Enrichment Mechanism of Lithium in Geothermal Waters from a Bedrock Reservoir in Xiong’an New Area, China
Abstract
:1. Introduction
2. Geological Background
3. Materials and Methods
4. Results
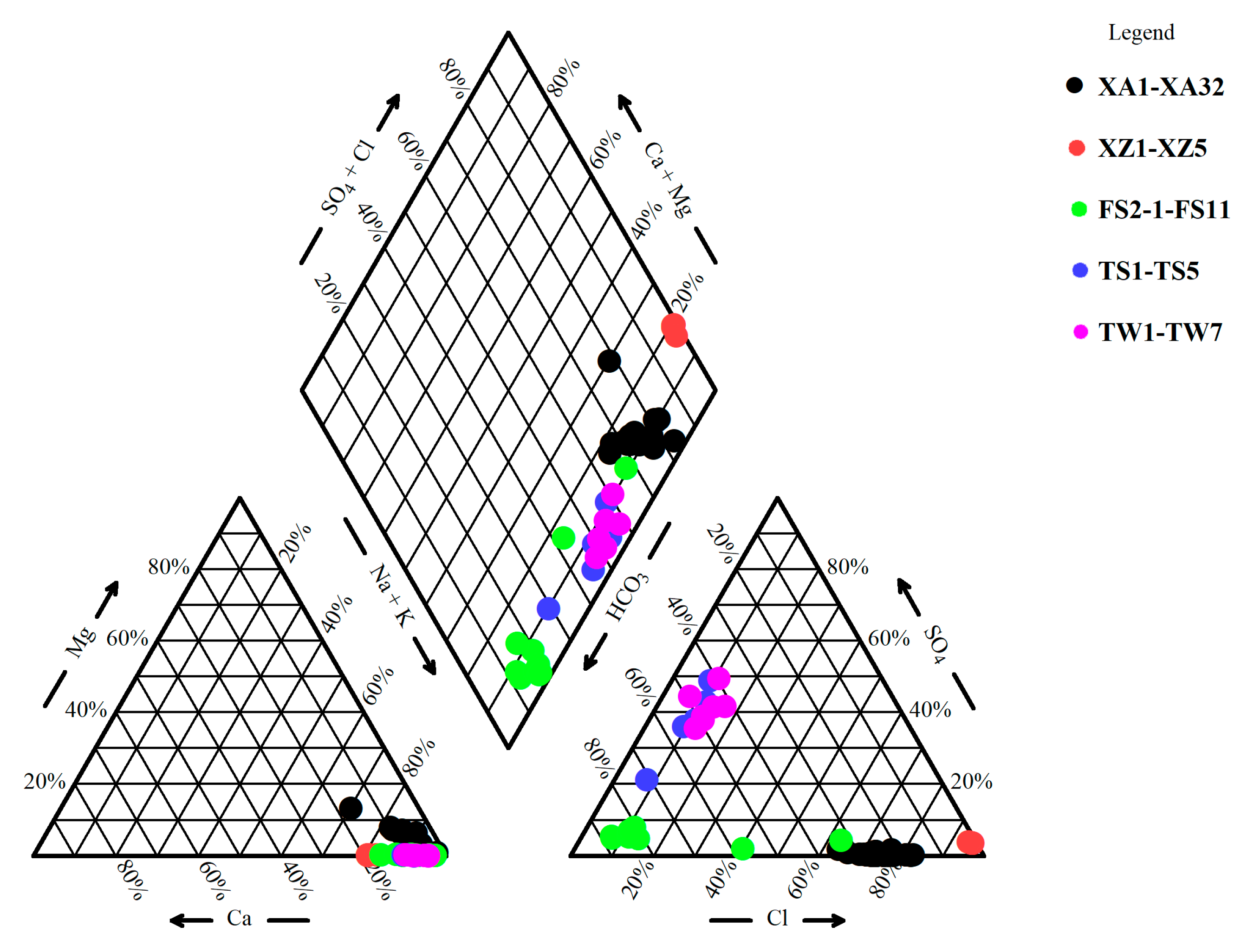
Hydrochemical Characteristics
5. Discussion
5.1. Factors Affecting the Lithium Concentration
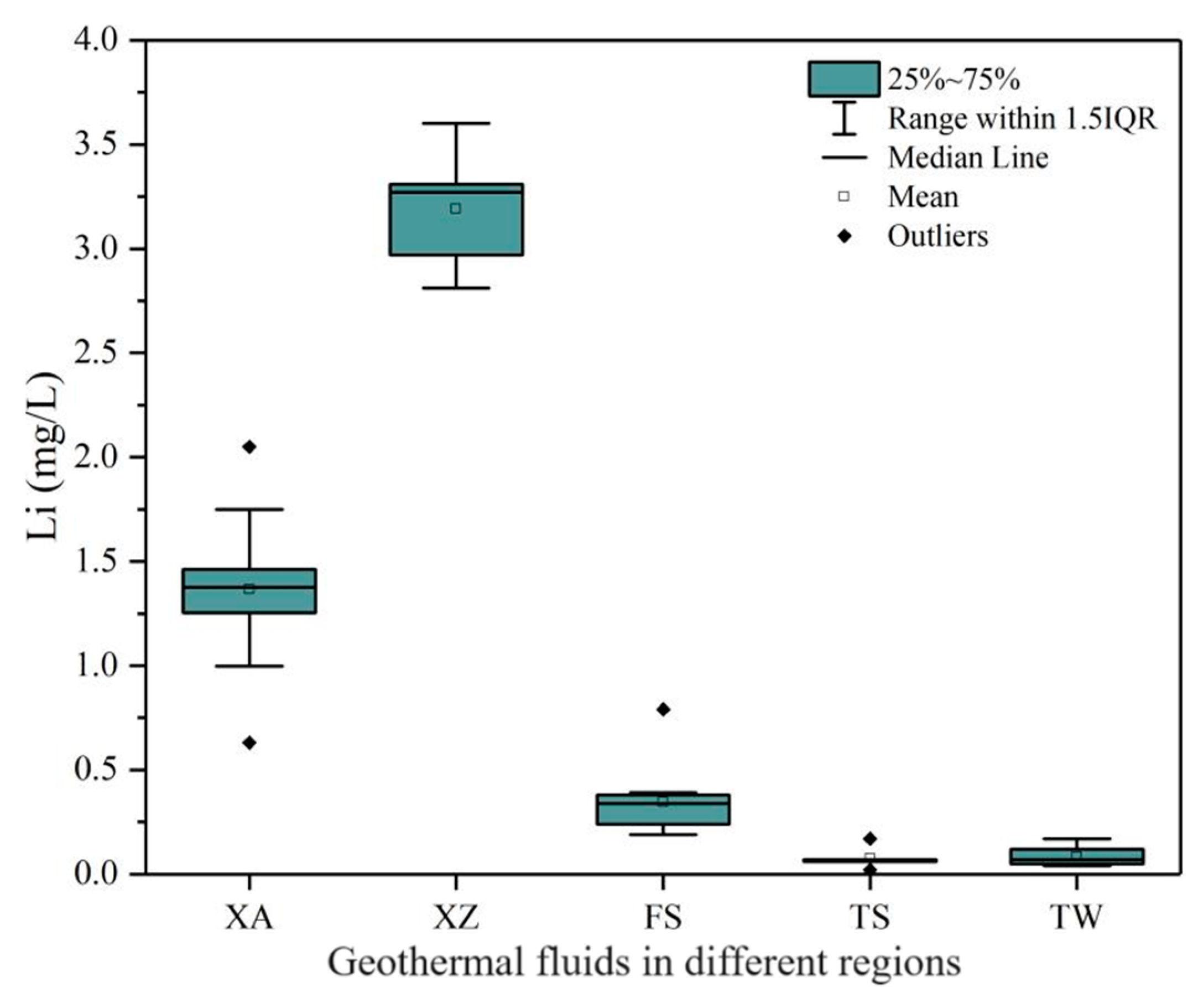
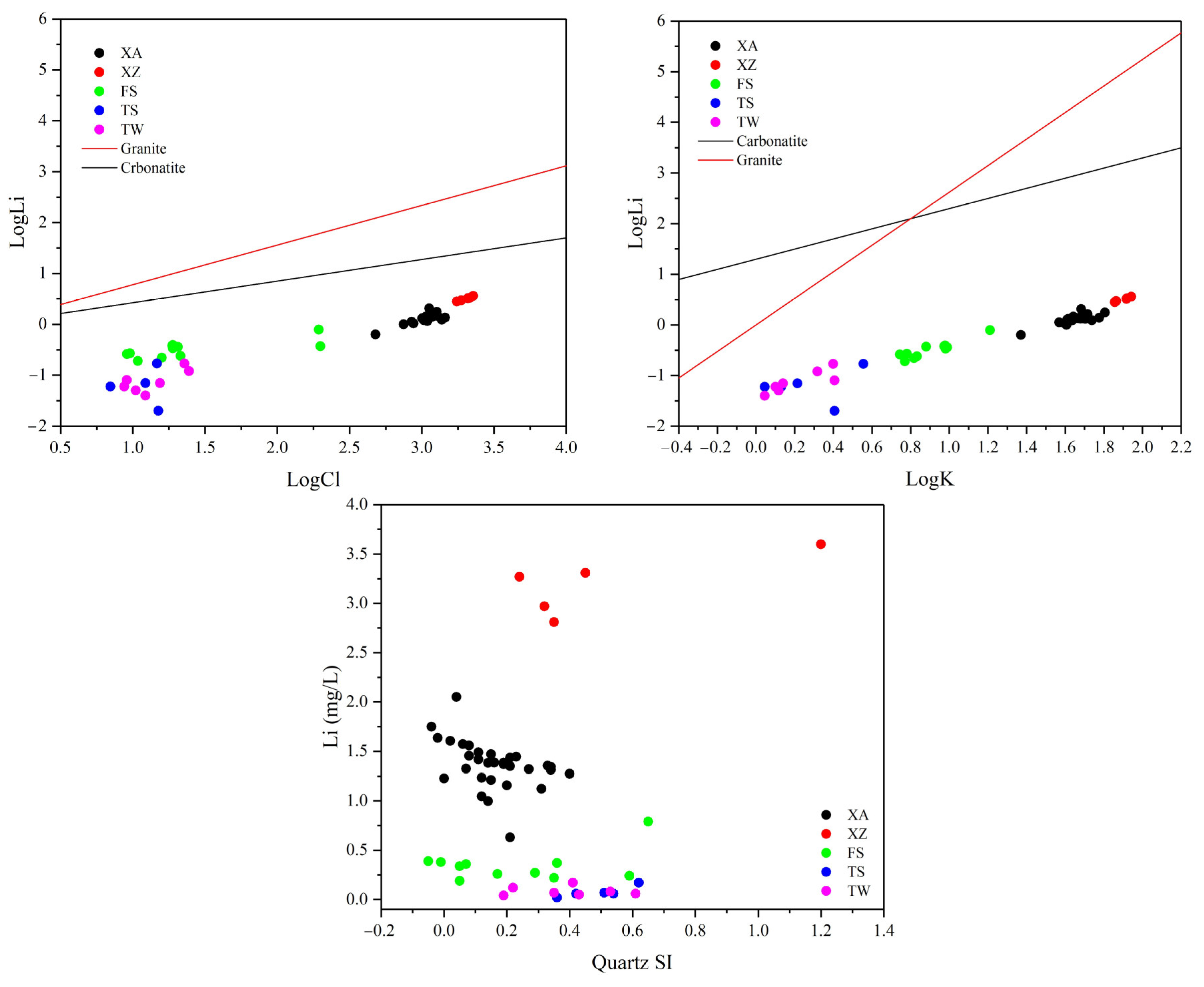
5.1.1. Reservoir Lithology
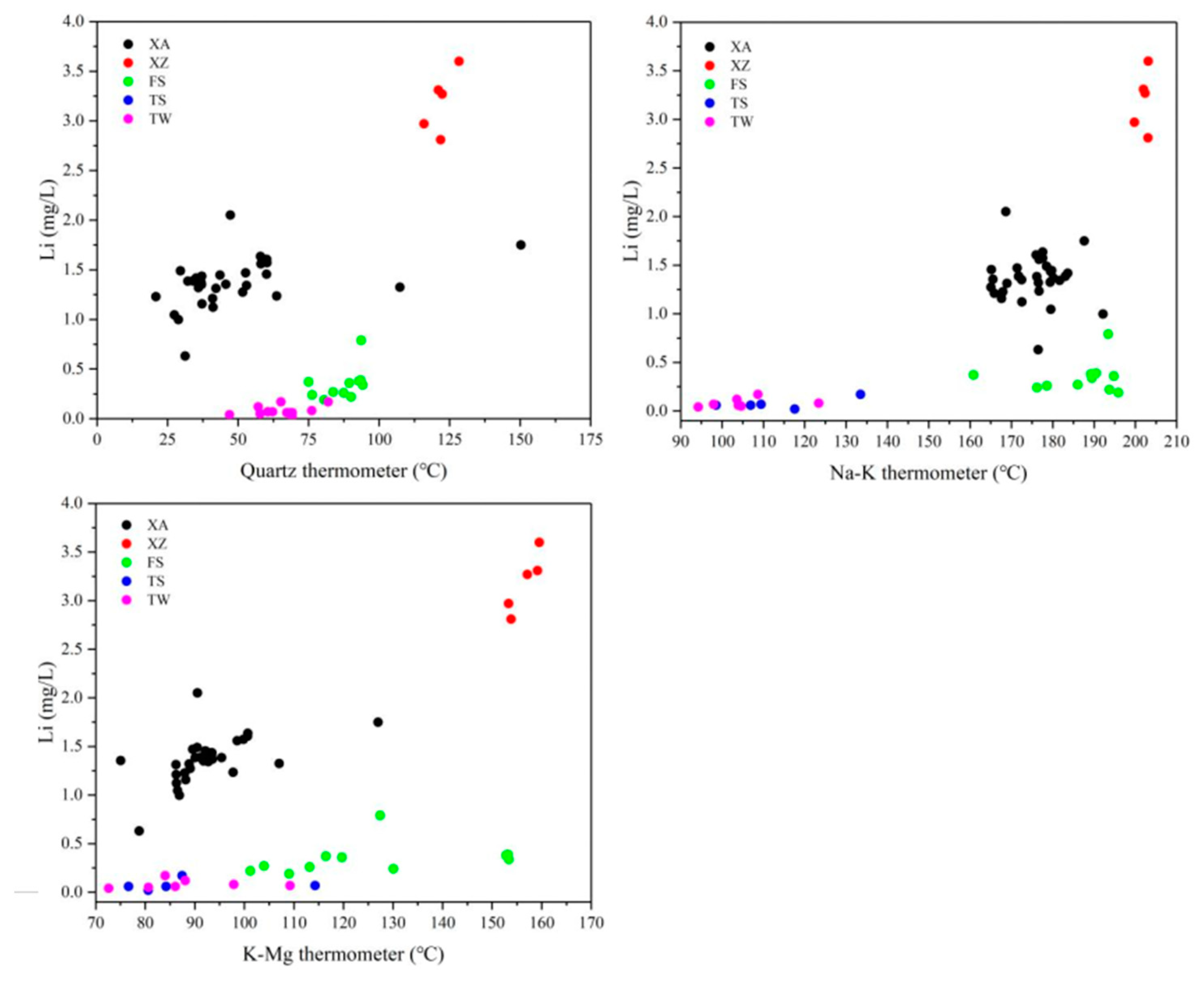
5.1.2. Temperature
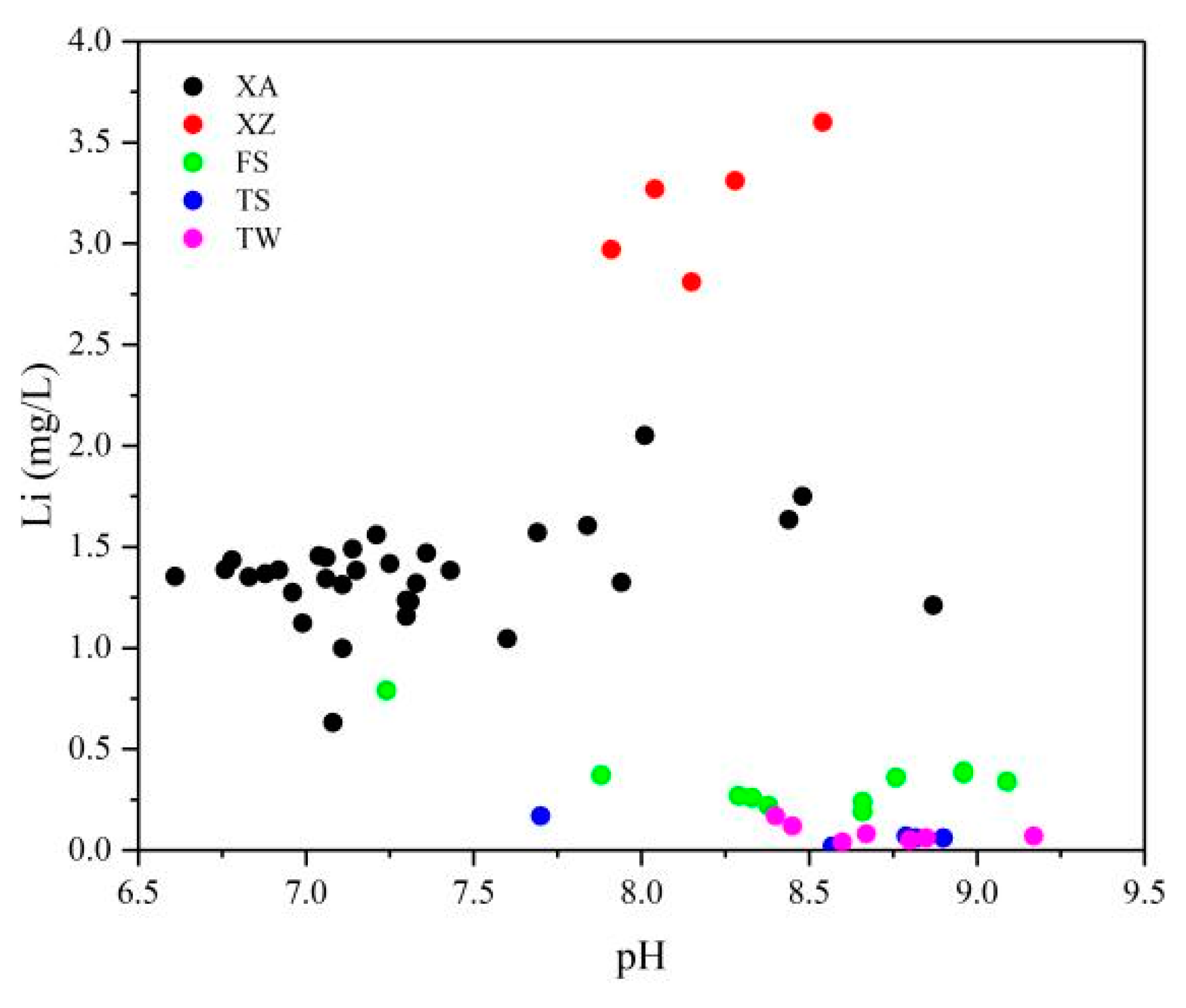
5.1.3. pH
5.1.4. Water–Rock Interaction
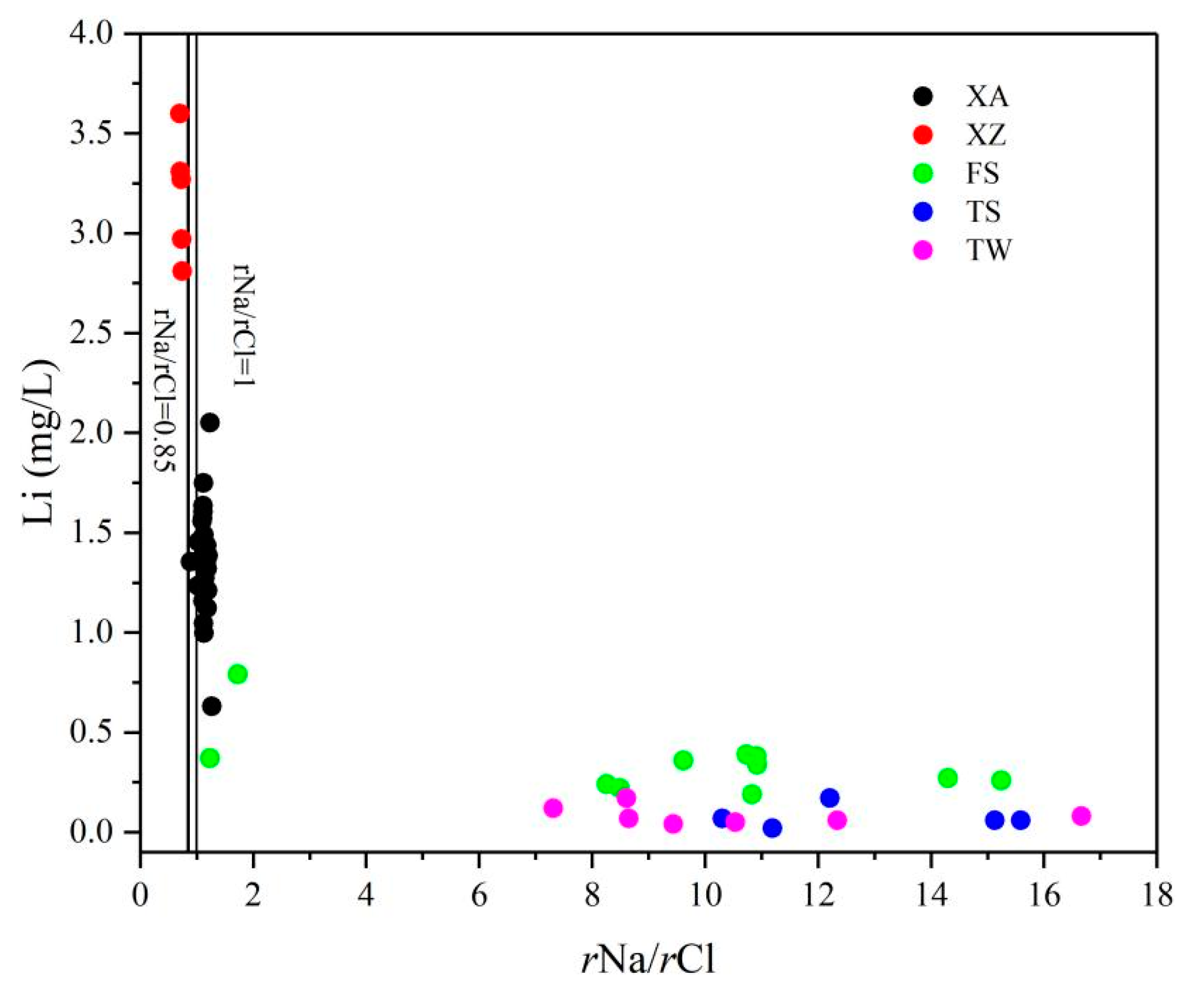
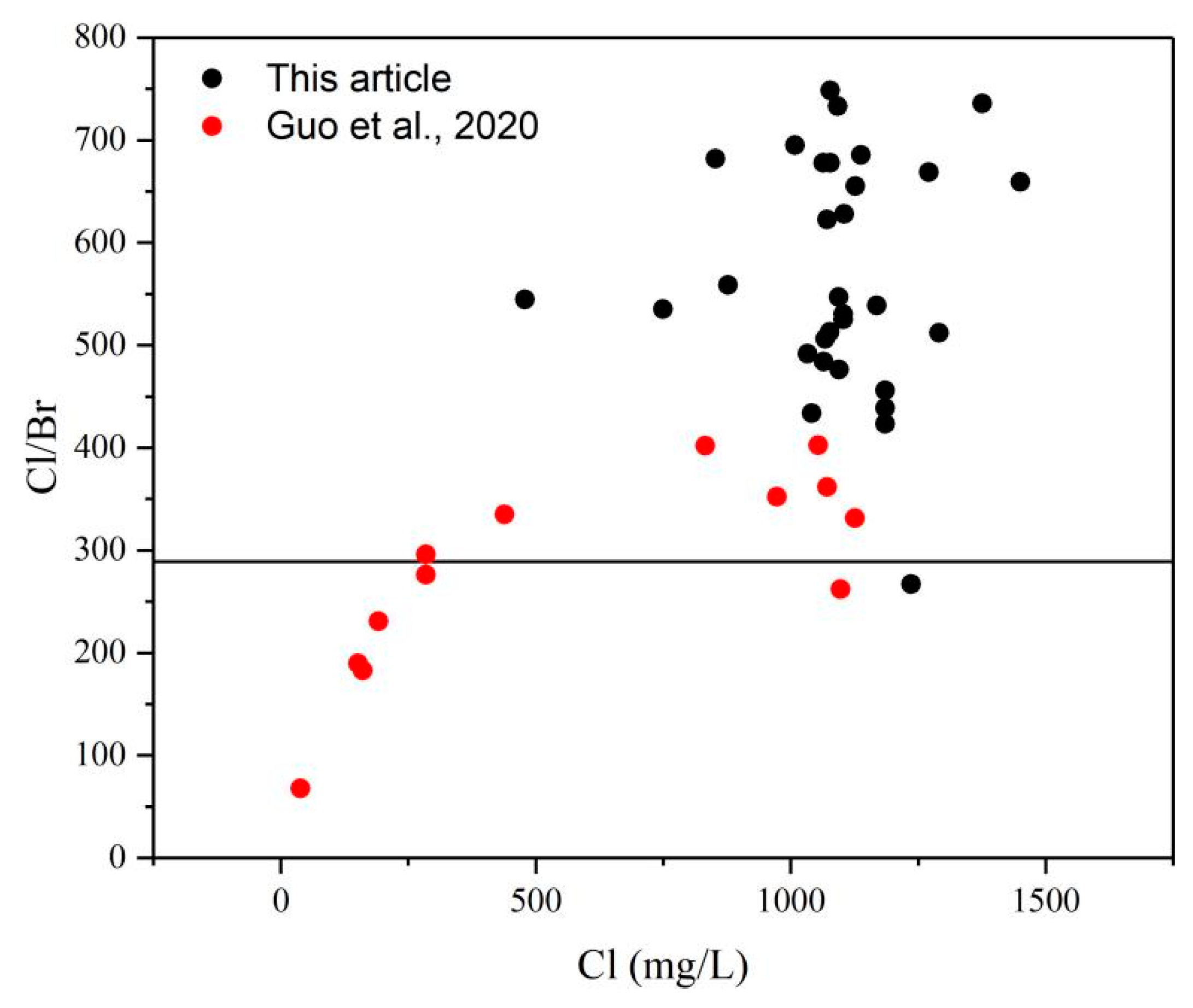
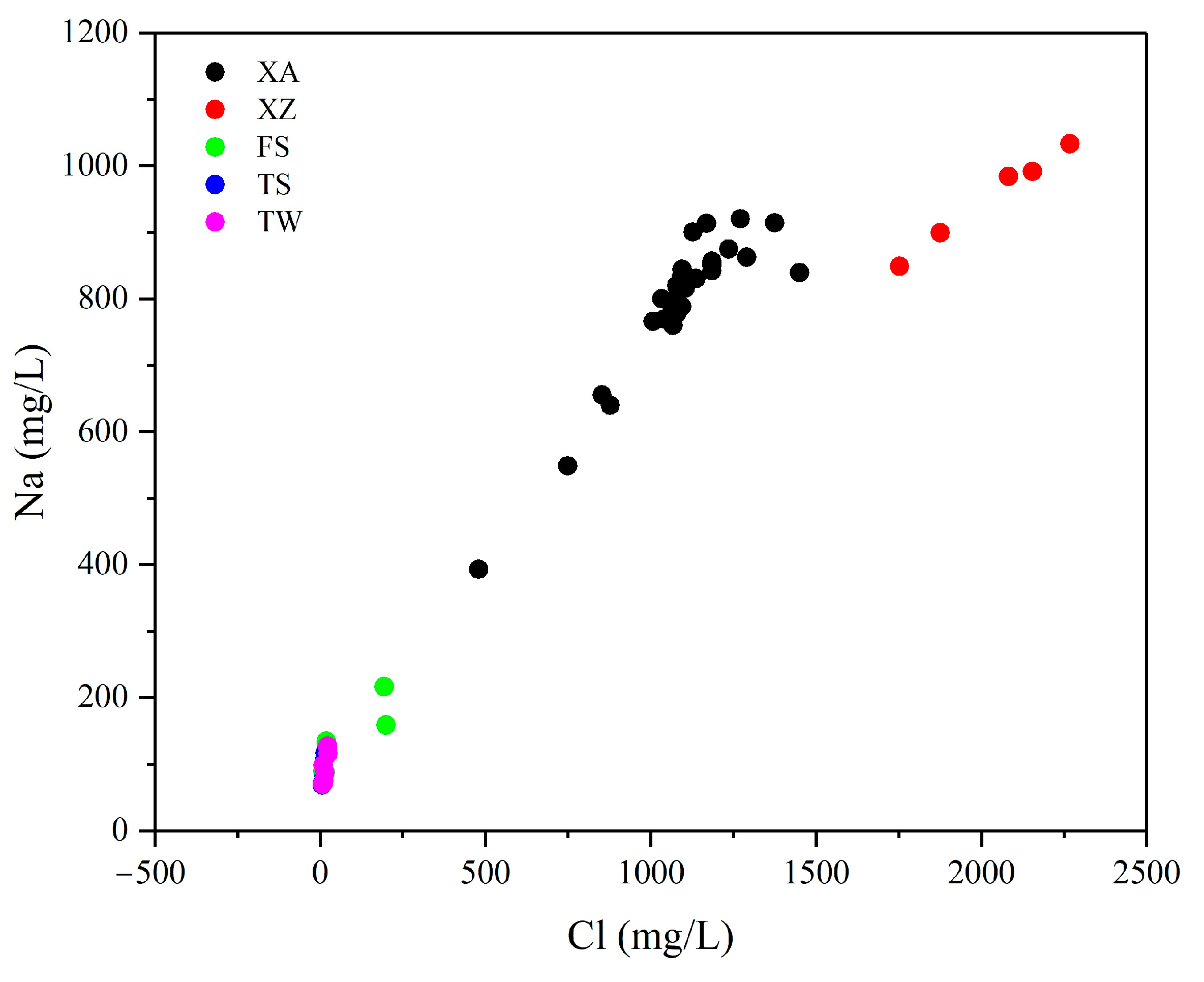
5.2. Evidence of the Influence of Paleo-Seawater on Li Concentrations
5.2.1. rNa/rCl
5.2.2. Cl/Br
5.2.3. Cl Concentrations
5.3. Enrichment Mechanism of Lithium
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut. Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, F.; Wang, Y.; Forson, K.; Cao, L.; Zhang, C.; Zhou, C.; Zhao, B.; Chen, J. Experimental investigation on the high-pressure sand suspension and adsorption capacity of guar gum fracturing fluid in low-permeability shale reservoirs: Factor analysis and mechanism disclosure. Environ. Sci. Pollut. Res. 2022, 29, 53050–53062. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiao, Z.; Liu, X.; Ren, J.; Xing, T.; Li, Z.; Li, X.; Chen, Y. Strategic design of cellulose nanofibers@zeolitic imidazolate frameworks derived mesoporous carbon-supported nanoscale CoFe2O4/CoFe hybrid composition as trifunctional electrocatalyst for Zn-air battery and self-powered overall water-splitting. J. Power Sources 2022, 521, 230925. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Zhang, X.; Wu, Q.; Liu, X.; Ren, J.; Chen, S. Geothermal-type lithium resources in Southern Tibetan Plateau. Sci. Technol. Rev. 2020, 38, 24–36. (In Chinese) [Google Scholar]
