Towards a Better Understanding of the Back-Side Illumination Mode on Photocatalytic Metal–Organic Chemical Vapour Deposition Coatings Used for Treating Wastewater Polluted by Pesticides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. TiO2 Coatings by MOCVD
2.3. Photocatalytic Degradation Experiments
2.4. Characterisation of Materials (TiO2 Coatings and Diuron)
2.5. Analytical Methods
3. Results and Discussion
3.1. Characterisation of TiO2 Coatings
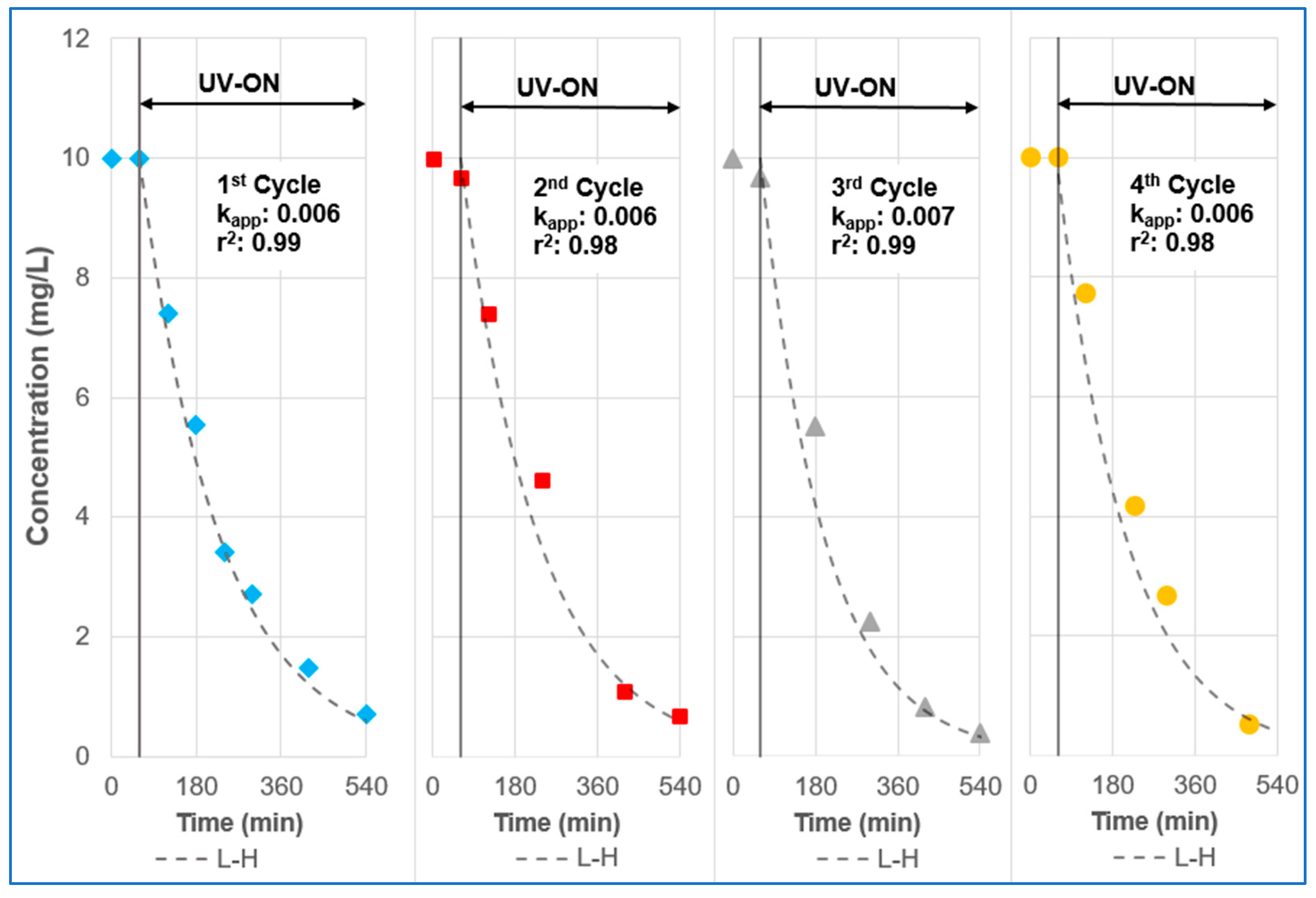
3.2. Photocatalytic Degradation and Kinetics Model
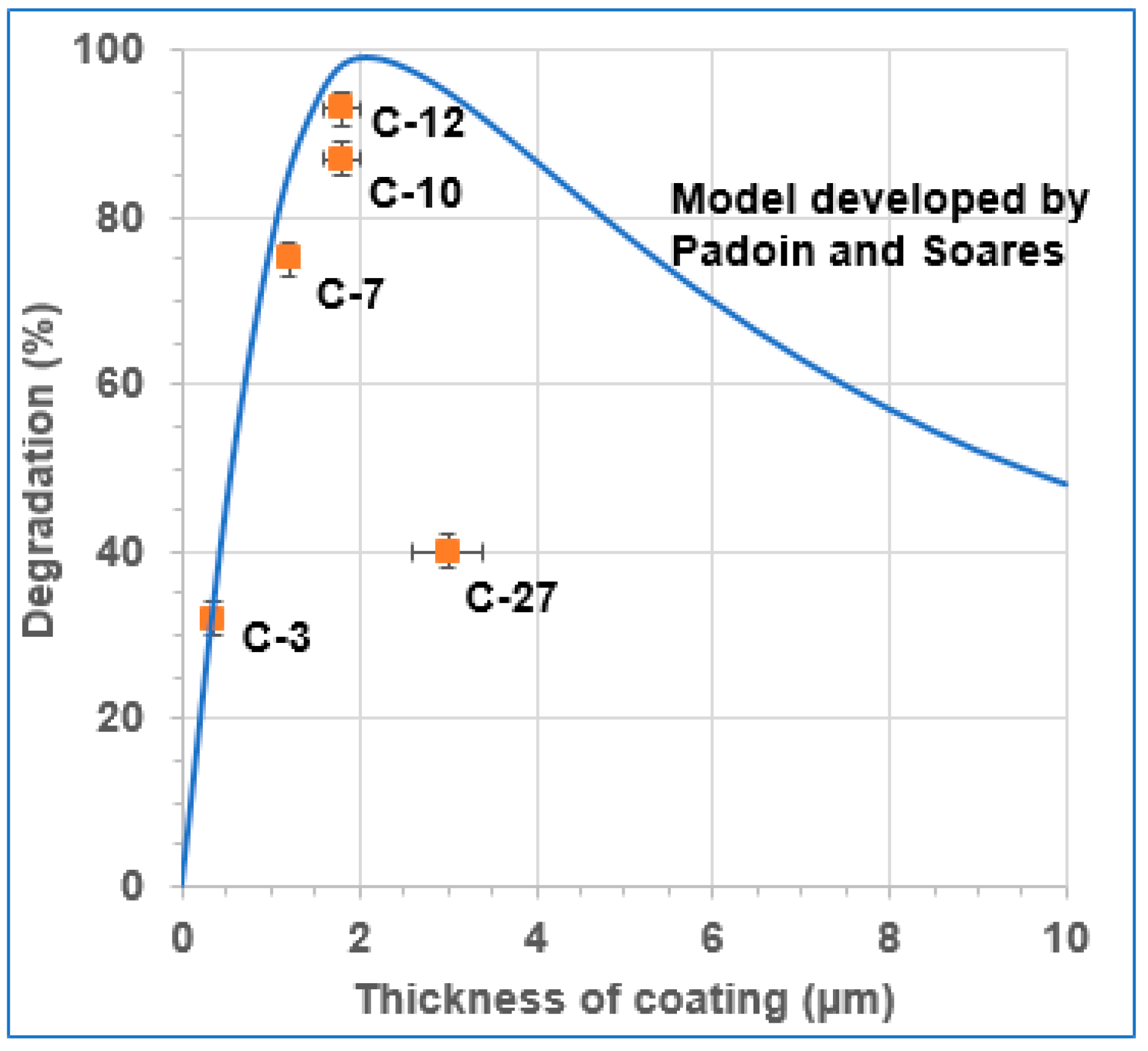
3.3. Relationship of TiO2 Coatings with Degradation Kinetics
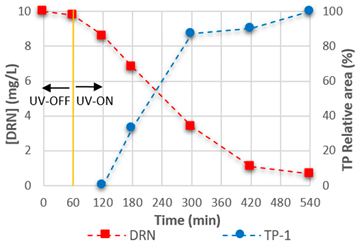
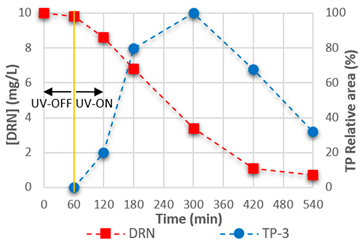
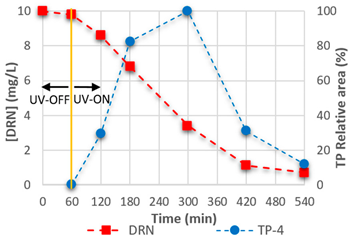
3.4. Mineralisation and TPs
3.5. Reuse and Leaching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roulová, N.; Hrdá, K.; Kašpar, M.; Peroutková, P.; Josefová, D.; Palarčík, J. Removal of Chloroacetanilide Herbicides from Water Using Heterogeneous Photocatalysis with TiO2/UV-A. Catalysts 2022, 12, 597. [Google Scholar] [CrossRef]
- Shukla, R.; Ahammad, S.Z. Performance assessment of a modified trickling filter and conventional activated sludge process along with tertiary treatment in removing emerging pollutants from urban sewage. Sci. Total Environ. 2023, 858, 159833. [Google Scholar] [CrossRef]
- Choi, Y.; Jung, E.-Y.; Lee, W.; Choi, S.; Son, H.; Lee, Y. In vitro bioanalytical assessment of the occurrence and removal of bioactive chemicals in municipal wastewater treatment plants in Korea. Sci. Total Environ. 2023, 858, 159724. [Google Scholar] [CrossRef]
- Blessing, M.; Baran, N. A review on environmental isotope analysis of aquatic micropollutants: Recent advances, pitfalls and perspectives. TrAC-Trends Anal. Chem. 2022, 157, 116730. [Google Scholar] [CrossRef]
- Goh, P.S.; Ahmad, N.A.; Wong, T.W.; Yogarathinam, L.T.; Ismail, A.F. Membrane technology for pesticide removal from aquatic environment: Status quo and way forward. Chemosphere 2022, 307, 136018. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, P.; Lu, S.; Liu, X.; Zhai, J.; Xu, J.; Wang, Y.; Zhang, G.; Liu, X.; Wan, Z. Occurrence and risk evaluation of organophosphorus pesticides in typical water bodies of Beijing, China. Environ. Sci. Pollut. Res. 2021, 28, 1454–1463. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M.; Hosny, M. Chapter 4—Feasibility and challenges of biopesticides application. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–97. ISBN 978-0-323-85581-5. [Google Scholar]
- Pham, T.H.; Bui, H.M.; Bui, T.X. Chapter 13—Advanced oxidation processes for the removal of pesticides. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Tyagi, R.D., Ngo, H.H., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 309–330. ISBN 978-0-12-819594-9. [Google Scholar]
