Photodegradation of Rhodamine B and Phenol Using TiO2/SiO2 Composite Nanoparticles: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SiO2 Powder
2.2. Synthesis of TiO2 Powder
2.3. Synthesis of TiO2/SiO2 Composite Powder
2.4. Characterization of the TiO2/SiO2 Composite Powder
2.5. Photocatalytic Activity Study of the TiO2/SiO2 Composite Powder
3. Results
3.1. Characterization of the TiO2/SiO2 Composite Powder
3.1.1. XRD Analysis
3.1.2. Micro-Raman Analysis
3.1.3. FTIR Analysis
3.1.4. BET Analysis
3.1.5. Dynamic Light Scattering (DLS) Analysis
3.1.6. Diffuse Reflectance UV–Vis Spectroscopy Analysis (DRS)
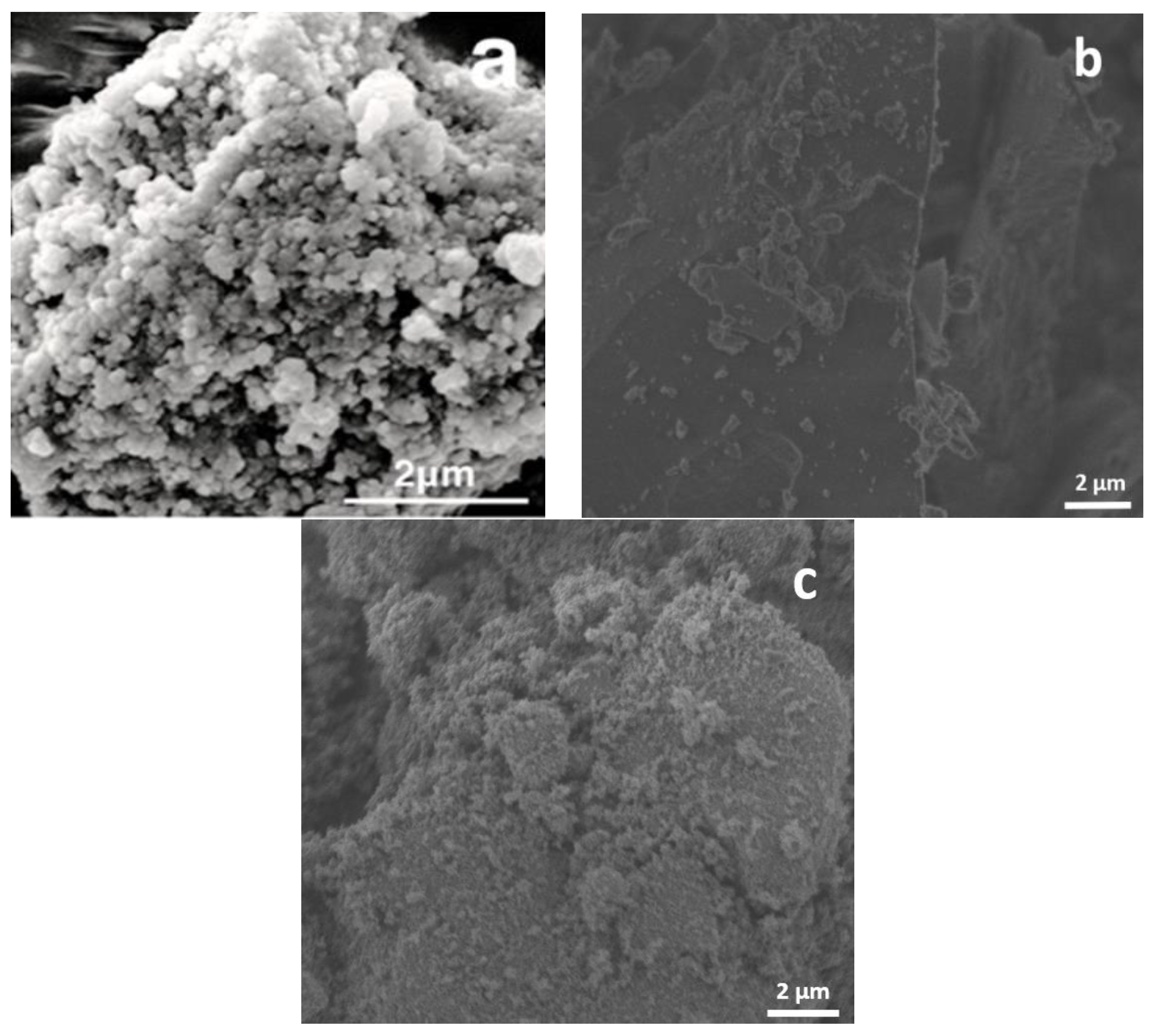
3.1.7. FESEM Analysis
3.2. Photocatalytic Study of TiO2/SiO2 Composite Powder
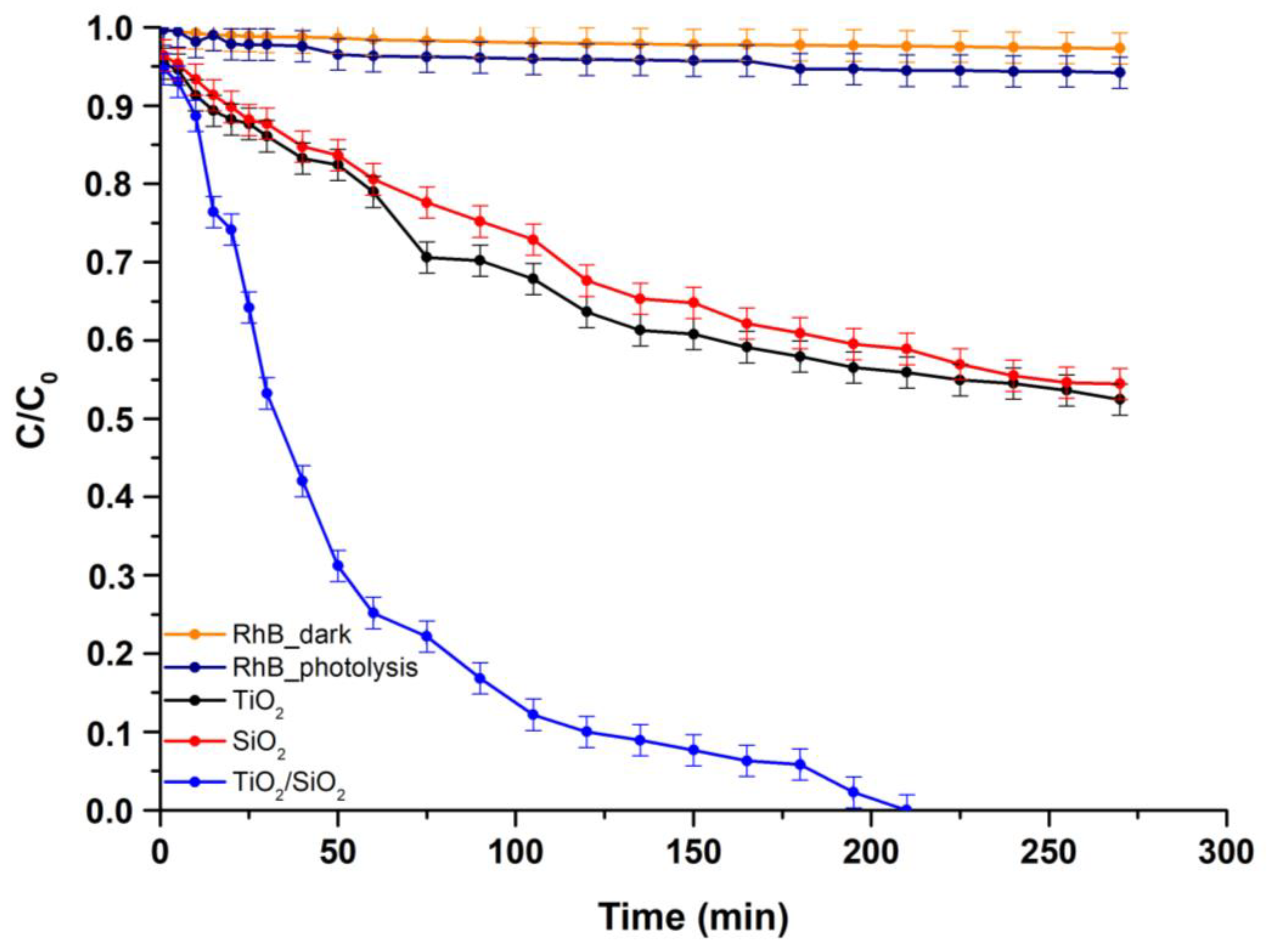
3.2.1. Study of the Photocatalytic Efficiency of TiO2/SiO2 towards RhB Degradation
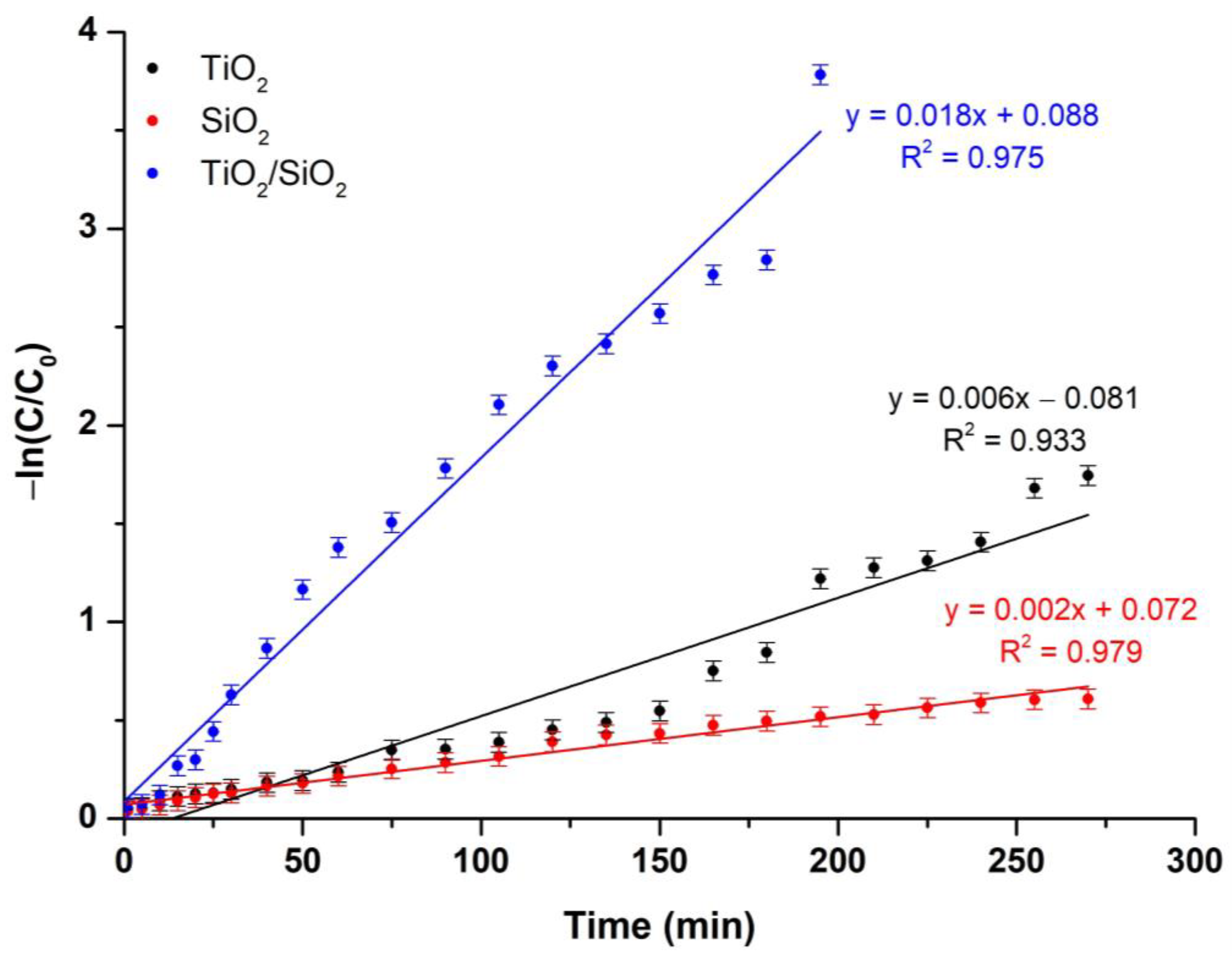
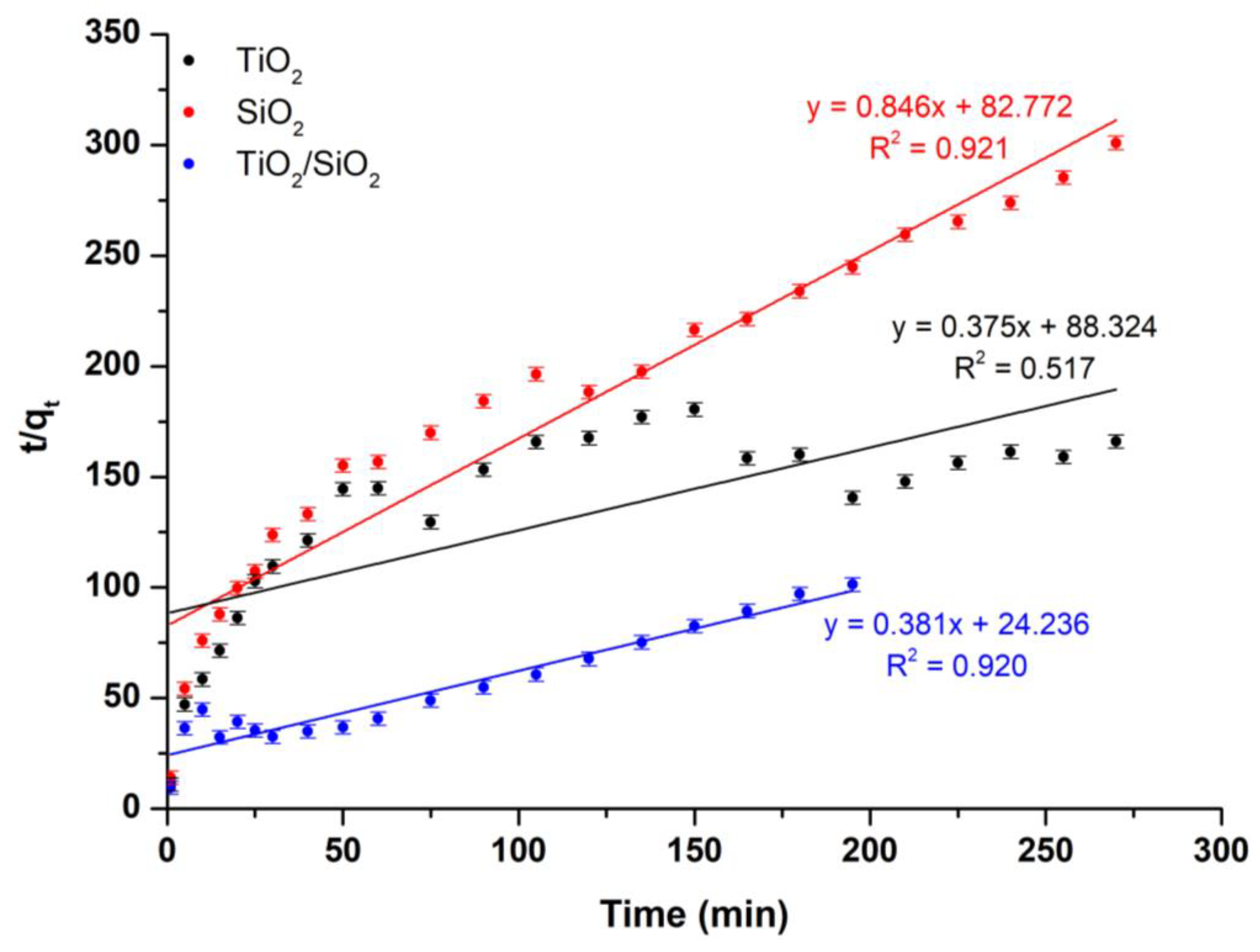
Kinetic Model Study
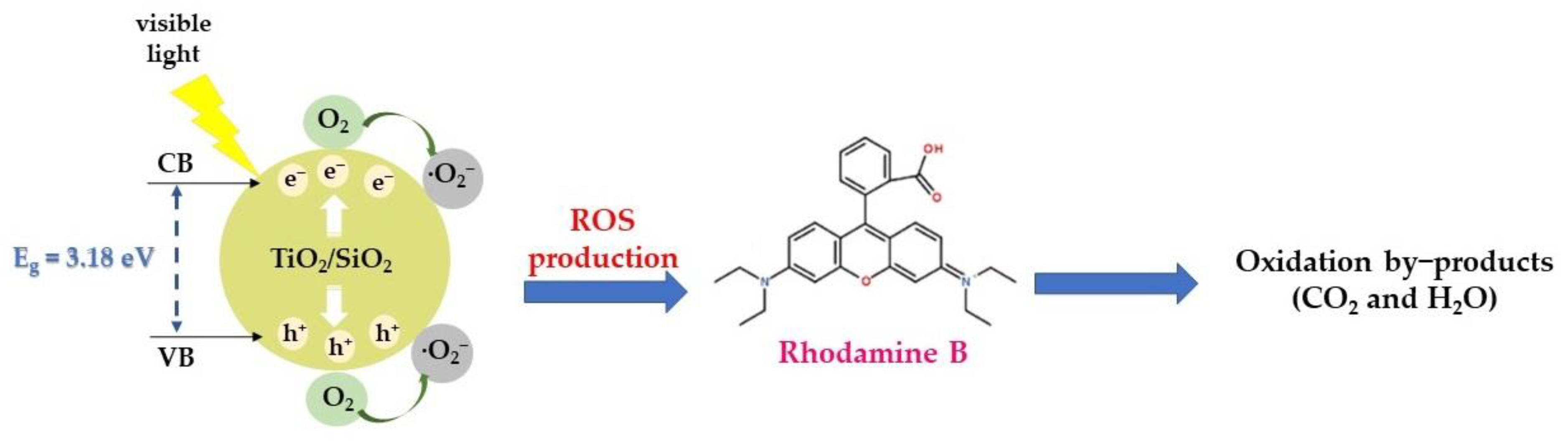
Study of the Photocatalytic Mechanism
Reusability Study
3.2.2. Photocatalytic Efficiency towards Phenol Degradation
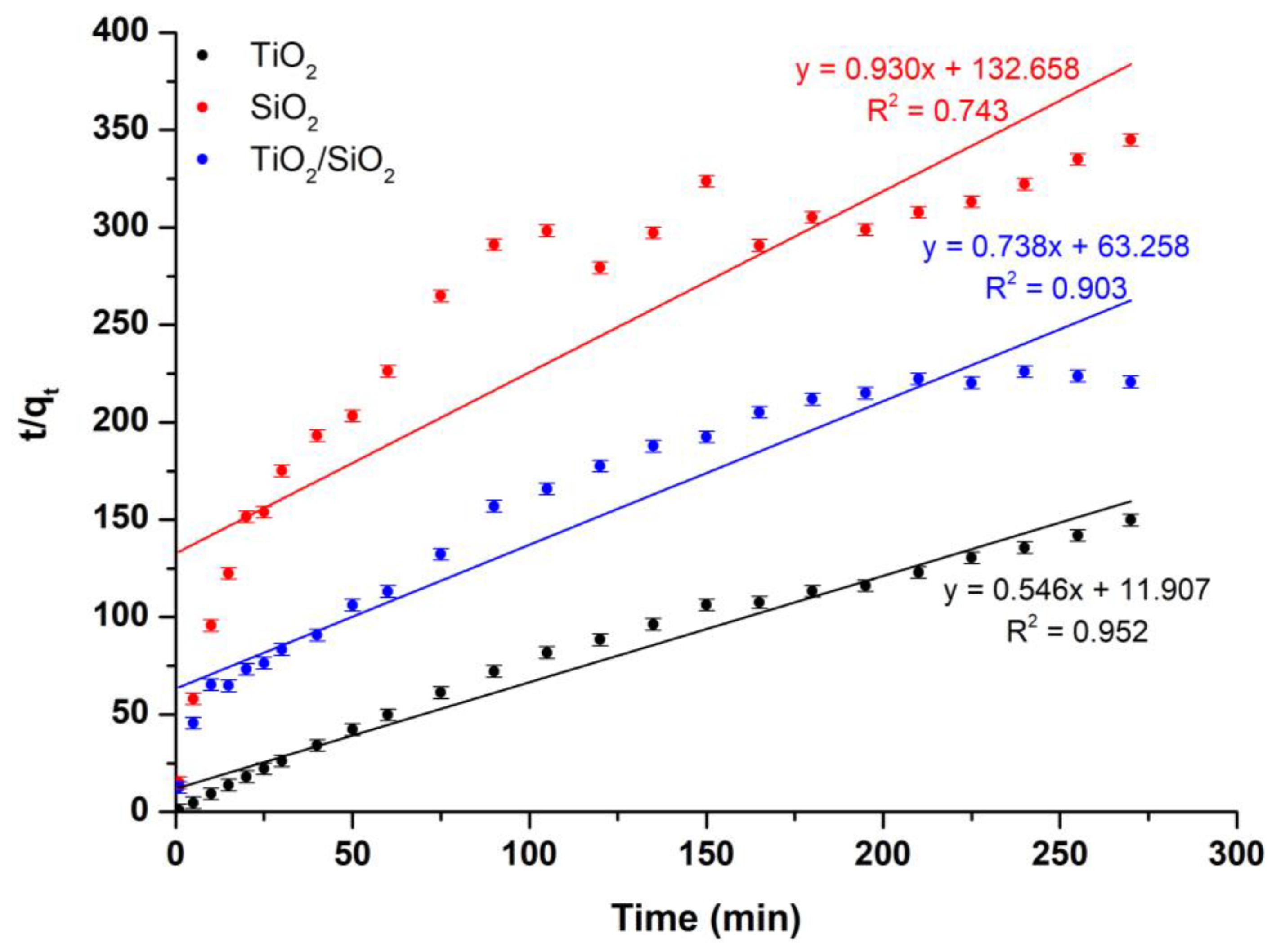
Study of the Kinetic Model
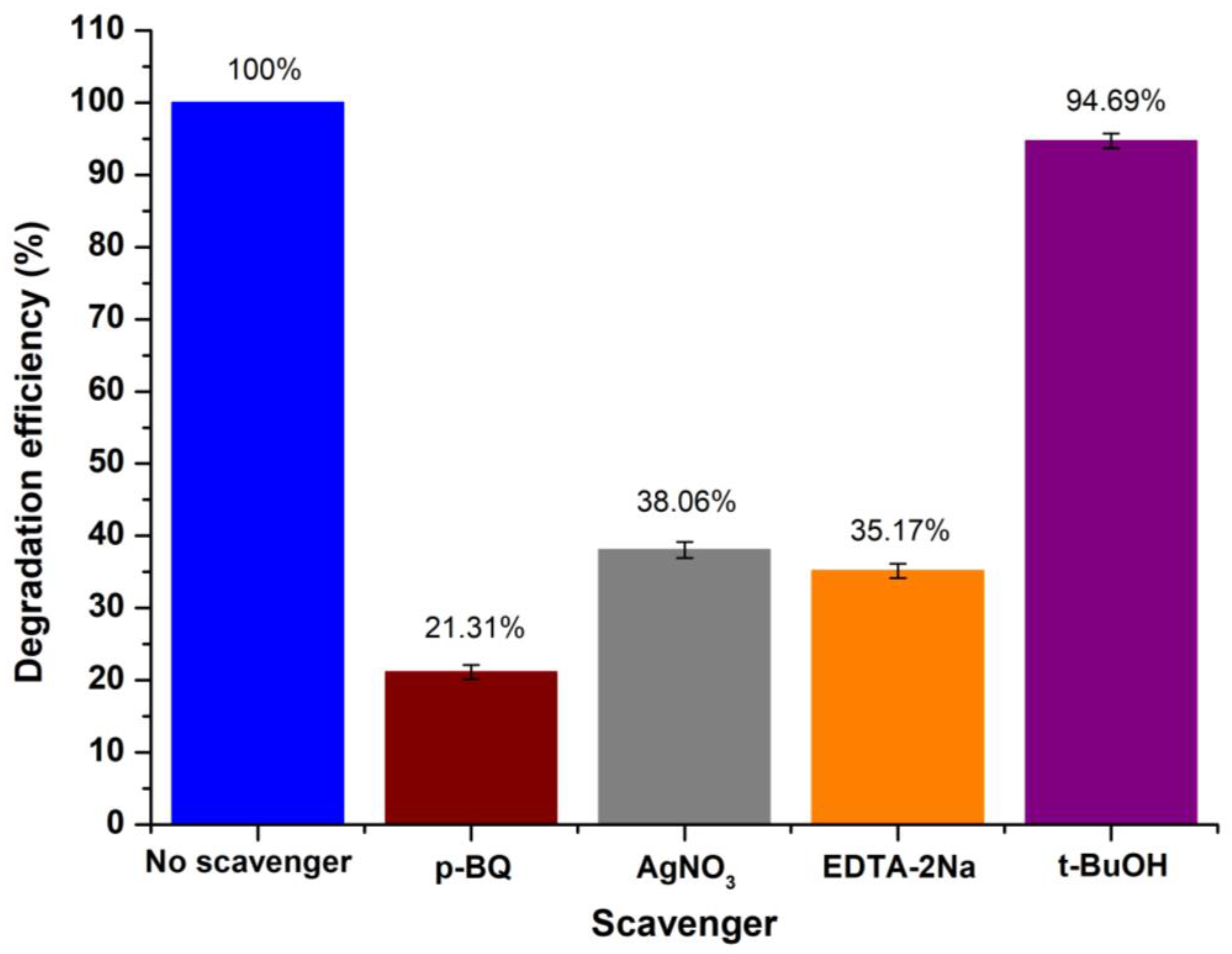
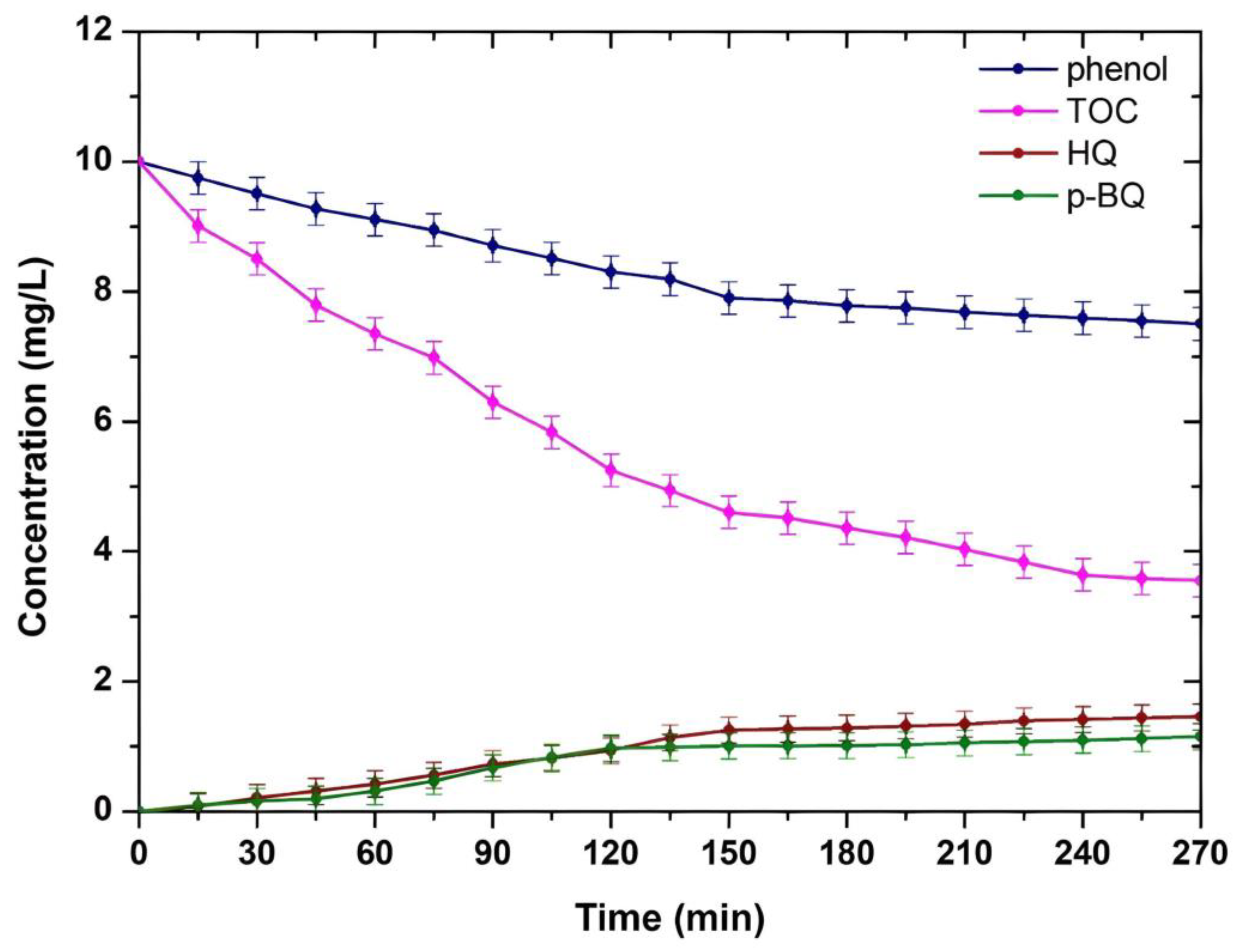
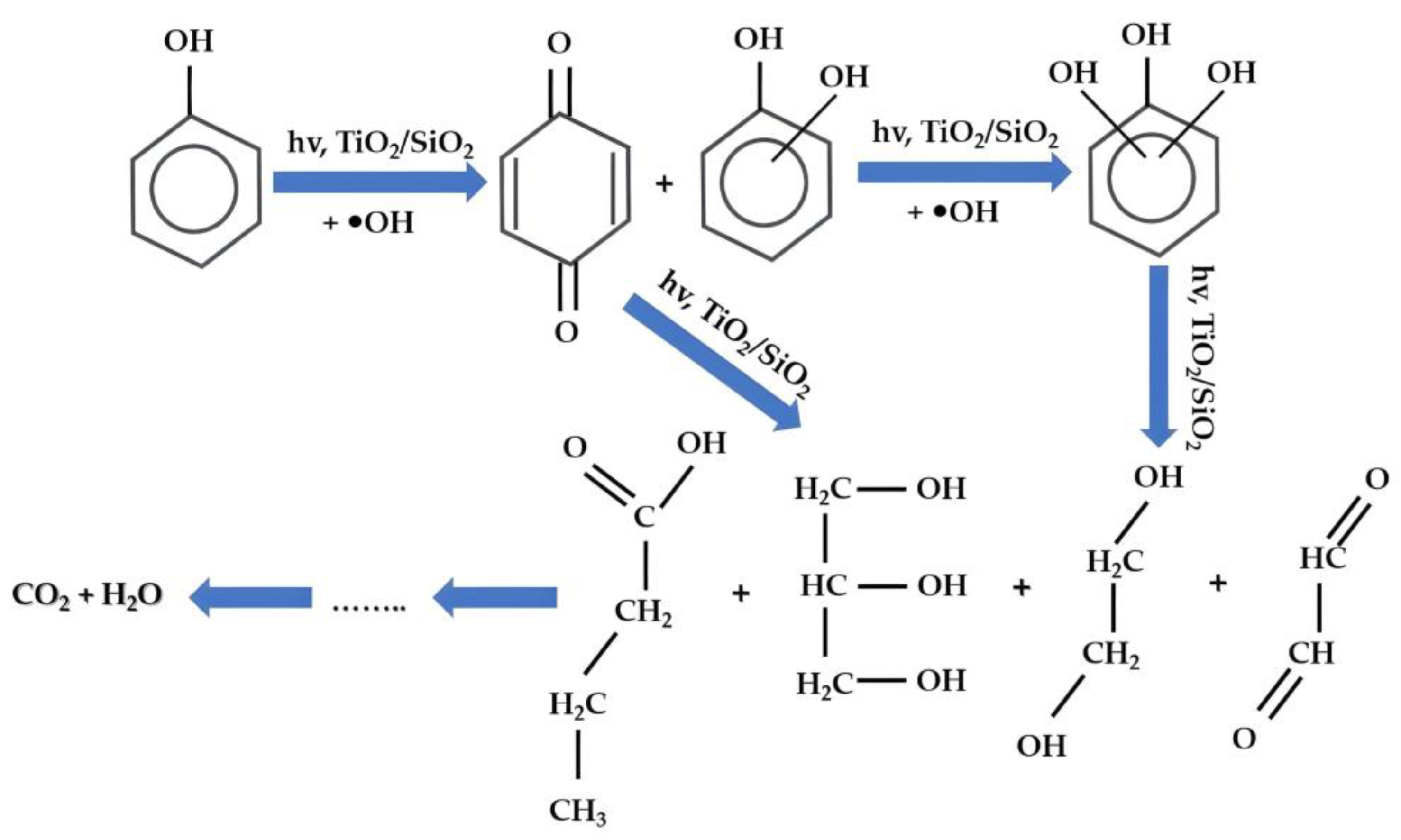
Study of the Photocatalytic Mechanism
Reusability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNESCO. The United Nations World Water Development Report 2020 Water and Climate Change; UNESCO: Paris, France, 2020. [Google Scholar]
- Khan, S.; Malik, A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ. Sci. Pollut. Res. 2018, 25, 4446–4458. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, I.; Adeel, M.; Akram, N.; Muneer, M. Synthesis of a CoO–ZnO photocatalyst for enhanced visible-light assisted photodegradation of methylene blue. New J. Chem. 2022, 46, 2224–2231. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, N.; Priyadarshini, A.; Sahoo, M.M.; Verma, A.K.; Daverey, A.; Sahoo, N.K. A comprehensive review on eco-toxicity and biodegradation of phenolics: Recent progress and future outlook. Environ. Technol. Innov. 