- Sanjuan, B.; Gourcerol, B.; Millot, R.; Rettenmaier, D.; Elodie, J.; Aurélien, R. Lithium-rich geothermal brines in Europe: An up-date about geochemical characteristics and implications for potential Li resources. Geothermics 2022, 101, 102385. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Ruan, C.; Gideon, S.; Li, J. Enrichment mechanisms of lithium for the geothermal springs in the southern Tibet, China. J. Hydrol. 2022, 612, 128022. [Google Scholar] [CrossRef]
- Lindsey, B.D.; Belitz, K.; Cravotta, C.A.; Toccalino, P.L.; Dubrovsky, N.M. Lithium in groundwater used for drinking-water supply in the United States. Sci. Total Environ. 2021, 767, 144691. [Google Scholar] [CrossRef]
- Ji, T.; Jiang, X.; Gou, L.; Jin, Z.; Zhang, H.; Wan, L.; Han, G.; Guo, H.; Wang, X. Behaviors of lithium and its isotopes in groundwater with different concentrations of dissolved CO2. Geochim. Cosmochim. Acta 2022, 326, 313–327. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, W.; Tian, Y.; Wang, J.; Ding, R. Genetic Analysis on High-temperature Geothermal Water in Niutuo Geothermal Field, Heibei Province. Urban Geol. 2016, 11, 59–64. (In Chinese) [Google Scholar]
- Guo, Y.; Zhang, Y.; Ji, P.; Li, C. The Analysis of Geological Characteristics and Genetic Model of Niutuozhen Geothermal Field, Hebei Province. Sichuan Nonferrous Met. 2017, 3, 13–16. (In Chinese) [Google Scholar]