- Carravieri, A.; Cherel, Y.; Brault-Favrou, M.; Churlaud, C.; Peluhet, L.; Labadie, P.; Budzinski, H.; Chastel, O.; Bustamante, P. From Antarctica to the subtropics: Contrasted geographical concentrations of selenium, mercury, and persistent organic pollutants in skua chicks (Catharacta spp.). Environ. Pollut. 2017, 228, 464–473. [Google Scholar] [CrossRef]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Mrema, E.J.; Rubino, F.M.; Brambilla, G.; Moretto, A.; Tsatsakis, A.M.; Colosio, C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 2013, 307, 74–88. [Google Scholar] [CrossRef]
- Farkas, J.; Náfrádi, M.; Hlogyik, T.; Pravda, B.C.; Schrantz, K.; Hernádi, K.; Alapi, T. Comparison of advanced oxidation processes in the decomposition of diuron and monuron—Efficiency, intermediates, electrical energy per order and the effect of various matrices. Environ. Sci. Water Res. Technol. 2018, 4, 1345–1360. [Google Scholar] [CrossRef]
- Perkins, R.; Whitehead, M.; Civil, W.; Goulson, D. Potential role of veterinary flea products in widespread pesticide contamination of English rivers. Sci. Total Environ. 2021, 755, 143560. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.; Collins, C.M.T. A rapid evidence assessment of the potential risk to the environment presented by active ingredients in the UK’s most commonly sold companion animal parasiticides. Environ. Sci. Pollut. Res. 2022, 29, 45070–45088. [Google Scholar] [CrossRef]
- Malnes, D.; Ahrens, L.; Köhler, S.; Forsberg, M.; Golovko, O. Occurrence and mass flows of contaminants of emerging concern (CECs) in Sweden’s three largest lakes and associated rivers. Chemosphere 2022, 294, 133825. [Google Scholar] [CrossRef] [PubMed]
- Schostag, M.D.; Gobbi, A.; Fini, M.N.; Ellegaard-Jensen, L.; Aamand, J.; Hansen, L.H.; Muff, J.; Albers, C.N. Combining reverse osmosis and microbial degradation for remediation of drinking water contaminated with recalcitrant pesticide residue. Water Res. 2022, 216, 118352. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Basave, B.; Brito-Hernández, A.; Saldarriaga-Noreña, H.A.; Romero-Aguilar, M.; Vergara-Sánchez, J.; Moeller-Chávez, G.E.; Díaz-Torres, J.d.J.; Rosales-Rivera, M.; Murillo-Tovar, M.A. Occurrence and Risk Assessment of Atrazine and Diuron in Well and Surface Water of a Cornfield Rural Region. Water 2022, 14, 3790. [Google Scholar] [CrossRef]
- Webb, D.T.; Zhi, H.; Kolpin, D.W.; Klaper, R.D.; Iwanowicz, L.R.; LeFevre, G.H. Emerging investigator series: Municipal wastewater as a year-round point source of neonicotinoid insecticides that persist in an effluent-dominated stream. Environ. Sci. Process. Impacts 2021, 23, 678–688. [Google Scholar] [CrossRef]
- Blum, K.M.; Haglund, P.; Gao, Q.; Ahrens, L.; Gros, M.; Wiberg, K.; Andersson, P.L. Mass fluxes per capita of organic contaminants from on-site sewage treatment facilities. Chemosphere 2018, 201, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Oluwalana, A.E.; Musvuugwa, T.; Sikwila, S.T.; Sefadi, J.S.; Whata, A.; Nindi, M.M.; Chaukura, N. The screening of emerging micropollutants in wastewater in Sol Plaatje Municipality, Northern Cape, South Africa. Environ. Pollut. 2022, 314, 120275. [Google Scholar] [CrossRef]