2022, 27, 102423. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Asiedu, T.; Agyei-Tuffour, B.; Nyankson, E.; Obada, D.; Mwabora, J.M. Photocatalytic degradation of rhodamine dyes using zinc oxide nanoparticles. Mater. Today Proc. 2021, 38, 809–815. [Google Scholar] [CrossRef]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal synthesis of ZnO photocatalyst for detoxification of anionic azo dyes and antibiotic. J. Phys. Chem. Solids 2022, 160, 110353. [Google Scholar] [CrossRef]
- Vaez, Z.; Javanbakht, V. Synthesis, characterization and photocatalytic activity of ZSM-5/ZnO nanocomposite modified by Ag nanoparticles for methyl orange degradation. J. Photochem. Photobiol. A 2020, 388, 112064. [Google Scholar] [CrossRef]
- Khataee, A.R.; Pons, M.N.; Zahraa, O. Photocatalytic degradation of three azo dyes using immobilized TiO2 nanoparticles on glass plates activated by UV light irradiation: Influence of dye molecular structure. J. Hazard. Mater. 2009, 168, 451–457. [Google Scholar] [CrossRef]
- John Peter, I.; Praveen, E.; Vignesh, G.; Nithiananthi, P. ZnO nanostructures with different morphology for enhanced photocatalytic activity. Mater. Res. Express 2017, 4, 124003. [Google Scholar] [CrossRef]
- Zhang, D.E.; Ren, L.Z.; Hao, X.Y.; Pan, B.B.; Wang, M.Y.; Ma, J.J.; Li, F.; Li, S.A.; Tong, Z.W. Synthesis and photocatalytic property of multilayered Co3O4. Appl. Surf. Sci. 2015, 355, 547–552. [Google Scholar] [CrossRef]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic degradation of pollutants in petroleum refinery wastewater by TiO2- and ZnO-based photocatalysts: Recent development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Sinar Mashuri, S.I.; Ibrahim, M.L.; Kasim, M.F.; Mastuli, M.S.; Rashid, U.; Abdullah, A.H.; Islam, A.; Asikin Mijan, N.; Tan, Y.H.; Mansir, N.; et al. Photocatalysis for organic wastewater treatment: From the basis to current challenges for society. Catalysts 2020, 10, 1260. [Google Scholar] [CrossRef]
- Tanji, K.; Navio, J.A.; Chaqroune, A.; Naja, J.; Puga, F.; Hidalgo, M.C.; Kherbeche, A. Fast photodegradation of rhodamine B and caffeine using ZnO-hydroxyapatite composites under UV-light illumination. Catal. Today 2022, 388–389, 176–186. [Google Scholar] [CrossRef]
