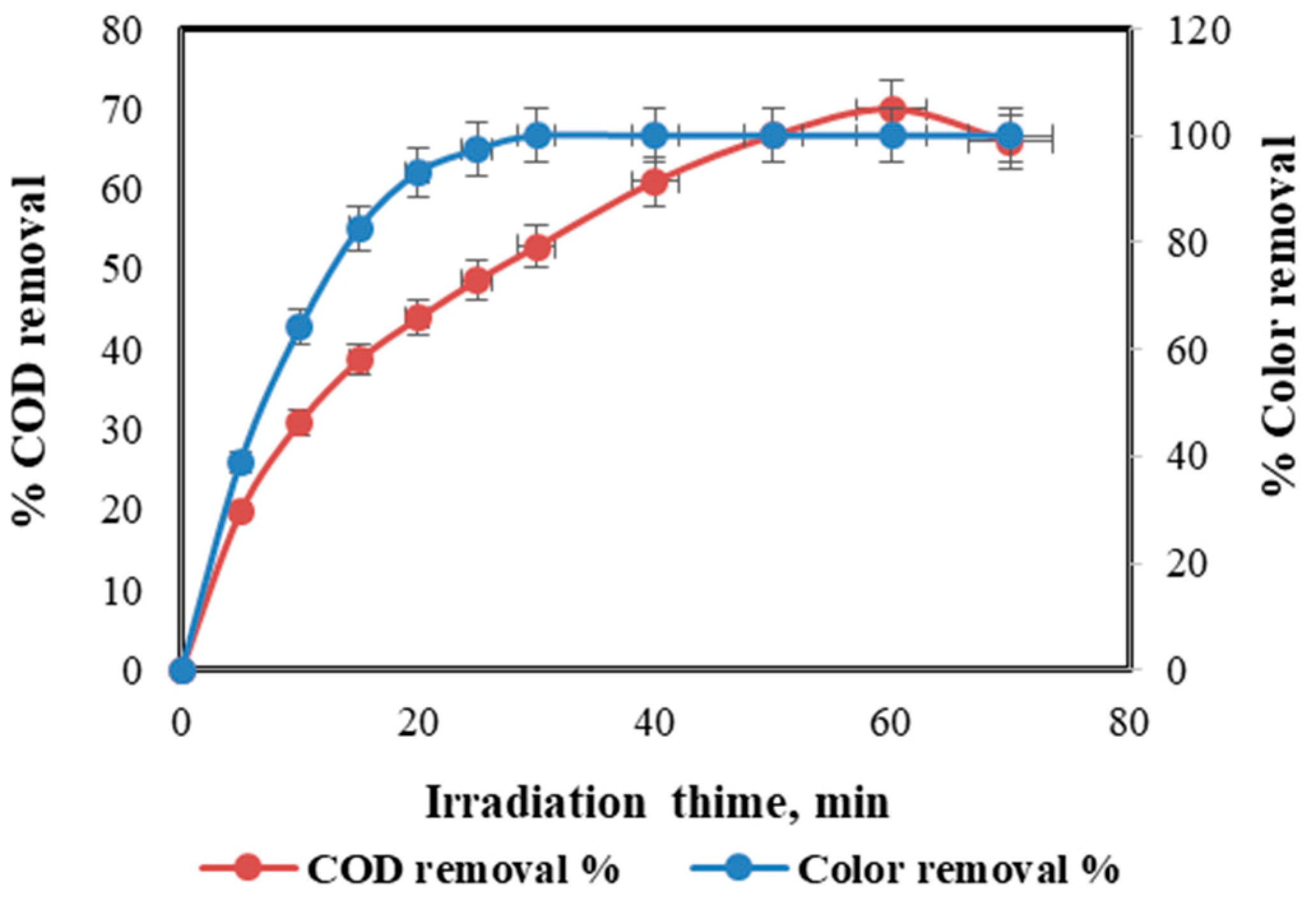
4.1. Effect of Irradiation Time on COD and Color Removal Percent
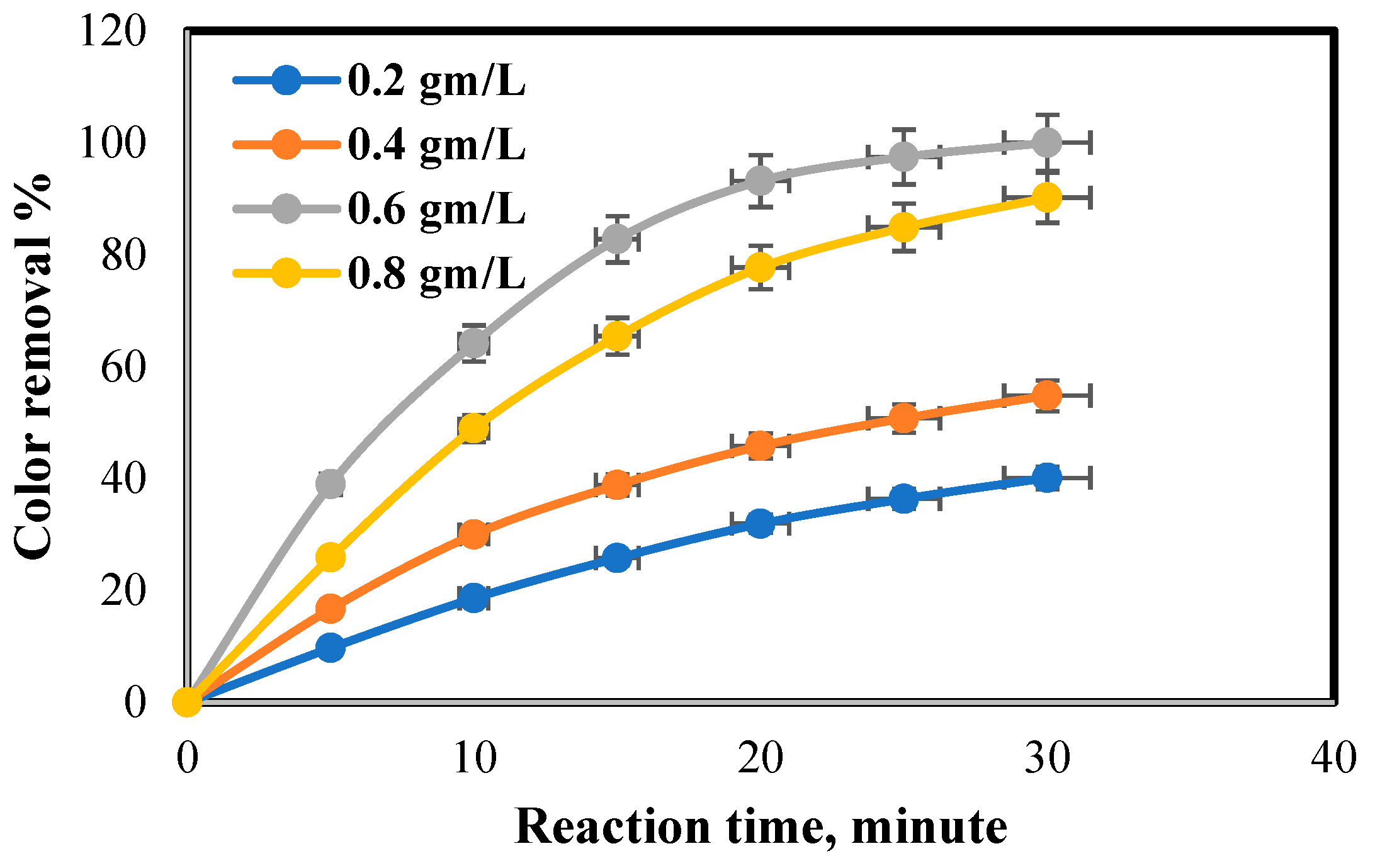
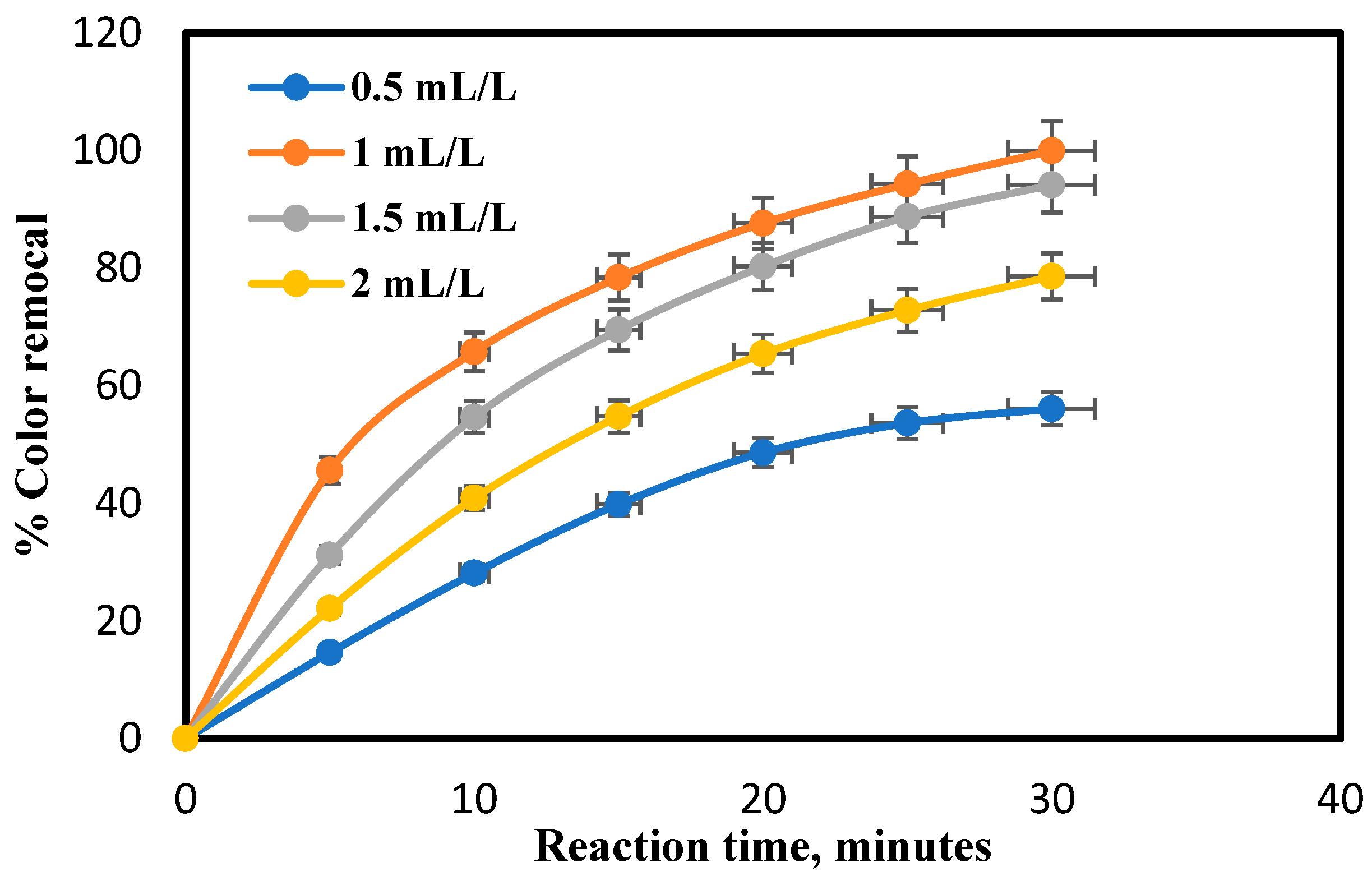
4.2. Effect of PhotoCatalyst Dose on COD and Color Removal Percent
4.3. Effect of Hydrogen Peroxide Amount on COD and Color Removal Percent
4.4. Effect of Wastewater Flow Rate on COD and Color Removal Percent
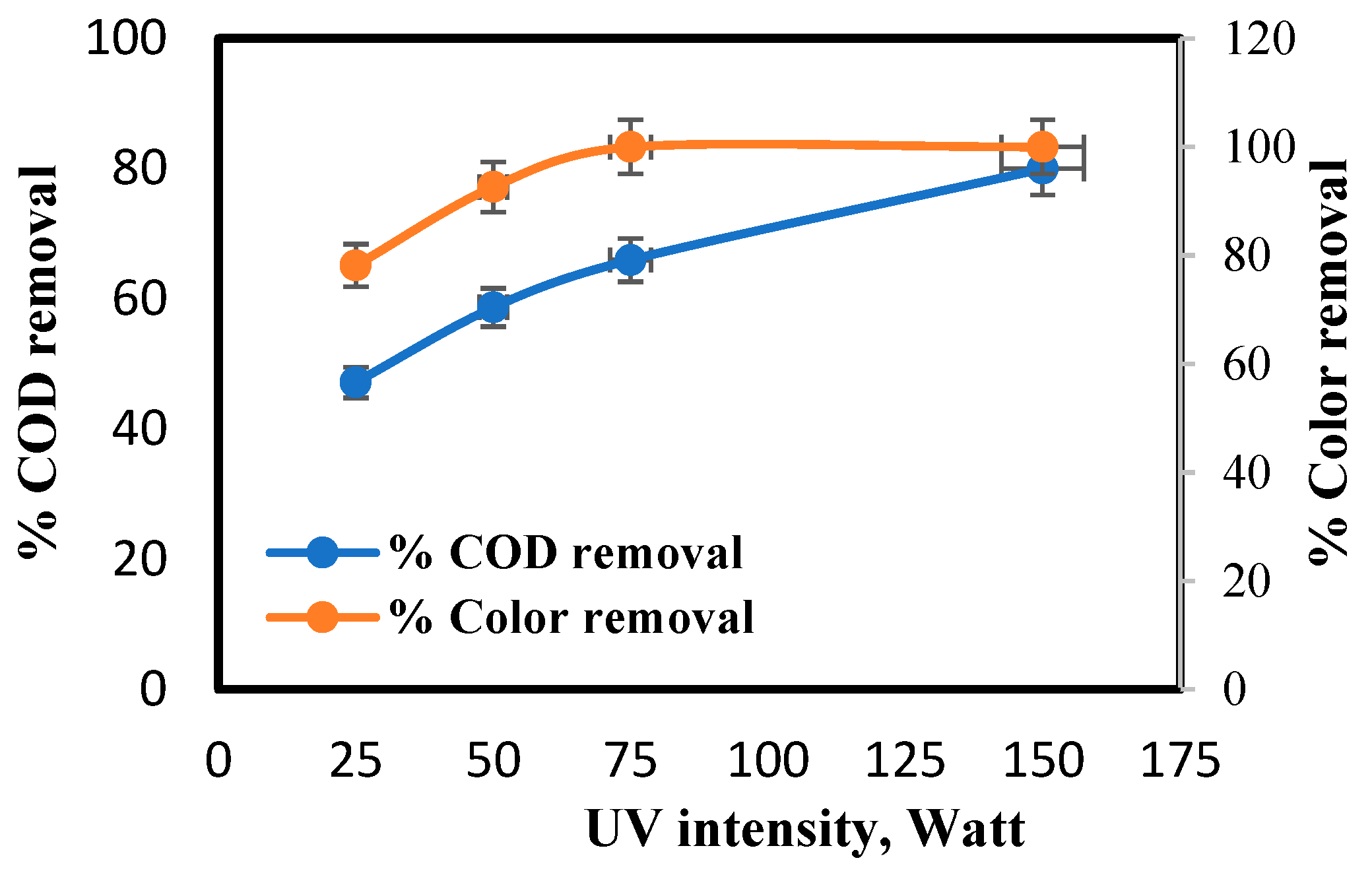
4.5. Effect of Light Intensity on Irradiation Time, COD and Color Removal Percent
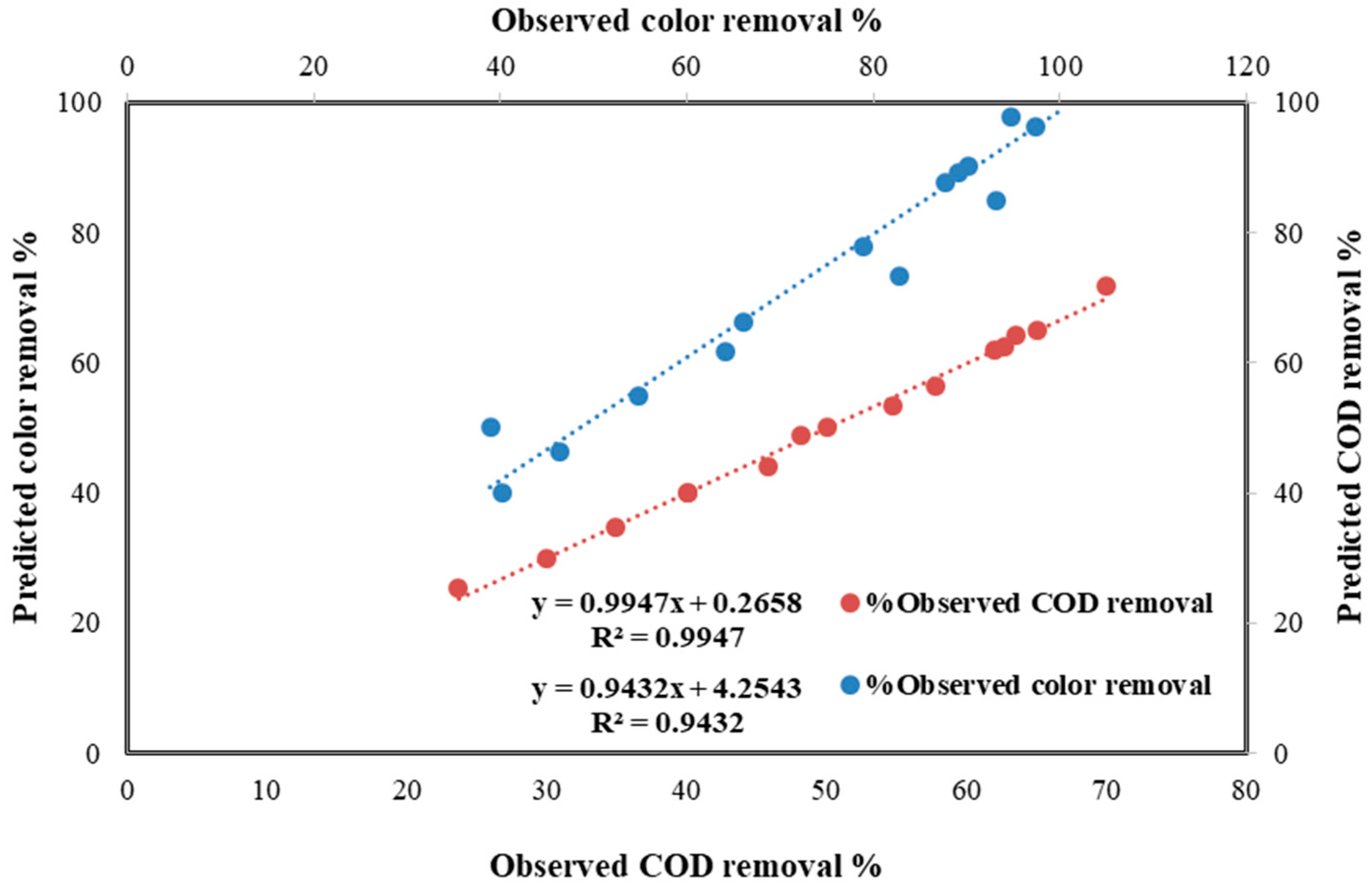
4.6. Statistic Study for the Photo-Fenton Process
4.7. Comparison between % COD Removal and % Color Removal Using Several Techniques
4.8. Economic Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altinbas, U.; Dökmeci, S.; Baristiran, A. Treability Study of Wastewater from Textile Industry. Environ. Technol. 1995, 16, 389–394. [Google Scholar] [CrossRef]
- Mersal, M.; Zedan, A.F.; Mohamed, G.G.; Hassan, G.K. Fabrication of Nitrogen Doped TiO2/Fe2O3 Nanostructures for Photocatalytic Oxidation of Methanol Based Wastewater. Sci. Rep. 2023, 13, 4431. [Google Scholar] [CrossRef]
- El-Gawad, H.A.; Hassan, G.K.; Aboelghait, K.M.; Mahmoud, W.H.; Mohamed, R.M.; Afify, A.A. Removal of Chromium from Tannery Industry Wastewater Using Iron-Based Electrocoagulation Process: Experimental; Kinetics; Isotherm and Economical Studies. Sci. Rep. 2023, 13, 19597. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Batool, F.; Mohyuddin, A.; Goh, H.H.; Othman, M.H.D.; Aziz, F.; Anouzla, A.; Al-Hazmi, H.E.; Chew, K.W. Chitosan-Coated Coconut Shell Composite: A Solution for Treatment of Cr(III)-Contaminated Tannery Wastewater. J. Taiwan Inst. Chem. Eng. 2024, 105478, in press. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Gupta, R.; Singh, R.L. Treatment and Recycling of Wastewater from Textile Industry. In Advances in Biological Treatment of Industrial Waste Water and Their Recycling for a Sustainable Future; Springer: Singapore, 2019; pp. 225–266. [Google Scholar]
- Donkadokula, N.Y.; Kola, A.K.; Naz, I.; Saroj, D. A Review on Advanced Physico-Chemical and Biological Textile Dye Wastewater Treatment Techniques. Rev. Environ. Sci. Bio/Technol. 2020, 19, 543–560. [Google Scholar] [CrossRef]
- Afify, A.A.; Hassan, G.K.; Al-Hazmi, H.E.; Kamal, R.M.; Mohamed, R.M.; Drewnowski, J.; Majtacz, J.; Mąkinia, J.; El-Gawad, H.A. Electrochemical Production of Sodium Hypochlorite from Salty Wastewater Using a Flow-by Porous Graphite Electrode. Energies 2023, 16, 4754. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Hassan, G.K.; Kurniawan, T.A.; Śniatała, B.; Joseph, T.M.; Majtacz, J.; Piechota, G.; Li, X.; El-Gohary, F.A.; Saeb, M.R.; et al. Technological Solutions to Landfill Management: Towards Recovery of Biomethane and Carbon Neutrality. J. Environ. Manag. 2024, 354, 120414. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Maktabifard, M.; Grubba, D.; Majtacz, J.; Hassan, G.K.; Lu, X.; Piechota, G.; Mannina, G.; Bott, C.B.; Mąkinia, J. An Advanced Synergy of Partial Denitrification-Anammox for Optimizing Nitrogen Removal from Wastewater: A Review. Bioresour. Technol. 