- Masiá, A.; Ibáñez, M.; Blasco, C.; Sancho, J.V.; Picó, Y.; Hernández, F. Combined use of liquid chromatography triple quadrupole mass spectrometry and liquid chromatography quadrupole time-of-flight mass spectrometry in systematic screening of pesticides and other contaminants in water samples. Anal. Chim. Acta 2013, 761, 117–127. [Google Scholar] [CrossRef]
- Lopez-Herguedas, N.; González-Gaya, B.; Castelblanco-Boyacá, N.; Rico, A.; Etxebarria, N.; Olivares, M.; Prieto, A.; Zuloaga, O. Characterization of the contamination fingerprint of wastewater treatment plant effluents in the Henares River Basin (central Spain) based on target and suspect screening analysis. Sci. Total Environ. 2022, 806, 151262. [Google Scholar] [CrossRef]
- Alexa, E.T.; Bernal-Romero del Hombre Bueno, M.d.l.Á.; González, R.; Sánchez, A.V.; García, H.; Prats, D. Occurrence and Removal of Priority Substances and Contaminants of Emerging Concern at the WWTP of Benidorm (Spain). Water 2022, 14, 4129. [Google Scholar] [CrossRef]
- Weng, B.; Qi, M.-Y.; Han, C.; Tang, Z.-R.; Xu, Y.-J. Photocorrosion Inhibition of Semiconductor-Based Photocatalysts: Basic Principle, Current Development, and Future Perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar] [CrossRef]
- Agustina, T.E.; Habiburrahman, M.; Amalia, F.; Arita, S.; Faizal, M.; Novia, N.; Gayatri, R. Reduction of Copper, Iron, and Lead Content in Laboratory Wastewater Using Zinc Oxide Photocatalyst under Solar Irradiation. J. Ecol. Eng. 2022, 23, 107–115. [Google Scholar] [CrossRef]
- Li, N.; Gao, X.; Su, J.; Gao, Y.; Ge, L. Metallic WO2-decorated g-C3N4 nanosheets as noble-metal-free photocatalysts for efficient photocatalysis. Chin. J. Catal. 2023, 47, 161–170. [Google Scholar] [CrossRef]
- Jamshaid, M.; Khan, H.M.; Nazir, M.A.; Wattoo, M.A.; Shahzad, K.; Malik, M.; Rehman, A.-U. A novel bentonite–cobalt doped bismuth ferrite nanoparticles with boosted visible light induced photodegradation of methyl orange: Synthesis, characterization and analysis of physiochemical changes. Int. J. Environ. Anal. Chem. 2022, 0, 1–16. [Google Scholar] [CrossRef]
- Andriantsiferana, C.; Mohamed, E.F.; Delmas, H. Photocatalytic degradation of an azo-dye on TiO2/activated carbon composite material. Environ. Technol. 2014, 35, 355–363. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Anandan, S. Review on the recent improvements in sonochemical and combined sonochemical oxidation processes—A powerful tool for destruction of environmental contaminants. Renew. Sustain. Energy Rev. 2016, 55, 426–454. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Degradation of traditional and new emerging pesticides in water by nanomaterials: Recent trends and future recommendations. Int. J. Environ. Sci. Technol. 2018, 15, 1347–1380. [Google Scholar] [CrossRef]