- Lagopati, N.; Tsilibary, E.P.; Falaras, P.; Papazafiri, P.; Pavlatou, E.A.; Kotsopoulou, E.; Kitsiou, P. Effect of nanostructured TiO2 crystal phase on photoinduced apoptosis of breast cancer epithelial cells. Int. J. Nanomed. 2014, 9, 3219–3230. [Google Scholar] [CrossRef] [Green Version]
- Lagopati, N.; Kitsiou, P.; Kontos, A.; Venieratos, P.; Kotsopoulou, E.; Kontos, A.; Dionysiou, D.; Pispas, S.; Tsilibary, E.; Falaras, P. Photo-induced treatment of breast epithelial cancer cells using nanostructured titanium dioxide solution. J. Photochem. Photobiol. A Chem. 2010, 214, 215–223. [Google Scholar] [CrossRef]
- Anitha, B.; Khadar, M.A. Anatase-rutile phase transformation and photocatalysis in peroxide gel route prepared TiO2 nanocrystals: Role of defect states. Solid State Sci. 2020, 108, 106392. [Google Scholar] [CrossRef]
- Lagopati, N.; Kotsinas, A.; Veroutis, D.; Evangelou, K.; Papaspyropoulos, A.; Arfanis, M.; Falaras, P.; Kitsiou, P.V.; Pateras, I.; Bergonzini, A.; et al. Biological effect of silver-modified nanostructured titanium dioxide in cancer. Cancer Genom. Proteom. 2021, 18 (Suppl. S3), 425–439. [Google Scholar] [CrossRef]
- Nur, A.S.M.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Xu, T.; Wang, P.; Wang, D.; Zhao, K.; Wei, M.; Liu, X.; Liu, H.; Cao, J.; Chen, Y.; Fan, H.; et al. Ultrasound-assisted synthesis of hyper-dispersed type-II tubular Fe3O4@SiO2@ZnO/ZnS core/shell heterostructure for improved visible-light photocatalysis. J. Alloys Compd. 2020, 838, 155689. [Google Scholar] [CrossRef]
- Dong, C.; Ji, J.; Yang, Z.; Xiao, Y.; Xing, M.; Zhang, J. Research progress of photocatalysis based on highly dispersed in mesoporous SiO2. Chin. Chem. Lett. 2019, 30, 853–862. [Google Scholar] [CrossRef]
- Eddy, D.R.; Ishmah, S.N.; Permana, M.D.; Firdaus, M.L.; Rahayu, I.; El-Badry, Y.A.; Hussein, E.E.; El-Bahy, Z.M. Photocatalytic phenol degradation by silica-modified titanium dioxide. Appl. Sci. 2021, 11, 9033. [Google Scholar] [CrossRef]