- Li, F.; Zeng, J. Characterization of Origin and Evolution of Formation Water in Buried Hill of Jizhong Depression, China, Using Multivariate Statistical Analysis of Geochemical Data. Geofluids 2017, 2017, 5290686. [Google Scholar] [CrossRef]
- Yang, L.; Liu, F.; Jia, Z.; Yuan, H.; Xu, Q.; Hu, Y. The hydrochemical and δ2H-δ18O characteristics of two geothermal fields in Niutuozhen of Hebei province and Tianjin and their environmental significance. Acta Geosci. Sin. 2018, 39, 71–78. (In Chinese) [Google Scholar]
- Zhao, J.; Zhang, W.; Ma, F.; Zhu, X.; Zhang, H.; Wang, G. Geochemical characteristics of the geothermal fluid in the Rongcheng geothermal field, Xiong’an New Area. Acta Geol. Sin. 2020, 94, 1991–2001. (In Chinese) [Google Scholar]
- Liu, M.; He, T.; Wu, Q.; Guo, Q. Hydrogeochemistry of geothermal waters from Xiongan New Area and its indicating significance. Earth Sci. 2020, 45, 2221–2231. (In Chinese) [Google Scholar]
- Guo, Q.; He, T.; Wu, Q.; Liu, M. Constraints of major ions and arsenic on the geological genesis of geothermal water: Insight from a comparison between Xiong’an and Yangbajain, two hydrothermal systems in China. Appl. Geochem. 2020, 117, 104589. [Google Scholar] [CrossRef]
- Dong, Y.; Zeng, J.; Zhao, X.; Wang, Y.; Chen, T.; Zhang, Y.; Feng, S. The Temperature Evaluation of the Buried Hill Geothermal Reservoirs in the Jizhong Depression, Bohai Bay Basin, China. Geofluids 2020, 2020, 4814515. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, G.; Ma, F.; Zhang, W.; Zhang, Q.; Zhang, H. Hydrogeochemistry of geothermal waters from Taihang Mountain-Xiong’an New Area and its indicating significance. Earth Sci. 2021, 46, 2594–2608. (In Chinese) [Google Scholar]