- Kirkland, D.; Aardema, M.J.; Battersby, R.V.; Beevers, C.; Burnett, K.; Burzlaff, A.; Czich, A.; Donner, E.M.; Fowler, P.; Johnston, H.J.; et al. A weight of evidence review of the genotoxicity of titanium dioxide (TiO2). Regul. Toxicol. Pharmacol. 2022, 136, 105263. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Rey, A.; Álvarez, P.M.; Beltrán, F.J.; Plucinski, P.K. Enhanced activity and reusability of TiO2 loaded magnetic activated carbon for solar photocatalytic ozonation. Appl. Catal. B Environ. 2014, 144, 96–106. [Google Scholar] [CrossRef]
- Galenda, A.; Natile, M.M.; El Habra, N. Large-Scale MOCVD Deposition of Nanostructured TiO2 on Stainless Steel Woven: A Systematic Investigation of Photoactivity as a Function of Film Thickness. Nanomaterials 2022, 12, 992. [Google Scholar] [CrossRef]
- Triquet, T.; Tendero, C.; Latapie, L.; Manero, M.-H.; Richard, R.; Andriantsiferana, C. TiO2 MOCVD coating for photocatalytic degradation of ciprofloxacin using 365 nm UV LEDs—Kinetics and mechanisms. J. Environ. Chem. Eng. 2020, 8, 104544. [Google Scholar] [CrossRef]
- Maury, F.; Duminica, F.-D. TiOxNy coatings grown by atmospheric pressure metal organic chemical vapor deposition. Surf. Coat. Technol. 2010, 205, 1287–1293. [Google Scholar] [CrossRef]
- Jung, S.-C.; Bang, H.-J.; Lee, H.; Ha, H.-H.; Yu, Y.H.; Kim, S.-J.; Park, Y.-K. Assessing the photocatalytic activity of europium doped TiO2 using liquid phase plasma process on acetylsalicylic acid. Catal. Today 2022, 388–389, 365–371. [Google Scholar] [CrossRef]
- Arconada, N.; Durán, A.; Suárez, S.; Portela, R.; Coronado, J.M.; Sánchez, B.; Castro, Y. Synthesis and photocatalytic properties of dense and porous TiO2-anatase thin films prepared by sol–gel. Appl. Catal. B Environ. 2009, 86, 1–7. [Google Scholar] [CrossRef]
- He, J.; Burt, S.P.; Ball, M.R.; Hermans, I.; Dumesic, J.A.; Huber, G.W. Catalytic C-O bond hydrogenolysis of tetrahydrofuran-dimethanol over metal supported WOx/TiO2 catalysts. Appl. Catal. B Environ. 2019, 258, 117945. [Google Scholar] [CrossRef]
- Yilleng, M.T.; Gimba, E.C.; Ndukwe, G.I.; Bugaje, I.M.; Rooney, D.W.; Manyar, H.G. Batch to continuous photocatalytic degradation of phenol using TiO2 and Au-Pd nanoparticles supported on TiO2. J. Environ. Chem. Eng. 2018, 6, 6382–6389. [Google Scholar] [CrossRef]
- Padoin, N.; Soares, C. An explicit correlation for optimal TiO2 film thickness in immobilized photocatalytic reaction systems. Chem. Eng. J. 2017, 310, 381–388. [Google Scholar] [CrossRef]