- Moji, R.G.; Kroon, R.E.; Motloung, S.V.; Motaung, T.E.; Koao, L.F. Morphology, structural and luminescent properties of sol-gel synthesized SiO2 powders co-doped with Sr2+ and Tb3+. Phys. B Condens. 2020, 580, 411817. [Google Scholar] [CrossRef]
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. STAM 2007, 8, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of Calcination Temperature on Photocatalytic Activity of Synthesized TiO2 Nanoparticles via Wet Ball Milling Sol-Gel Method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Limón-Rocha, I.; Guzmán-González, C.A.; Anaya-Esparza, L.M.; Romero-Toledo, R.; Rico, J.L.; González-Vargas, O.A.; Pérez-Larios, A. Effect of the Precursor on the Synthesis of ZnO and Its Photocatalytic Activity. Inorganics 2022, 10, 16. [Google Scholar] [CrossRef]
- Levin, A.A.; Narykova, M.V.; Lihachev, A.I.; Kardashev, B.K.; Kadomtsev, A.G.; Brunkov, P.N.; Panfilov, A.G.; Prasolov, N.D.; Sultanov, M.M.; Kuryanov, V.N.; et al. Modification of the Structural, Microstructural, and Elastoplastic Properties of Aluminum Wires after Operation. Metals 2021, 11, 1955. [Google Scholar] [CrossRef]
- Eddy, D.R.; Ishmah, S.N.; Permana, M.D.; Firdaus, M.L. Synthesis of Titanium Dioxide/Silicon Dioxide from Beach Sand as Photocatalyst for Cr and Pb Remediation. Catalysts 2020, 10, 1248. [Google Scholar] [CrossRef]
- Prabhu, R.R.; Abdul Khadar, M. Study of optical phonon modes of CdS nanoparticles using Raman spectroscopy. Bull. Mater. Sci. 2008, 31, 511–515. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Singh, M.K.; Mathpal, M.C.; Mishra, S.K.; Agarwal, A. Study of structural transformation in TiO2 nanoparticles and its optical properties. J. Alloys Compd. 2013, 549, 114–120. [Google Scholar] [CrossRef]
- Reddy, A.J.; Kokila, M.K.; Nagabhushana, H.; Chakradhar, R.P.S.; Shivakumara, C.; Rao, J.L.; Nagabhushana, B.M. Structural, optical and EPR studies on ZnO: Cu nanopowders prepared via low temperature solution combustion synthesis. J. Alloys Compd. 2011, 509, 5349–5355. [Google Scholar] [CrossRef]