- Xing, Y.; Wang, H.; Li, J.; Teng, Y.; Zhang, B.; Li, Y.; Wang, G. Chemical field characteristics of geothermal water in Xiong’an New Area, and analysis on the influencing factors. Geol. China 2021, 49, 1711–1722. (In Chinese) [Google Scholar]
- Gan, H.; Wang, G.; Wang, X.; Lin, W.; Yue, G. Research on the Hydrochemistry and Fault Control Mechanism of Geothermal Water in Northwestern Zhangzhou Basin. Geofluids 2019, 2019, 3925462. [Google Scholar] [CrossRef]
- Li, J.; Sagoe, G.; Yang, G.; Lu, G. Evaluation of mineral-aqueous chemical equilibria of felsic reservoirs with low-medium temperature: A comparative study in Yangbajing geothermal field and Guangdong geothermal fields. J. Volcanol. Geotherm. Res. 2018, 352, 92–105. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Wang, J.; Deng, X.; Yang, S.; Xiong, L.; Zhang, J. The characteristics of the geothermal field and its formation mechanism in the North China down-fault basin. Acta Geol. Sin. 1990, 64, 80–91. (In Chinese) [Google Scholar]
- Chen, M. Geothermics of North China; Science Press: Beijing, China, 1988; pp. 1–214. (In Chinese) [Google Scholar]
- Pang, J.; Pang, Z.; Lv, M.; Tian, J.; Kong, Y. Geochemical and Isotopic Characteristics of Fluids in the Niutuozhen Geothermal Field, North China. Environ. Earth Sci. 2018, 77, 1–21. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Wang, J.; Li, X.; Liu, H. Occurrence, genesis and travertine deposition of the Adong hot springs in northwestern Yunnan of China. Geothermics 2020, 87, 101851. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Liu, Y.; Xu, H.; Wu, Y.; Zhuo, L. Major, trace and rare earth elements geochemistry of geothermal waters from the Rehai high-temperature geothermal field in Tengchong of China. Appl. Geochem. 2020, 119, 104639. [Google Scholar] [CrossRef]