- Vezzoli, M.; Farrell, T.; Baker, A.; Psaltis, S.; Martens, W.N.; Bell, J.M. Optimal catalyst thickness in titanium dioxide fixed film reactors: Mathematical modelling and experimental validation. Chem. Eng. J. 2013, 234, 57–65. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Santos, S.G.S.; Boaventura, R.A.R.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J.P. Intensification of heterogeneous TiO2 photocatalysis using the NETmix mili-photoreactor under microscale illumination for oxytetracycline oxidation. Sci. Total Environ. 2019, 681, 467–474. [Google Scholar] [CrossRef]
- da Costa Filho, B.M.; Araujo, A.L.P.; Padrão, S.P.; Boaventura, R.A.R.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J.P. Effect of catalyst coated surface, illumination mechanism and light source in heterogeneous TiO2 photocatalysis using a mili-photoreactor for n-decane oxidation at gas phase. Chem. Eng. J. 2019, 366, 560–568. [Google Scholar] [CrossRef]
- Gerbasi, R.; Bolzan, M.; Habra, N.E.; Rossetto, G.; Schiavi, L.; Strini, A.; Barison, S. Photocatalytic Activity Dependence on the Structural Orientation of MOCVD TiO2 Anatase Films. J. Electrochem. Soc. 2009, 156, K233. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C.; García-Muñoz, P.; Keller, N. TiO2 and TiO2-Carbon Hybrid Photocatalysts for Diuron Removal from Water. Catalysts 2021, 11, 457. [Google Scholar] [CrossRef]
- Rico-Santacruz, M.; García-Muñoz, P.; Keller, V.; Batail, N.; Pham, C.; Robert, D.; Keller, N. Alveolar TiO2-β-SiC photocatalytic composite foams with tunable properties for water treatment. Catal. Today 2019, 328, 235–242. [Google Scholar] [CrossRef]
- Kouamé, N.A.; Robert, D.; Keller, V.; Keller, N.; Pham, C.; Nguyen, P. TiO2/[beta]-SiC foam-structured photoreactor for continuous wastewater treatment. Environ. Sci. Pollut. Res. Int. 2012, 19, 3727–3734. [Google Scholar] [CrossRef]
- Panis, C.; Candiotto, L.Z.P.; Gaboardi, S.C.; Gurzenda, S.; Cruz, J.; Castro, M.; Lemos, B. Widespread pesticide contamination of drinking water and impact on cancer risk in Brazil. Environ. Int. 2022, 165, 107321. [Google Scholar] [CrossRef]
- Wilkinson, A.D.; Collier, C.J.; Flores, F.; Negri, A.P. Acute and additive toxicity of ten photosystem-II herbicides to seagrass. Sci. Rep. 2015, 5, 17443. [Google Scholar] [CrossRef]
- Taucare, G.; Bignert, A.; Kaserzon, S.; Thai, P.; Mann, R.M.; Gallen, C.; Mueller, J. Detecting long temporal trends of photosystem II herbicides (PSII) in the Great Barrier Reef lagoon. Mar. Pollut. Bull. 2022, 177, 113490. [Google Scholar] [CrossRef]
- Lopes, J.Q.; Salgado, B.C.B. Photocatalytic performance of titanium and zinc oxides in diuron degradation. Environ. Chall. 2021, 4, 100186. [Google Scholar] [CrossRef]
- Lopes, J.Q.; Cardeal, R.A.; Araújo, R.S.; Assunção, J.C.D.C.; Salgado, B.C.B. Application of titanium dioxide as photocatalyst in diuron degradation: Operational variables evaluation and mechanistic study. Eng. Sanit. E Ambient. 2021, 26, 61–68. [Google Scholar] [CrossRef]
- De Oliveira, P.L.; Lima, N.S.; Costa, A.C.F.d.M.; Cavalcanti, E.B.; Conrado, L.d.S. Obtaining TiO2:CoFe2O4 nanocatalyst by Pechini method for diuron degradation and mineralization. Ceram. Int. 2020, 46, 9421–9435. [Google Scholar] [CrossRef]