2023, 381, 129168. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Hassan, G.K.; Maktabifard, M.; Grubba, D.; Majtacz, J.; Mąkinia, J. Integrating Conventional Nitrogen Removal with Anammox in Wastewater Treatment Systems: Microbial Metabolism, Sustainability and Challenges. Environ. Res. 2022, 215, 114432. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent Advances in the Treatment of Dye-Containing Wastewater from Textile Industries: Overview and Perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Afify, A.A.-M. Modified Ultrasonic Irradiation Reactor: Application on Produced Water Treatment. Egypt. J. Chem. 2023, 66, 443–451. [Google Scholar] [CrossRef]
- Gosavi, V.D.; Sharma, S. A General Review on Various Treatment Methods for Textile Wastewater. J. Environ. Sci. Comput. Sci. Eng. Technol. 2014, 3, 29–39. [Google Scholar]
- Al-Sayed, A.; Hellal, M.S.; Al-Shemy, M.T.; Hassan, G.K. Performance Evaluation of Submerged Membrane Bioreactor for Municipal Wastewater Treatment: Experimental Study and Model Validation with GPS-X Software Simulator. Water Environ. J. 2023, 37, 480–492. [Google Scholar] [CrossRef]
- El-kebeer, A.A.; Mahmoud, U.F.; Ismail, S.; Jalal, A.A.E.; Kowal, P.; Al-Hazmi, H.E.; Hassan, G.K. Maximizing Bio-Hydrogen and Energy Yields Obtained in a Self-Fermented Anaerobic Bioreactor by Screening of Different Sewage Sludge Pretreatment Methods. Processes 2024, 12, 118. [Google Scholar] [CrossRef]