- Kaasalainen, H.; Stefánsson, A. The chemistry of trace elements in surface geothermal waters and steam, Iceland. Chem. Geol. 2012, 330–331, 60–85. [Google Scholar] [CrossRef]
- Wrage, J.; Tardani, D.; Reich, M.; Daniele, L.; Arancibia, G.; Cembrano, J.; Sánchez-Alfaro, P.; Morata, D.; Pérez-Moreno, R. Geochemistry of thermal waters in the Southern Volcanic Zone, Chile-Implications for structural controls on geothermal fluid composition. Chem. Geol. 2017, 466, 545–561. [Google Scholar] [CrossRef]


| ID | T (°C) | pH | K | Na | Ca | Mg | Cl | SO4 | HCO3 | Li | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| XA1 | 60.5 | 7.25 | 51.68 | 784.1 | 58.75 | 28.62 | 1065 | 16.21 | 674.8 | 1.417 | 35.14 |
| XA2 | 50 | 7.08 | 23.5 | 393.1 | 40.02 | 18.73 | 479.3 | 19.08 | 442.7 | 0.631 | 31.224 |
| XA3 | 52 | 6.92 | 43.46 | 777.3 | 49.29 | 26.15 | 1078 | 1.49 | 651.8 | 1.386 | 32.234 |
| XA4 | 52 | 6.96 | 40.71 | 801.3 | 48.39 | 24.77 | 1077 | 5.74 | 645.1 | 1.274 | 51.62 |
| XA5 | 57 | 7.06 | 48.74 | 759.8 | 48.67 | 26.88 | 1068 | 5.14 | 646.7 | 1.342 | 53.02 |
| XA6 | 51 | 6.99 | 37.08 | 655.4 | 51.29 | 25.54 | 852.6 | 7.71 | 598 | 1.123 | 41.13 |
| XA7 | 53.1 | 6.61 | 42.95 | 839.5 | 176.9 | 85.43 | 1450 | 42.95 | 699.8 | 1.354 | 45.66 |
| XA8 | 52 | 7.11 | 40.46 | 548.7 | 58.41 | 29.01 | 749.3 | 12.33 | 628.1 | 0.998 | 28.86 |
| XA9 | 57 | 7.15 | 59.69 | 913.4 | 66.59 | 32.91 | 1169 | 4.74 | 777.7 | 1.384 | 35.07 |
| XA10 | 56.5 | 6.88 | 51.77 | 823.6 | 52.56 | 28.48 | 1103 | 3.63 | 682 | 1.368 | 36.95 |
| XA11 | 55.5 | 6.83 | 46.33 | 819.8 | 46.16 | 26.12 | 1078 | 24.13 | 646.4 | 1.351 | 37.07 |
| XA12 | 55.1 | 6.78 | 51.78 | 831.5 | 58.06 | 28.71 | 1092 | 9.15 | 676.6 | 1.436 | 37.15 |
