Effect of Nitrogen Application and Microbial Fertilizer on Nitrogen Conversion Processes in Saline Farmland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Area Where the Soil Samples Are Located
2.2. Test Materials
2.3. Experiment Design
2.4. Measurement Methods and Calculations
2.5. Data Analysis Method
3. Results
3.1. Effect of Microbial Fertilizer and Different Nitrogen Applications on the Ammonification Process of Nitrogen Fertilizer
3.2. Effect of Microbial Fertilizers and Different Nitrogen Applications on the Nitrification Rate of Nitrogen Fertilizers
3.3. Changes in Nitrogen Content of Salinized Farmland Soils as a Result of Microbial Fertilizers and Different Nitrogen Applications
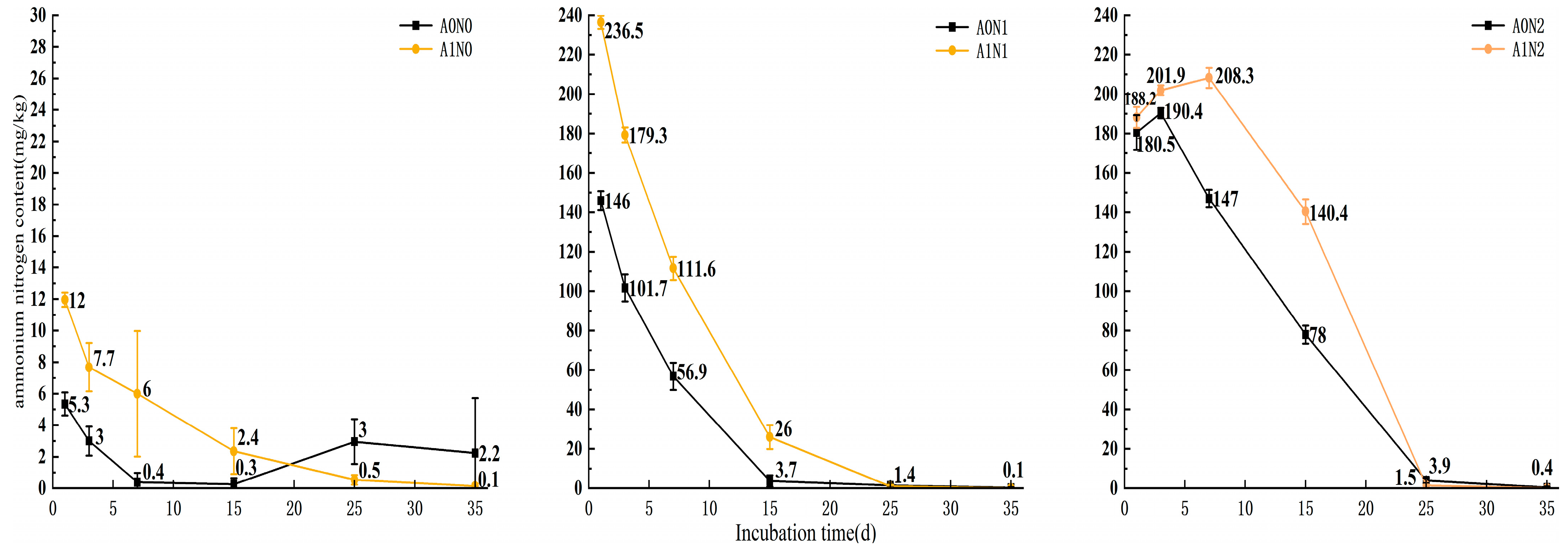
3.3.1. Effect of Microbial Fertilizer and Different Application Rates of Nitrogen on the Change in Ammonium Nitrogen Content in Salinized Farmland Soils
3.3.2. Effect of Microbial Fertilizers and Different N Application Rates on Changes in Nitrate N Content of Saline Farmland Soils
3.3.3. Effect of Microbial Fertilizers and Different N Applications on Changes in the Inorganic N Content of Saline Farmland Soils
4. Discussion
4.1. Effect of Microbial Fertilizers and Different Nitrogen Applications on the Rate of Ammonification of Nitrogen Fertilizers
4.2. Effect of Microbial Fertilizers and Different Nitrogen Applications on Nitrification Rates of Nitrogen Fertilizers
4.3. Effect of Microbial Fertilizers and Different N Application Rates on Soil N Content
5. Conclusions
- (i)
- When no microbial fertilizer was applied, an increased nitrogen fertilizer application promoted the ammonification and nitrification of nitrogen fertilizer and reduced the maximum nitrification rate under high nitrogen fertilizer application. A high nitrogen fertilizer application prolonged the process of nitrogen transformation, with ammonification and nitrification reactions as the main effects. From the perspective of fertilizer utilization and the ecological environment, reducing nitrogen application can suppress the intensity and duration of soil nitrification reactions, thereby decreasing the content of nitrate nitrogen in the soil and reducing nitrate leaching losses.