- Hassan, G.; Alaneny, A.; Afify, A.; El-Liethy, M.A.; El-Gohary, F. Production of biofuels (H2&CH4) from food leftovers via dual-stage anaerobic digestion: Enhancement of bioenergy production and determination of metabolic fingerprinting of microbial communities. Egypt. J. Chem. 2021, 64, 4105–4115. [Google Scholar]
- Basha, C.A.; Selvakumar, K.V.; Prabhu, H.J.; Sivashanmugam, P.; Lee, C.W. Degradation Studies for Textile Reactive Dye by Combined Electrochemical, Microbial and Photocatalytic Methods. Sep. Purif. Technol. 2011, 79, 303–309. [Google Scholar] [CrossRef]
- Hassan, G.K.; Gad-Allah, T.A.; Badawy, M.I.; El-Gohary, F.A. Remediation of Ammonia-Stripped Sanitary Landfill Leachate by Integrated Heterogeneous Fenton Process and Aerobic Biological Methods. Int. J. Environ. Anal. Chem. 2023, 103, 7287–7300. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Xu, M.; Wu, C.; Zhou, Y. Advancements in the Fenton Process for Wastewater Treatment. Adv. Oxid. Process 2020, 61, 61–77. [Google Scholar]
- Hansson, H.; Kaczala, F.; Marques, M.; Hogland, W. Photo-Fenton and Fenton Oxidation of Recalcitrant Industrial Wastewater Using Nanoscale Zero-Valent Iron. Int. J. Photoenergy 2012, 2012, 531076. [Google Scholar] [CrossRef]
- Qiu, M.; Huang, C. A Comparative Study of Degradation of the Azo Dye CI Acid Blue 9 by Fenton and Photo-Fenton Oxidation. Desalin. Water Treat. 2010, 24, 273–277. [Google Scholar] [CrossRef]
- El-Gawad, H.A.; Ebrahiem, E.E.; Ghaly, M.Y.; Afify, A.A.; Mohamed, R.M. An Application of Advanced Oxidation Process on Industrial Crude Oily Wastewater Treatment. Sci. Rep. 2023, 13, 3420. [Google Scholar] [CrossRef] [PubMed]