- Šćepanović, M.; Aškrabić, S.; Berec, V.; Golubović, A.; Dohčević-Mitrović, Z.; Kremenović, A.; Popović, Z.V. Characterization of La-Doped TiO2 Nanopowders by Raman Spectroscopy. Acta Phys. Pol. 2009, 115, 771–774. [Google Scholar] [CrossRef]
- Zanatta, A.R. A fast-reliable methodology to estimate the concentration of rutile or anatase phases of TiO2. AIP Adv. 2017, 7, 075201. [Google Scholar] [CrossRef] [Green Version]
- Jasinski, J.J.; Lubas, M.; Suchorab, K.; Gawęda, M.; Kurpaska, L.; Brykala, M.; Kosinska, A.; Sitarz, M.; Jagielski, J. Qualitative and semi-quantitative phase analysis of TiO2 thin layers by Raman imaging. J. Mol. Struct. 2022, 1260, 132803. [Google Scholar] [CrossRef]
- Lu, X.; Lv, X.; Sun, Z.; Zheng, Y. Nanocomposites of poly(l-lactide) and surface-grafted TiO2 nanoparticles: Synthesis and characterization. Eur. Polym. J. 2008, 44, 2476–2481. [Google Scholar] [CrossRef]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles—Synthesized via sol–gel route. Spectrochim Acta A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Olurode, K.; Neelgund, G.M.; Oki, A.; Luo, Z. A facile hydrothermal approach for construction of carbon coating on TiO2 nanoparticles. Spectrochim Acta A Mol. Biomol. Spectrosc. 2012, 89, 333–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Pudukudy, M.; Yaakob, Z.; Narayananc, B.; Gopalakrishnan, A.; Tasirin, S.M. Facile synthesis of bimodal mesoporous spinel Co3O4 nanomaterials and their structural properties. Superlattices Microstruct. 2013, 64, 15–26. [Google Scholar] [CrossRef]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Lagopati, N.; Vagena, I.-A.; Gazouli, M.; Pavlatou, E.A. ZnO Nanoparticles from Different Precursors and Their Photocatalytic Potential for Biomedical Use. Nanomaterials 2023, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, F.; Jiang, Y.; Li, F.; Wei, C. The effect of calcination temperature on the structure and activity of TiO2/SiO2 composite catalysts derived from titanium sulfate and fly ash acid sludge. Colloids Surf. 2018, 554, 81–85. [Google Scholar] [CrossRef]