| XA13 | 55 | 7.14 | 51.06 | 830.8 | 68.75 | 34.92 | 1138 | 5.88 | 774.4 | 1.49 | 29.57 |
| XA14 | 51.5 | 7.6 | 39.86 | 639.7 | 56.29 | 28.97 | 877.5 | 6.76 | 646.1 | 1.046 | 27.41 |
| XA15 | 56 | 6.76 | 44.52 | 795.5 | 43.85 | 25.39 | 1064 | 2.3 | 640.3 | 1.387 | 33.98 |
| XA16 | 59 | 7.06 | 50.99 | 815.7 | 61.33 | 30.15 | 1105 | 1.37 | 704.5 | 1.446 | 43.58 |
| XA17 | 59 | 7.43 | 47.38 | 796.2 | 54.45 | 25.98 | 1071 | 4.01 | 676.6 | 1.382 | 35.67 |
| XA18 | 123.4 | 8.48 | 63.99 | 920.6 | 17.01 | 4.3 | 1271 | 5.41 | 448.7 | 1.75 | 150.31 |
| XA19 | 85 | 7.69 | 51.53 | 849.6 | 52.09 | 17.67 | 1185 | 2.81 | 647.5 | 1.572 | 60.25 |
| XA20 | 83.2 | 7.21 | 50.5 | 842.4 | 52.49 | 18.63 | 1185 | 2.12 | 653.8 | 1.56 | 58.15 |
| XA21 | 88 | 7.84 | 50.62 | 852.9 | 37.65 | 16.08 | 1185 | 2.64 | 610.2 | 1.605 | 60.15 |
| XA22 | 82 | 8.44 | 51.98 | 856.6 | 48.07 | 16.91 | 1185 | 2.69 | 567.8 | 1.635 | 57.95 |
| XA23 | 83 | 7.3 | 54.8 | 914.3 | 48.1 | 23.3 | 1375.6 | 3.9 | 505.2 | 1.235 | 63.68 |
| XA24 | 85 | 7.04 | 43.9 | 862.7 | 47.3 | 22.8 | 1290.5 | 6.3 | 507.7 | 1.455 | 60.08 |
| XA25 | 72 | 7.36 | 48.7 | 875 | 35.9 | 34.1 | 1236 | 4.8 | 491.8 | 1.47 | 52.69 |
| XA26 | 74.5 | 8.01 | 48.2 | 900.9 | 68.8 | 31 | 1127.3 | 15.6 | 727.3 | 2.05 | 47.23 |
| XA27 | 50 | 7.33 | 50.5 | 844.5 | 62.95 | 38.75 | 1095.4 | 14.05 | 702.43 | 1.32 | 35.92 |
| XA28 | 52 | 8.87 | 41.14 | 800.3 | 62 | 31.52 | 1033 | 23.96 | 618.8 | 1.211 | 41 |
| XA29 | 52 | 7.31 | 43.37 | 819 | 64.31 | 30.58 | 1103 | 0.96 | 708.3 | 1.228 | 20.86 |
| XA30 | 50 | 7.11 | 41.17 | 766.3 | 64.3 | 31.68 | 1008 | 5.4 | 686.5 | 1.312 | 42.2 |
| XA31 | 109.2 | 7.94 | 47.83 | 769.2 | 35.5 | 9.1 | 1041 | 12.77 | 540.7 | 1.325 | 107.38 |