- Benassi, J.C.; Sánchez, A.A.C.; da Costa, S.A.; da Costa, S.M. Evaluation of Color Removal Efficiencies and Kinetic Parameters of Fenton (H2O2/Fe2+) and Photo-Fenton (H2O2/Fe2+/UV) Processes in the Treatment of a Textile Wastewater Containing Indigo Blue/Avaliação Da Eficiência de Remoção de Cor e Parâmetros Cinético. Braz. J. Dev. 2021, 7, 102327–102347. [Google Scholar] [CrossRef]
- Hafezi, M.; Mozaffarian, M.; Jafarikojour, M.; Mohseni, M.; Dabir, B. Application of Impinging Jet Atomization in UV/H2O2 Reactor Operation: Design, Evaluation, and Optimization. J. Photochem. Photobiol. A Chem. 2020, 389, 112198. [Google Scholar] [CrossRef]
- Yu, X.; Somoza-Tornos, A.; Graells, M.; Pérez-Moya, M. An Experimental Approach to the Optimization of the Dosage of Hydrogen Peroxide for Fenton and Photo-Fenton Processes. Sci. Total Environ. 2020, 743, 140402. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Raut, P.D. Treatment of Textile Wastewater by Fenton’s Process as a Advanced Oxidation Process. IOSR J. Environ. Sci. Toxicol. Food. Technol 2014, 8, 29–32. [Google Scholar] [CrossRef]
- Ilhan, F.; Ulucan-Altuntas, K.; Dogan, C.; Kurt, U. Treatability of Raw Textile Wastewater Using Fenton Process and Its Comparison with Chemical Coagulation. Desalin. Water Treat 2019, 162, 142–148. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Electro Fenton Oxidation for the Removal of Rhodamine B from Aqueous Solution in a Bubble Column Reactor under Continuous Mode. Desalin. Water Treat. 2015, 55, 263–271. [Google Scholar] [CrossRef]