- Galata, E.; Georgakopoulou, E.A.; Kassalia, M.-E.; Papadopoulou-Fermeli, N.; Pavlatou, E.A. Development of Smart Composites Based on Doped-TiO2 Nanoparticles with Visible Light Anticancer Properties. Materials 2019, 12, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Fang, Y.; Suna, S.; Wang, Y. Surface co-modification of TiO2 with N doping and Ag loading for enhanced visible-light photoactivity. RSC Adv. 2016, 6, 12272–12279. [Google Scholar] [CrossRef]
- Babyszko, A.; Wanag, A.; Sadłowski, M.; Kusiak-Nejman, E.; Morawski, A.W. Synthesis and Characterization of SiO2/TiO2 as Photocatalyst on Methylene Blue Degradation. Catalysts 2022, 12, 1372. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A 2021, 404, 112866. [Google Scholar] [CrossRef]
- Rasalingam, S.; Wu, C.M.; Koodali, R.T. Modulation of pore sizes of titanium dioxide photocatalysts by a facile template free hydrothermal synthesis method: Implications for photocatalytic degradation of rhodamine B. ACS Appl. Mater. Interfaces 2015, 7, 4368–4380. [Google Scholar] [CrossRef]
- Trenczek-Zajac, A.; Synowiec, M.; Zakrzewska, K.; Zazakowny, K.; Kowalski, K.; Dziedzic, A.; Radecka, M. Scavenger-Supported Photocatalytic Evidence of an Extended Type I Electronic Structure of the TiO2@Fe2O3 Interface. ACS Appl. Mater. Interfaces 2022, 14, 38255–38269. [Google Scholar] [CrossRef]
- Farhadian, N.; Akbarzadeh, R.; Pirsaheb, M.; Jen, T.-C.; Fakhri, Y.; Asadi, A. Chitosan modified N, S-doped TiO2 and N, S-doped ZnO for visible light photocatalytic degradation of tetracycline. Int. J. Biol. Macromol. 2019, 132, 360–373. [Google Scholar] [CrossRef]
- Niu, P.; Wu, G.; Chen, P.; Zheng, H.; Cao, Q.; Jiang, H. Optimization of Boron Doped TiO2 as an Efficient Visible Light-Driven Photocatalyst for Organic Dye Degradation With High Reusability. Front. Chem. 2020, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yao, Y.; Yang, X.; Zhou, X.; Lei, R.; He, S. Degradation of Phenol by Photocatalysis Using TiO2/Montmorillonite Composites Under UV Light. Res. Sq. 2021, 29, 68293–68305. [Google Scholar] [CrossRef] [PubMed]
- Sobczyński, A.; Duczmal, Ł.; Zmudziński, W. Phenol destruction by photocatalysis on TiO2: An attempt to solve the reaction mechanism. J. Mol. Catal. A Chem. 2004, 213, 225–230. [Google Scholar] [CrossRef]