| XA32 | 56 | 7.3 | 41.6 | 788.8 | 57.01 | 27.78 | 1094 | 2 | 688.3 | 1.157 | 37.2 |
| ID | T (°C) | pH | K | Na | Ca | Mg | Cl | SO4 | HCO3 | Li | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| XZ1 | 85.8 | 7.91 | 73.08 | 899.5 | 190.3 | 1.17 | 1875.55 | 97.93 | 48.78 | 2.97 | 115.93 |
| XZ2 | / | 8.54 | 87.47 | 1033 | 220.8 | 1.19 | 2268.2 | 112.1 | 34.96 | 3.6 | 128.36 |
| XZ3 | 94.8 | 8.04 | 82.56 | 984.1 | 211.6 | 1.21 | 2082.57 | 105.25 | 49.8 | 3.27 | 122.36 |
| XZ4 | 72.4 | 8.28 | 82.8 | 991.8 | 214 | 1.09 | 2154.43 | 107.61 | 42.87 | 3.31 | 121.07 |
| XZ5 | 82.7 | 8.15 | 71.87 | 849.2 | 157.7 | 1.1 | 1752.23 | 96.07 | 60.35 | 2.81 | 121.82 |
| FS2-1 | 52.5 | 7.24 | 16.24 | 216.7 | 37.16 | 0.27 | 193.7 | 13.08 | 468.98 | 0.79 | 93.71 |
| FS2-2 | 66.3 | 7.88 | 7.59 | 159 | 16.74 | 0.12 | 198.92 | 18.58 | 175.33 | 0.37 | 75.02 |
| FS3 | 85.8 | 8.33 | 5.55 | 90.19 | 5.92 | 0.08 | 9.13 | 9.38 | 190.26 | 0.26 | 87.47 |
| FS4 | 74.3 | 8.29 | 6.04 | 88.75 | 7.37 | 0.18 | 9.58 | 8.6 | 190.97 | 0.27 | 83.70 |
| FS5 | 88.2 | 8.66 | 5.89 | 76.15 | 5.63 | 0.12 | 10.85 | 10.14 | 131.02 | 0.19 | 80.53 |
| FS6 | 71.2 | 8.38 | 6.57 | 87.38 | 10.9 | 0.26 | 15.88 | 9.61 | 196.52 | 0.22 | 90.09 |
| FS7 | 46.6 | 8.66 | 6.81 | 114.4 | 4.64 | 0.04 | 21.39 | 10.02 | 213.13 | 0.24 | 76.31 |
| FS8 | 89.6 | 8.96 | 9.42 | 132.8 | 3.41 | 0.02 | 18.78 | 16.45 | 245.68 | 0.38 | 92.91 |
| FS9 | 87.5 | 8.76 | 9.76 | 128 | 4.83 | 0.16 | 20.53 | 15.6 | 266.22 | 0.36 | 89.44 |
| FS10 | 92.4 | 8.96 | 9.52 | 131.9 | 3.43 | 0.02 | 18.96 | 15.89 | 246.99 | 0.39 | 93.51 |
| FS11 | 81.4 | 9.09 | 9.57 | 134.4 | 3.36 | 0.02 | 18.99 | 15.92 | 237.54 | 0.339 | 94.26 |
| TS1 | 53 | 8.82 | 1.35 | 70.69 | 2.84 | 0.04 | 7 | 36.42 | 70.31 | 0.06 | 67.26 |