- Moghadam, F.; Kohbanan, N.N. Removal of Reactive Blue 19 Dye Using Fenton from Aqueous Solution. Avicenna J. Environ. Health Eng. 2018, 5, 50–55. [Google Scholar] [CrossRef]
- Sreeja, P.H.; Sosamony, K.J. A Comparative Study of Homogeneous and Heterogeneous Photo-Fenton Process for Textile Wastewater Treatment. Procedia Technol. 2016, 24, 217–223. [Google Scholar] [CrossRef]
- Metin, S.; Çifçi, D.İ. Chemical Industry Wastewater Treatment by Coagulation Combined with Fenton and Photo-Fenton Processes. J. Chem. Technol. Biotechnol. 2023, 98, 1158–1165. [Google Scholar] [CrossRef]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of Various Advanced Oxidation Processes and Chemical Treatment Methods for COD and Color Removal from a Polyester and Acetate Fiber Dyeing Effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef]
- Çalık, Ç.; Çifçi, D.İ. Comparison of Kinetics and Costs of Fenton and Photo-Fenton Processes Used for the Treatment of a Textile Industry Wastewater. J. Environ. Manag. 2022, 304, 114234. [Google Scholar] [CrossRef]
- Rubio-Clemente, A.; Chica, E.; Peñuela, G.A. Petrochemical Wastewater Treatment by Photo-Fenton Process. Water Air Soil Pollut. 2015, 226, 62. [Google Scholar] [CrossRef]
- Wali, F.K.M. Color Removal and COD Reduction of Dyeing Bath Wastewater by Fenton Reaction. Int. J. Waste Resour 2015, 5, 171. [Google Scholar]
- Sajjad, T.A.; Al-zobai, K.M.M. Investigation the Effect of Intensity and Direction of Light on the Removal of Reactive Blue Dye from Simulated Wastewater Using Photo-Fenton Oxidation under UV Irradiation: Batch and Continuous Methods. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 022132. [Google Scholar] [CrossRef]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Degradation of Four Pharmaceuticals by Solar Photo-Fenton Process: Kinetics and Costs Estimation. J. Environ. Chem. Eng. 2015, 3, 46–51. [Google Scholar] [CrossRef]
- Wang, X.S.; Lu, Z.P.; Miao, H.H.; He, W.; Shen, H.L. Kinetics of Pb (II) Adsorption on Black Carbon Derived from Wheat Residue. Chem. Eng. J. 2011, 166, 986–993. [Google Scholar] [CrossRef]
- Alshamsi, H.A.H.; Shdood, A. UV-ClO2 Assisted Decolorization of Methylene Blue. J. Chem. Pharm. Res. 2015, 7, 36–44. [Google Scholar]
- Punjabi, P.B.; Ameta, R.; Kumar, A.; Jain, M. Visible Light Induced Photocatalytic Degradation of Some Xanthene Dyes Using Immobilized Anthracene. Bull. Chem. Soc. Ethiop. 2008, 22, 361–368. [Google Scholar] [CrossRef]
- Tawfik, A.; Al-sayed, A.; Hassan, G.K.; Nasr, M.; El-Shafai, S.A.; Alhajeri, N.S.; Khan, M.S.; Akhtar, M.S.; Ahmad, Z.; Rojas, P.; et al. Electron Donor Addition for Stimulating the Microbial Degradation of 1,4 Dioxane by Sequential Batch Membrane Bioreactor: A Techno-Economic Approach. Chemosphere 2022, 306, 135580. [Google Scholar] [CrossRef]
- El-Khateeb, M.; Hassan, G.K.; El-Liethy, M.A.; El-Khatib, K.M.; Abdel-Shafy, H.I.; Hu, A.; Gad, M. Sustainable municipal wastewater treatment using an innovative integrated compact unit: Microbial communities, parasite removal, and techno-economic analysis. Ann. Microbiol. 2023, 73, 35. [Google Scholar] [CrossRef]



| Materials | Ideal Dose/L | Cost for 1 L Treatment | ||
|---|---|---|---|---|