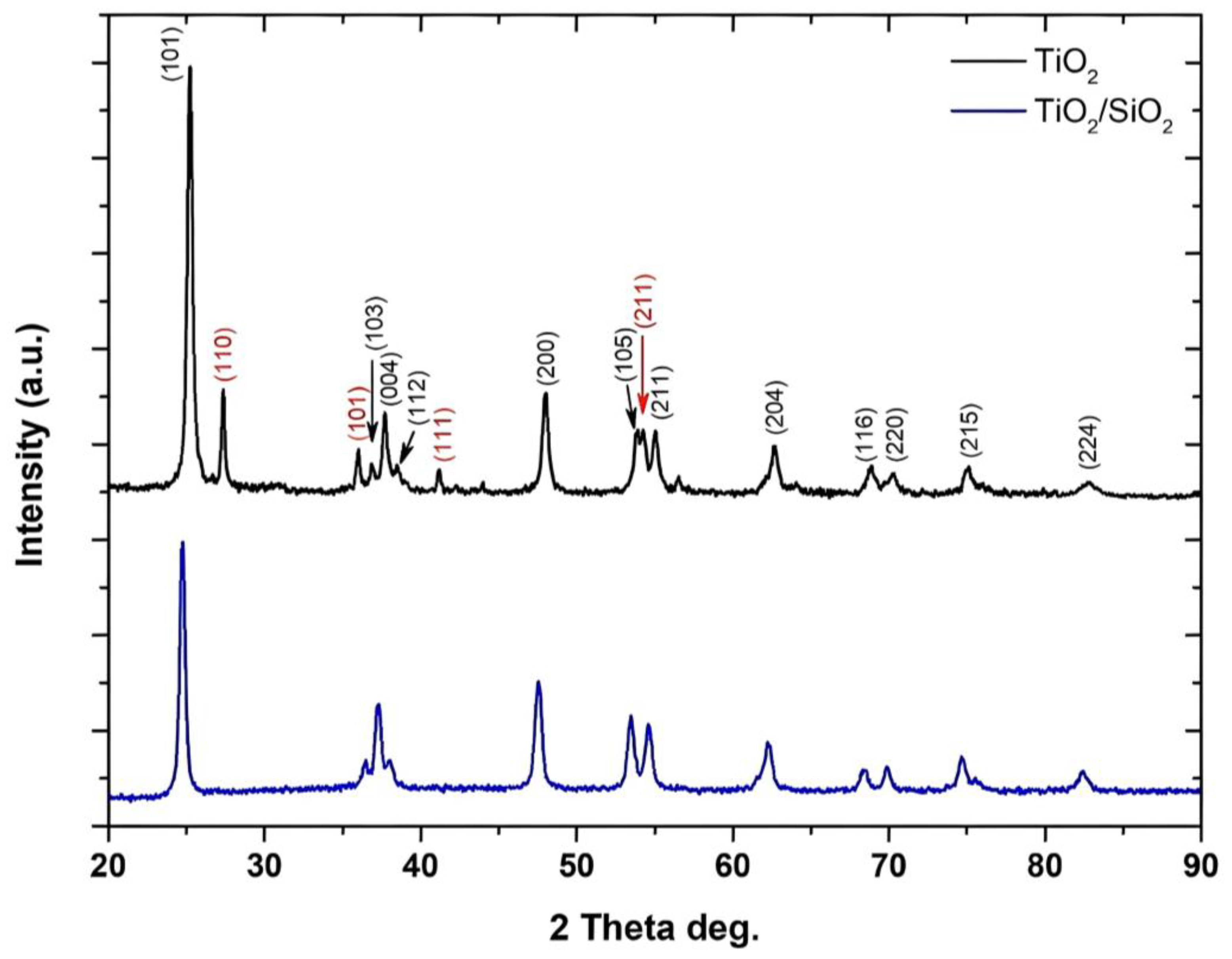

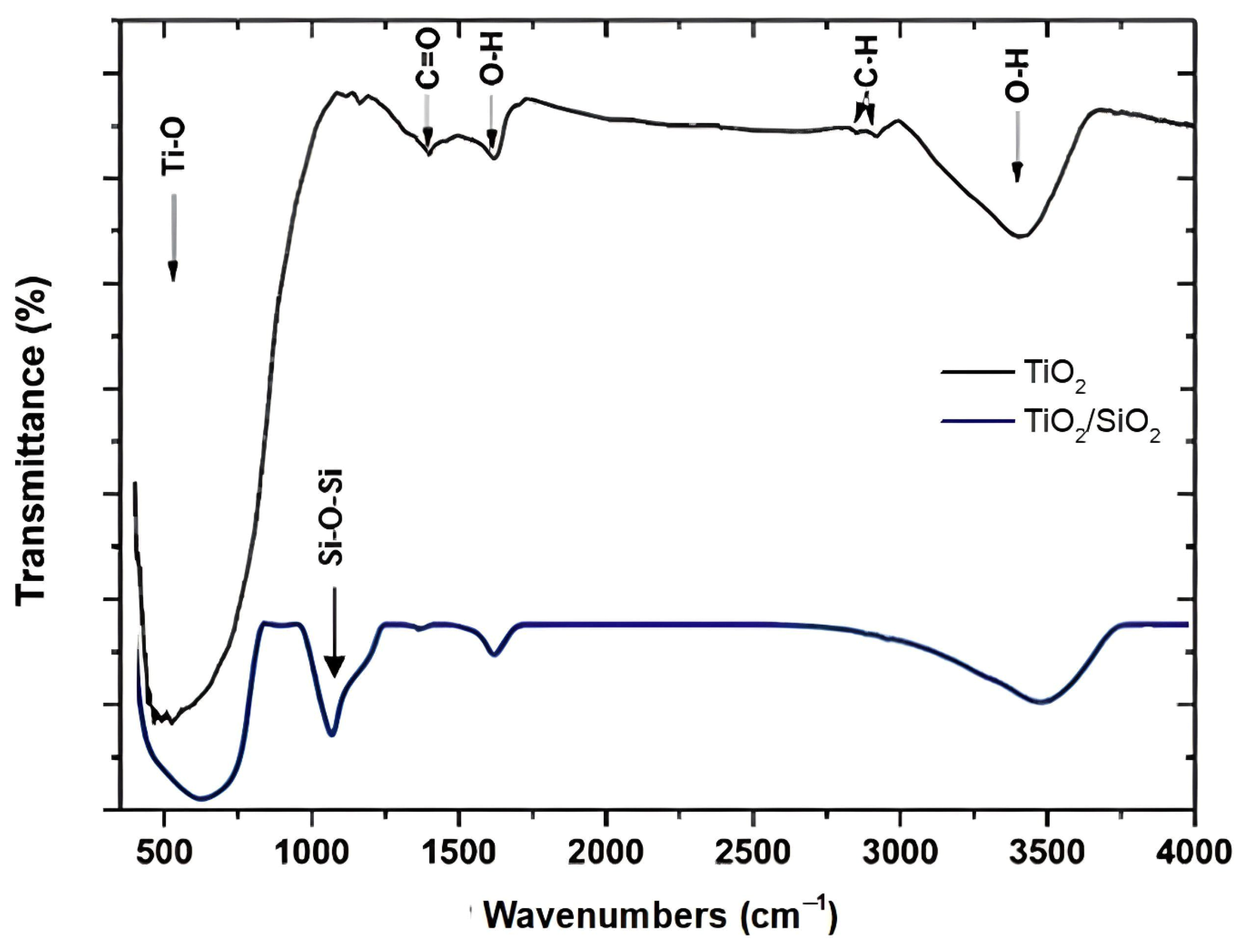
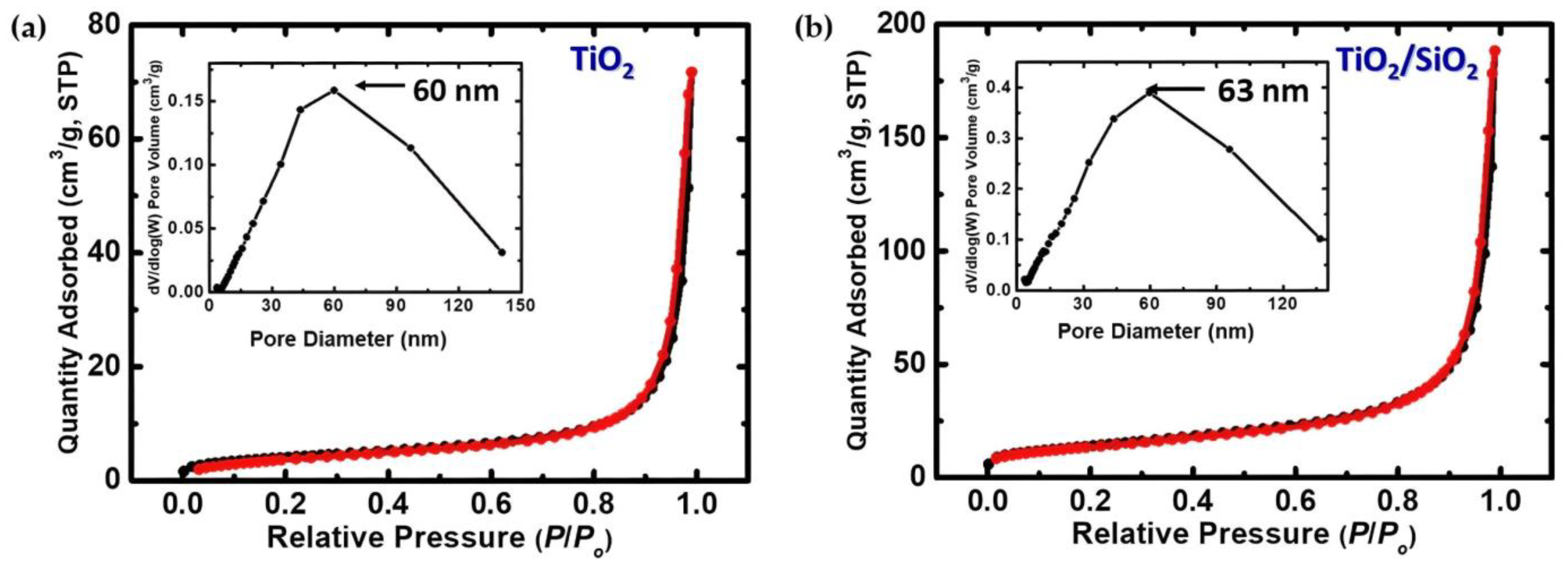
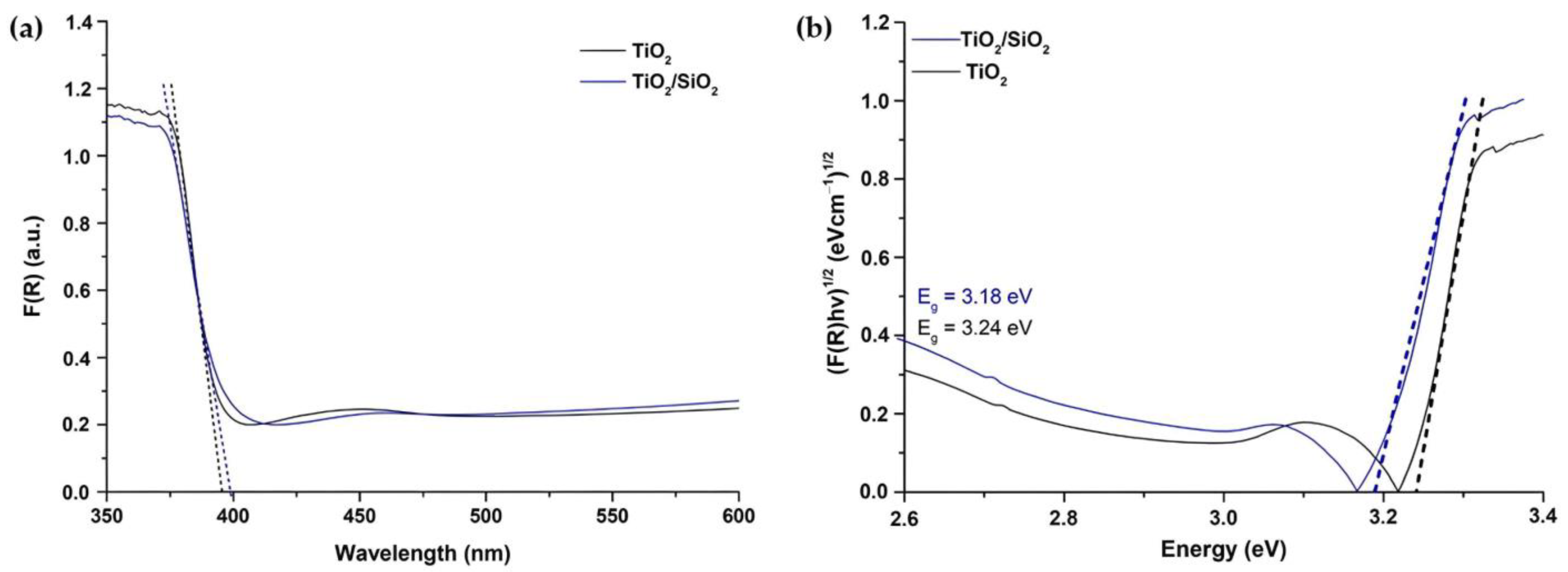




| Sample | Crystalline Phase | Space Group | Phases Percentage |
|---|---|---|---|
| TiO2 | Tetragonal (anatase) Tetragonal (rutile) | I41/a m d P42/m n m | 73.22% 26.78% |
| TiO2/SiO2 | Tetragonal (anatase) | I41/a m d | 100% |
| Bragg’s Angle | dhkl (Å) | dhkl (nm) | hkl | |
|---|---|---|---|---|
| 2θ | θ | |||
| 25.26 | 12.63 | 3.5339 | 0.3534 | 101 (A) |
| 27.36 | 13.68 | 3.2571 | 0.3257 | 110 (R) |
| 36.02 | 18.01 | 2.4914 | 0.2491 | 101 (R) |
| 36.87 | 18.44 | 2.4359 | 0.2436 | 103 (A) |
| 37.72 | 18.86 | 2.3829 | 0.2383 | 004 (A) |
| 38.48 | 19.24 | 2.3376 | 0.2338 | 112 (A) |
| 41.16 | 20.58 | 2.1914 | 0.2191 | 111 (R) |
| 47.99 | 23.99 | 1.8942 | 0.1894 | 200 (A) |
| 53.85 | 26.93 | 1.7011 | 0.1701 | 105 (A) |
| 54.25 | 27.13 | 1.6895 | 0.1689 | 211 (R) |
| 54.97 | 27.49 | 1.6691 | 0.1669 | 211 (A) |
| 62.67 | 31.34 | 1.4812 | 0.1481 | 204 (A) |
| 68.84 | 34.42 | 1.3627 | 0.1363 | 116 (A) |
| 70.29 | 35.15 | 1.3381 | 0.1338 | 220 (A) |
| 74.99 | 37.49 | 1.2655 | 0.1266 | 215 (A) |
| 82.81 | 41.41 | 1.1647 | 0.1165 | 224 (A) |
| Bragg’s Angle | dhkl (Å) | dhkl (nm) | hkl | |
|---|---|---|---|---|
| 2θ | θ | |||
| 25.26 | 12.63 | 3.5202 | 0.35202 | 101 (A) |
| 36.85 | 18.43 | 2.4894 | 0.24894 | 103 (A) |
| 37.80 | 18.90 | 2.3781 | 0.23781 | 004 (A) |
| 38.55 | 19.28 | 2.3335 | 0.23335 | 112 (A) |
| 48.05 | 24.03 | 1.8920 | 0.18920 | 200 (A) |
| 53.93 | 26.97 | 1.6988 | 0.16988 | 105 (A) |
| 55.07 | 27.54 | 1.6663 | 0.16663 | 211 (A) |
| 62.72 | 31.36 | 1.4802 | 0.14802 | 204 (A) |
| 68.94 | 34.47 | 1.3610 | 0.13610 | 116 (A) |
| 70.32 | 35.16 | 1.3376 | 0.13376 | 220 (A) |
| 75.09 | 37.55 | 1.2641 | 0.12641 | 215 (A) |
| 82.65 | 41.33 | 1.1665 | 0.11665 | 224 (A) |
| Sample | Crystal Lattice Index (a = b ≠ c) | Average Crystallite Size (nm) | FWHM | Crystallinity (%) | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| TiO2 | 3.7833 | 3.7833 | 9.4929 | 3.17 | 0.3330 | 69.49 |