- Katsumata, H.; Sada, M.; Nakaoka, Y.; Kaneco, S.; Suzuki, T.; Ohta, K. Photocatalytic degradation of diuron in aqueous solution by platinized TiO2. J. Hazard. Mater. 2009, 171, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Solís, R.R.; Rivas, F.J.; Martínez-Piernas, A.; Agüera, A. Ozonation, photocatalysis and photocatalytic ozonation of diuron. Intermediates identification. Chem. Eng. J. 2016, 292, 72–81. [Google Scholar] [CrossRef]
- Kovács, K.; Farkas, J.; Veréb, G.; Arany, E.; Simon, G.; Schrantz, K.; Dombi, A.; Hernádi, K.; Alapi, T. Comparison of various advanced oxidation processes for the degradation of phenylurea herbicides. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2016, 51, 205–214. [Google Scholar] [CrossRef]
- Sarantopoulos, C. Photocatalyseurs à Base de TIO2 Préparés par Infiltration Chimique en Phase Vapeur (CVI) Sur Supports Microfibreux. Ph.D. Thesis, INPT, Toulouse, France, 2007. [Google Scholar]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Miquelot, A.; Youssef, L.; Villeneuve-Faure, C.; Prud’homme, N.; Dragoe, N.; Nada, A.; Rouessac, V.; Roualdes, S.; Bassil, J.; Zakhour, M.; et al. In- and out-plane transport properties of chemical vapor deposited TiO2 anatase films. J. Mater. Sci. 2021, 56, 10458–10476. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, X.-T. Chemical Vapour Deposition: An Integrated Engineering Design for Advanced Materials; Engineering Materials and Processes; Springer: London, UK, 2010; ISBN 978-1-84882-893-3. [Google Scholar]
- Duminica, F.-D.; Maury, F.; Hausbrand, R. Growth of TiO2 thin films by AP-MOCVD on stainless steel substrates for photocatalytic applications. Surf. Coat. Technol. 2007, 201, 9304–9308. [Google Scholar] [CrossRef]
- Topka, K.C.; Chliavoras, G.A.; Senocq, F.; Vergnes, H.; Samelor, D.; Sadowski, D.; Vahlas, C.; Caussat, B. Large temperature range model for the atmospheric pressure chemical vapor deposition of silicon dioxide films on thermosensitive substrates. Chem. Eng. Res. Des. 2020, 161, 146–158. [Google Scholar] [CrossRef]
- Chin-Pampillo, J.S.; Perez-Villanueva, M.; Masis-Mora, M.; Mora-Dittel, T.; Carazo-Rojas, E.; Alcañiz, J.M.; Chinchilla-Soto, C.; Domene, X. Amendments with pyrolyzed agrowastes change bromacil and diuron’s sorption and persistence in a tropical soil without modifying their environmental risk. Sci. Total Environ. 2021, 772, 145515. [Google Scholar] [CrossRef]
- Silva, T.S.; Araújo de Medeiros, R.C.; Silva, D.V.; de Freitas Souza, M.; das Chagas, P.S.F.; Lins, H.A.; da Silva, C.C.; Souza, C.M.M.; Mendonça, V. Interaction between herbicides applied in mixtures alters the conception of its environmental impact. Environ. Sci. Pollut. Res. 2022, 29, 15127–15143. [Google Scholar] [CrossRef]
- Mercurio, P.; Mueller, J.F.; Eaglesham, G.; O’Brien, J.; Flores, F.; Negri, A.P. Degradation of Herbicides in the Tropical Marine Environment: Influence of Light and Sediment. PLoS ONE 2016, 11, e0165890. [Google Scholar] [CrossRef]