| Fenton | Photo-Fenton | Fenton | Photo-Fenton | |
| Ferrous sulphate, Kg | 0.6 gm/L | 0.4 gm/L | 0.0225 | 0.0151 |
| Hydrogen peroxide 30%, L | 1.5 mL/L | 1 mL/L | 1.2 | 0.83 |
| Sulfuric acid 98%, L | 0.05 mL/L | 0.05 mL/L | 1.5 | 1.5 |
| Sodium hydroxide 48%, Kg | 0.0213 gm | 0.0213 gm | 0.0093 | 0.00935 |
| Total cost of materials USD/L | 2.75 | 2.35 | ||
| Total cost of materials USD/L | 5.48 | 4.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, G.K.; Ghaly, M.Y.; Ahmed, G.E.; Mohamed, R.M.; El-Gawad, H.A.; Kowal, P.; Al-Hazmi, H.E.; Afify, A.A. A Comprehensive Approach to Azo Dichlorotriazine Dye Treatment: Assessing the Impact of Physical, Chemical, and Biological Treatment Methods through Statistical Analysis of Experimental Data. Water 2024, 16, 1327. https://doi.org/10.3390/w16101327
Hassan GK, Ghaly MY, Ahmed GE, Mohamed RM, El-Gawad HA, Kowal P, Al-Hazmi HE, Afify AA. A Comprehensive Approach to Azo Dichlorotriazine Dye Treatment: Assessing the Impact of Physical, Chemical, and Biological Treatment Methods through Statistical Analysis of Experimental Data. Water. 2024; 16(10):1327. https://doi.org/10.3390/w16101327
Chicago/Turabian StyleHassan, Gamal K., Montaser Y. Ghaly, Ghada E. Ahmed, Rehab M. Mohamed, Heba A. El-Gawad, Przemysław Kowal, Hussein E. Al-Hazmi, and Ahmed A. Afify. 2024. "A Comprehensive Approach to Azo Dichlorotriazine Dye Treatment: Assessing the Impact of Physical, Chemical, and Biological Treatment Methods through Statistical Analysis of Experimental Data" Water 16, no. 10: 1327. https://doi.org/10.3390/w16101327