| TS2 | 45.5 | 8.9 | 1.11 | 68.62 | 2.97 | 0.05 | 7 | 35.9 | 48.91 | 0.06 | 68.82 |
| TS3 | 45 | 8.79 | 1.64 | 81.8 | 6.14 | 0.0065 | 12.25 | 55.61 | 94.77 | 0.07 | 60.59 |
| TS4 | 50.1 | 7.7 | 3.6 | 116.4 | 10.85 | 0.22 | 14.71 | 53.89 | 229.3 | 0.17 | 81.92 |
| TS5 | 60.2 | 8.57 | 2.55 | 109.2 | 11.17 | 0.19 | 15.06 | 106.2 | 116.2 | 0.02 | 69.23 |
| TW1 | 42.1 | 8.85 | 1.26 | 69.98 | 2.87 | 0.03 | 8.75 | 36.44 | 58.08 | 0.06 | 69.18 |
| TW2 | 48.4 | 8.8 | 1.31 | 71.71 | 3.46 | 0.05 | 10.5 | 40.85 | 67.25 | 0.05 | 57.81 |
| TW3 | 55.8 | 8.4 | 2.51 | 127 | 12.46 | 0.14 | 22.76 | 135.9 | 137.6 | 0.17 | 65.20 |
| TW4 | 50.2 | 8.67 | 2.55 | 98.27 | 6.88 | 0.05 | 9.1 | 83.36 | 116.8 | 0.08 | 76.10 |
| TW5 | 50 | 9.17 | 1.38 | 86.36 | 3.48 | 0.0065 | 15.41 | 52.69 | 67.25 | 0.07 | 62.29 |
| TW6 | 65 | 8.45 | 2.08 | 116.2 | 9.25 | 0.07 | 24.51 | 100.4 | 137.6 | 0.12 | 57.16 |
| TW7 | 60 | 8.6 | 1.11 | 74.91 | 3.6 | 0.07 | 12.25 | 47.63 | 88.65 | 0.04 | 46.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, H.; Zhang, Y.; Wang, L.; Zhang, Z. Enrichment Mechanism of Lithium in Geothermal Waters from a Bedrock Reservoir in Xiong’an New Area, China. Water 2023, 15, 3518. https://doi.org/10.3390/w15193518
Li J, Zhang H, Zhang Y, Wang L, Zhang Z. Enrichment Mechanism of Lithium in Geothermal Waters from a Bedrock Reservoir in Xiong’an New Area, China. Water. 2023; 15(19):3518. https://doi.org/10.3390/w15193518
Chicago/Turabian StyleLi, Jun, Hanxiong Zhang, Yinmei Zhang, Laibin Wang, and Zhigang Zhang. 2023. "Enrichment Mechanism of Lithium in Geothermal Waters from a Bedrock Reservoir in Xiong’an New Area, China" Water 15, no. 19: 3518. https://doi.org/10.3390/w15193518