- Murgolo, S.; Yargeau, V.; Gerbasi, R.; Visentin, F.; El Habra, N.; Ricco, G.; Lacchetti, I.; Carere, M.; Curri, M.L.; Mascolo, G. A new supported TiO2 film deposited on stainless steel for the photocatalytic degradation of contaminants of emerging concern. Chem. Eng. J. 2017, 318, 103–111. [Google Scholar] [CrossRef]
- Gonçalves, M.S.T.; Oliveira-Campos, A.M.F.; Pinto, E.M.M.S.; Plasência, P.M.S.; Queiroz, M.J.R.P. Photochemical treatment of solutions of azo dyes containing TiO2. Chemosphere 1999, 39, 781–786. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Bizani, E.; Fytianos, K.; Poulios, I.; Tsiridis, V. Photocatalytic decolorization and degradation of dye solutions and wastewaters in the presence of titanium dioxide. J. Hazard. Mater. 2006, 136, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-C.; Kim, S.-J.; Imaishi, N.; Cho, Y.-I. Effect of TiO2 thin film thickness and specific surface area by low-pressure metal–organic chemical vapor deposition on photocatalytic activities. Appl. Catal. B Environ. 2005, 55, 253–257. [Google Scholar] [CrossRef]
- Meephon, S.; Rungrotmongkol, T.; Puttamat, S.; Praserthdam, S.; Pavarajarn, V. Heterogeneous photocatalytic degradation of diuron on zinc oxide: Influence of surface-dependent adsorption on kinetics, degradation pathway, and toxicity of intermediates. J. Environ. Sci. 2019, 84, 97–111. [Google Scholar] [CrossRef]
- de Matos, D.B.; Barbosa, M.P.R.; Leite, O.M.; Steter, J.R.; Lima, N.S.; Torres, N.H.; Marques, M.N.; de Alsina, O.L.S.; Cavalcanti, E.B. Characterization of a tubular electrochemical reactor for the degradation of the commercial diuron herbicide. Environ. Technol. 2020, 41, 1307–1321. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Baiocchi, C.; Hidaka, H.; Pelizzetti, E. Light-Induced Transformation of Alkylurea Derivatives in Aqueous TiO2 Dispersion. Chem. Eur. J. 2006, 12, 727–736. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Huovinen, M.; Vähäkangas, K.H. Toxicity of diuron metabolites in human cells. Environ. Toxicol. Pharmacol. 2020, 78, 103409. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, M.; Lei, L. Preparation of photocatalytic TiO2 coatings of nanosized particles on activated carbon by AP-MOCVD. Carbon 2005, 43, 1700–1708. [Google Scholar] [CrossRef]
- Hsuan-Fu, Y.; Chao-Wei, C. Enhancing photocatalytic ability of TiO2 films using gel-derived P/Si-TiO2 powder. J. Sol-Gel Sci. Technol. 2021, 97, 259–270. [Google Scholar] [CrossRef]
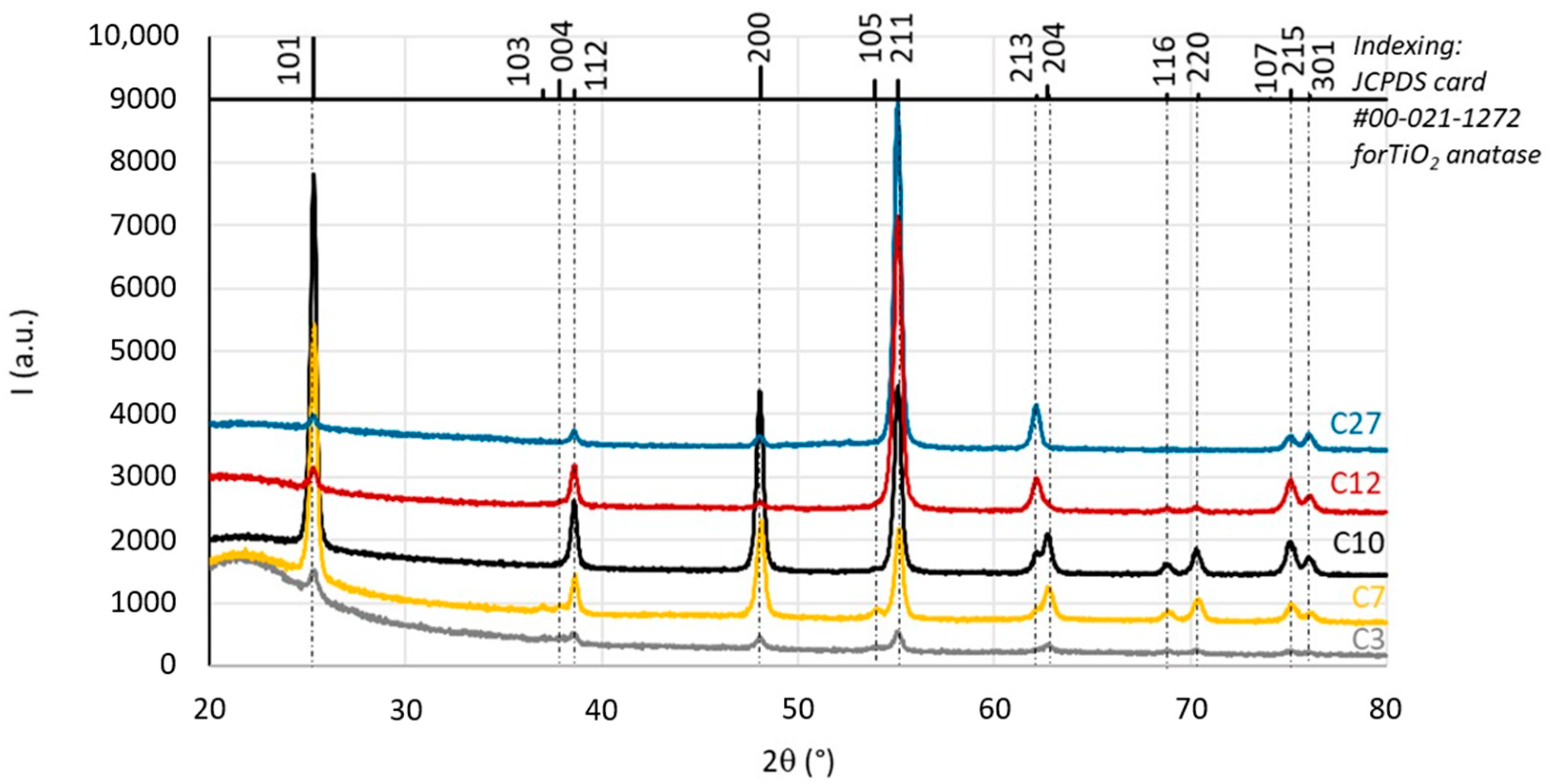
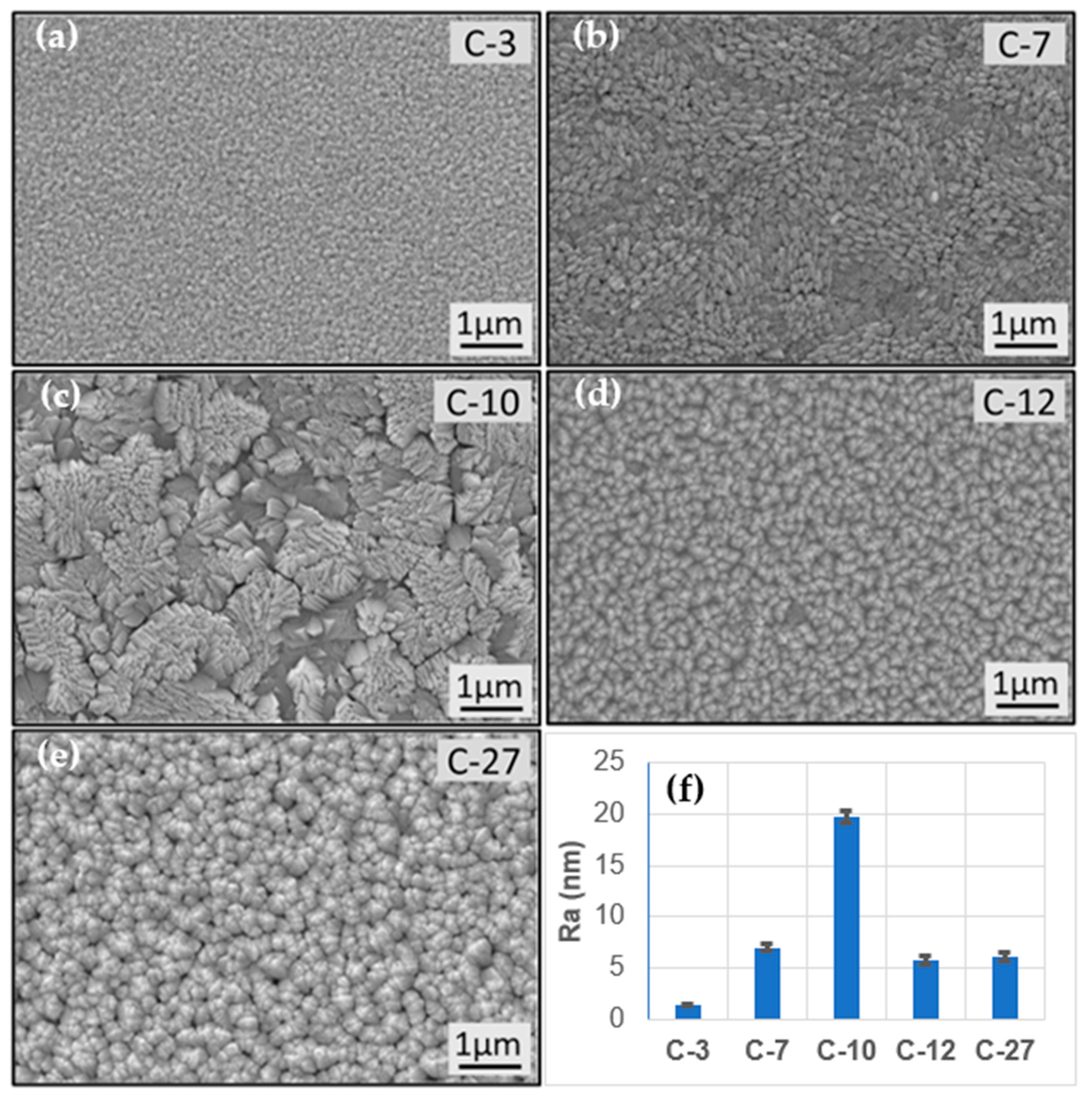

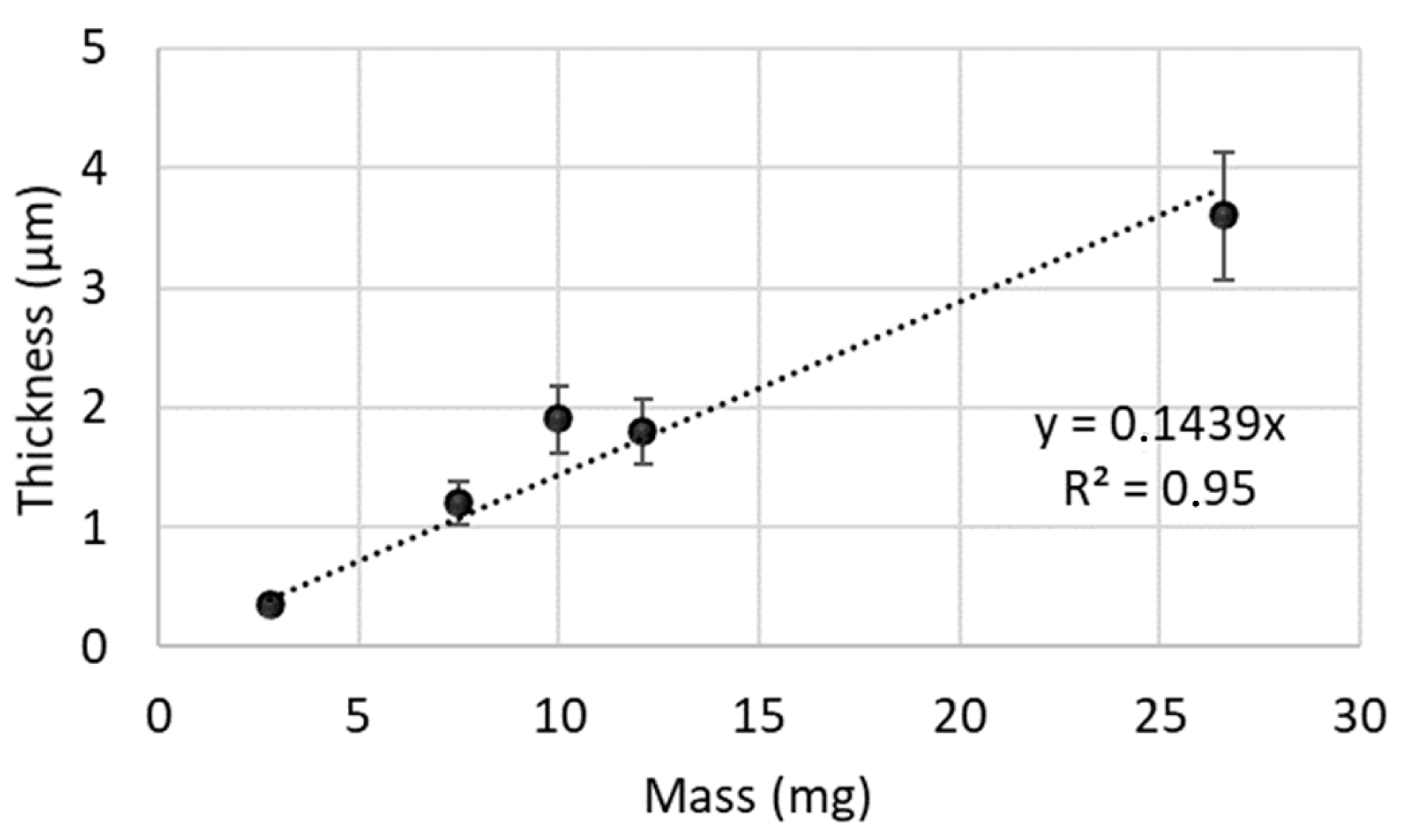
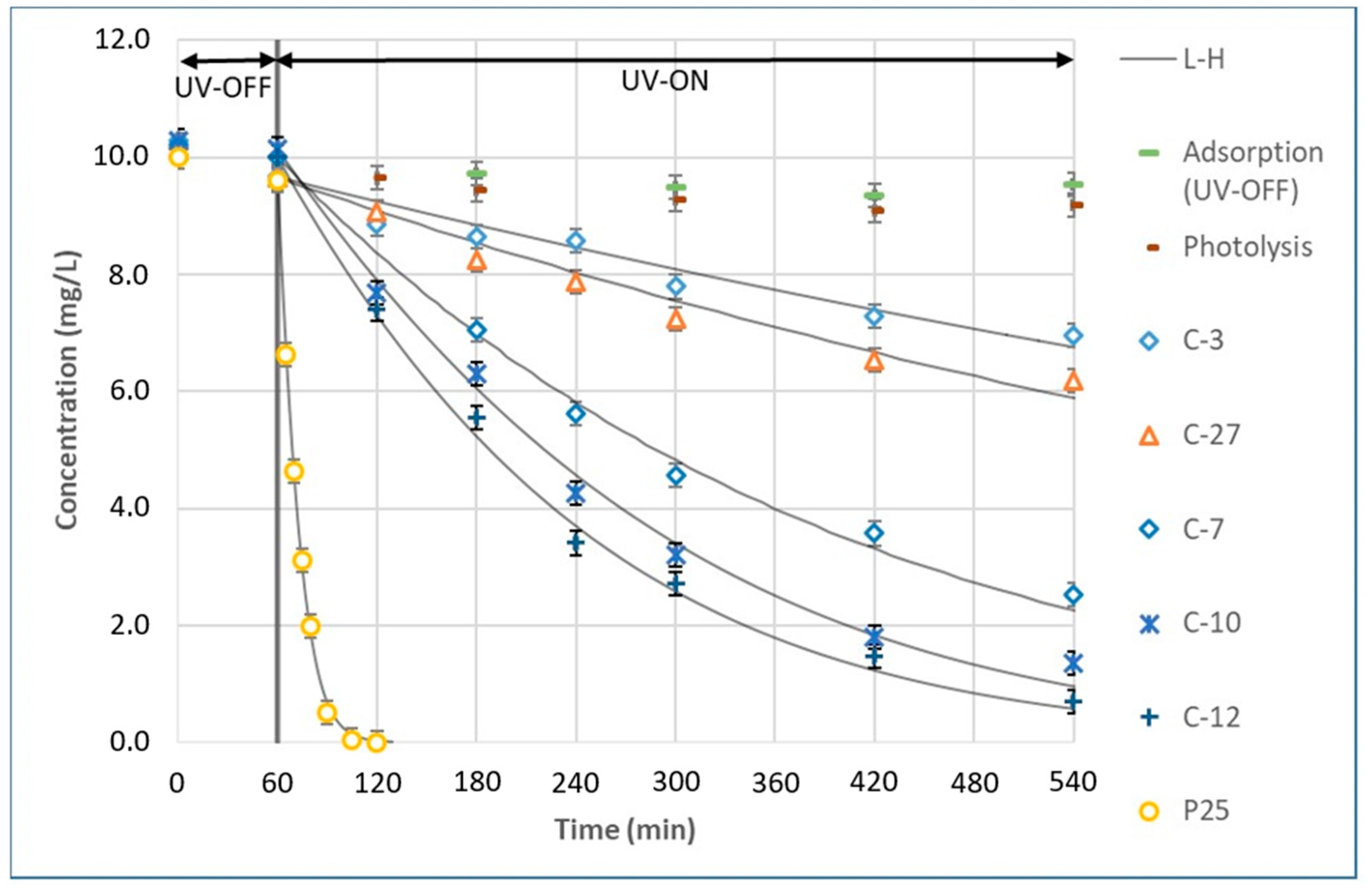

| Operating Conditions | Coatings | ||||
|---|---|---|---|---|---|
| C-3 | C-7 | C-10 | C-12 | C-27 | |
| Deposition time (min) | 2 × 20 | 2 × 60 | 2 × 60 | 2 × 75 | 2 × 100 |