| TiO2/SiO2 | 3.7885 | 3.7885 | 9.5317 | 1.82 | 0.4878 | 85.22 |
| Sample | Average Crystallite Size (D, nm) | Full Width at Half Maximum (β, FWHM) | Average Micro-Strain (ε, ×103) |
|---|---|---|---|
| TiO2 | 3.17 | 0.3330 | 3.39 |
| TiO2/SiO2 | 1.82 | 0.4878 | 5.67 |
| Sample | Calculated Phase Composition (%) | |||
|---|---|---|---|---|
| According to Maximum Intensity | According to Band Integral Intensity | |||
| Anatase | Rutile | Anatase | Rutile | |
| TiO2 | 71.25 | 28.75 | 72.88 | 27.12 |
| TiO2/SiO2 | 100 | - | 100 | - |
| Sample | BET Surface Area (m2/g) | Micropore Surface Area (m2/g) | Cumulative Volume (1.7–300 nm) (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| TiO2 | 15 | 2 | 0.1 | 30 |
| TiO2/SiO2 | 50 | 2 | 0.3 | 23 |
| SiO2 | 185 [22] | - | - | - |
| Sample | Hydrodynamic Diameter (Dh) (nm) | PdI * |
|---|---|---|
| TiO2 | 35.94 ± 1.17 | 0.263 ± 0.011 |
| TiO2/SiO2 | 27.03 ± 1.02 | 0.114 ± 0.009 |
| Sample Name | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g/mg·min) | R2 | |
| TiO2 | 0.006 | 0.933 | 0.375 | 0.517 |
| SiO2 | 0.002 | 0.979 | 0.846 | 0.921 |
| TiO2/SiO2 | 0.018 | 0.975 | 0.381 | 0.920 |
| Sample Description | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g/mg·min) | R2 | |
| TiO2 | 0.002 | 0.987 | 0.546 | 0.952 |
| SiO2 | 0.001 | 0.992 | 0.930 | 0.743 |
| TiO2/SiO2 | 0.003 | 0.975 | 0.738 | 0.903 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatou, M.-A.; Fiorentis, E.; Lagopati, N.; Pavlatou, E.A. Photodegradation of Rhodamine B and Phenol Using TiO2/SiO2 Composite Nanoparticles: A Comparative Study. Water 2023, 15, 2773. https://doi.org/10.3390/w15152773
Gatou M-A, Fiorentis E, Lagopati N, Pavlatou EA. Photodegradation of Rhodamine B and Phenol Using TiO2/SiO2 Composite Nanoparticles: A Comparative Study. Water. 2023; 15(15):2773. https://doi.org/10.3390/w15152773
Chicago/Turabian StyleGatou, Maria-Anna, Evangelos Fiorentis, Nefeli Lagopati, and Evangelia A. Pavlatou. 2023. "Photodegradation of Rhodamine B and Phenol Using TiO2/SiO2 Composite Nanoparticles: A Comparative Study" Water 15, no. 15: 2773. https://doi.org/10.3390/w15152773