| Precursor temperature in the bubbler (°C) | 37 | 34 | |||
| Deposition temperature (°C) | 475 | ||||
| Carrier gas (N2) flow rate (cm3 min−1) | 8 | 8 | 12 | 8 | 8 |
| Dilution gas (N2) flow rate (cm3 min−1) | 530 | ||||
| Deposition pressure (Torr) | 5 | ||||
| Inlet precursor mole fraction | 3.4 × 10−4 | 3.4 × 10−4 | 5.1 × 10−4 | 2.8 × 10−4 | 2.8 × 10−4 |
| Deposited mass (mg) | 2.8 ± 0.2 | 7.5 ± 0.2 | 10.0 ± 0.2 | 12.1 ± 0.2 | 26.6 ± 0.2 |
| Molecule | Formula | Proposed Structure | Molecule | Formula | Proposed Structure |
|---|---|---|---|---|---|
| TP-1 | C2H7N2O |  | TP-2 | C3H8N2O | 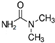 |
| RT (min) LC-MS | Measured mass [M+H]+ (m/z) | Maximum area detected | RT (min) LC-MS | Measured mass [M+H]+ (m/z) | Maximum area detected |
| 1.62 | 75.05613 | 81,178 | 1.84 | 89.07161 | 17,049,090 |
 |  | ||||
| Molecule | Formula | Proposed structure | Molecule | Formula | Proposed structure |
| TP-3 | C8H9N2O2Cl |  | TP-4 | C9H11O2N2Cl | 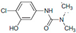 |
| RT (min) LC-MS | Measured mass [M+H]+ (m/z) | Maximum area detected | RT (min) LC-MS | Measured mass [M+H]+ (m/z) | Maximum area detected |
| 5.36 | 201.04262 | 176,634 | 5.96 | 215.05827 | 3,193,831 |
 |  | ||||
| Molecule | Formula | Proposed structure | Molecule | Formula | Proposed structure |
| TP-5 | C9H10N2O2Cl2 |  | TP-6 | C8H8Cl2N2O | 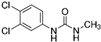 |
| RT (min) LC-MS | Measured mass [M+H]+ (m/z) | Maximum area detected | RT (min) LC-MS | Measured mass [M+H]+ (m/z) | Maximum area detected |
| 14.60 | 249.01920 | 900,214 | 15.23 | 219.00861 | 976,775 |
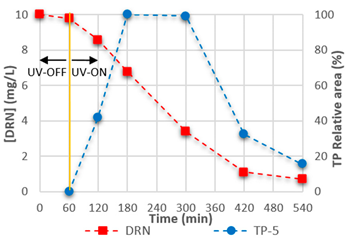 | 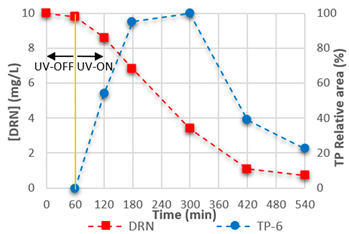 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Castañeda, C.Y.; Tendero, C.; Triquet, T.; Acevedo, P.A.; Latapie, L.; Sierra-Carrillo, M.M.; Andriantsiferana, C. Towards a Better Understanding of the Back-Side Illumination Mode on Photocatalytic Metal–Organic Chemical Vapour Deposition Coatings Used for Treating Wastewater Polluted by Pesticides. Water 2024, 16, 1. https://doi.org/10.3390/w16010001
Quintero-Castañeda CY, Tendero C, Triquet T, Acevedo PA, Latapie L, Sierra-Carrillo MM, Andriantsiferana C. Towards a Better Understanding of the Back-Side Illumination Mode on Photocatalytic Metal–Organic Chemical Vapour Deposition Coatings Used for Treating Wastewater Polluted by Pesticides. Water. 2024; 16(1):1. https://doi.org/10.3390/w16010001
Chicago/Turabian StyleQuintero-Castañeda, Cristian Yoel, Claire Tendero, Thibaut Triquet, Paola Andrea Acevedo, Laure Latapie, María Margarita Sierra-Carrillo, and Caroline Andriantsiferana. 2024. "Towards a Better Understanding of the Back-Side Illumination Mode on Photocatalytic Metal–Organic Chemical Vapour Deposition Coatings Used for Treating Wastewater Polluted by Pesticides" Water 16, no. 1: 1. https://doi.org/10.3390/w16010001