- (ii)
- The application of microbial fertilizer significantly increased the rate of ammonification and nitrification for the low N fertilizer dosage, the intensity of ammonification and nitrification for the low N fertilizer dosage was greater than the high N fertilizer dosage, and the application of microbial fertilizer prolonged the nitrogen transformation with ammonification and nitrification reactions as the main effects under the high N fertilizer dosage. When microbial bacterial fertilizers are applied together with low-nitrogen fertilizers, there is a higher presence of ammonium nitrogen in the soil. Conversely, when high-nitrogen fertilizers are applied, the soil maintains a consistently high level of nitrate nitrogen, significantly increasing the risk of nitrogen leaching.
- (iii)
- In terms of nitrogen conversion, the use of microbial fertilizers as a substitute for a portion of nitrogen fertilizers in salinized farmland effectively increases the nitrogen nutrient content required by crops. To capitalize on this benefit, appropriate timing of subsequent fertilization is crucial, allowing the substantial amount of ammonium nitrogen produced by microbial fertilizers to be efficiently utilized. By doing so, the risk of fertilizer loss and environmental pollution can be minimized.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J.; Zhang, L. Key processes and microbial mechanisms of soil nitrogen transformation. Microbiol. China 2013, 40, 98–108. [Google Scholar] [CrossRef]
- Li, H.; Yao, R.; Yang, J.; Wang, X.; Zheng, F.; Chen, Q.; Xie, W.; Zhang, X. Influencing mechanism of soil salinization on nitrogen transformation processes and efficiency improving methods for high efficient utilization of nitrogen in salinized farmland. Chin. J. Appl. Ecol. 2020, 31, 3915–3924. [Google Scholar] [CrossRef]
- Jiao, Y.; Qi, P.; Wang, X.; Wu, J.; Yao, Y.; Cai, L.; Zhang, R. Effects of Different Nitrogen Application Rates on Soil Organic Nitrogen Components and Enzyme Activities in Farmland. Sci. Agric. Sin. 2020, 53, 2423–2434. [Google Scholar]
- Wang, X.; Xu, J.; Long, C.; Zhu, S.; Lu, M.; Yang, W. Effect of nitrogen rates and soil water contents on soil nitrogen mineralization under ryegrass returning into red soil. J. Plant Nutr. Fertil. 2018, 24, 365–374. [Google Scholar]
- Tian, D.; Gao, M.; Xu, C. Effects of Soil Moisture and Nitrogen Addition on Nitrogen Mineralization and Soil pH in Purple Soil of Three Different Textures. J. Soil Water Conserv. 2016, 30, 255–261. [Google Scholar] [CrossRef]
- Luo, Q.; Gong, J.; Xu, S.; Baoyin, T.; Wang, Y.; Di, Z.; Pan, Y.; Liu, M.; Yang, L. Effects of N and P additions on net nitrogen mineralization in temperate typical grasslands in Nei Mongol, China. Chin. J. Plant Ecol. 2016, 40, 480–492. [Google Scholar]
- Lu, Y.; Zhou, L.; Long, G.; Tang, L. Effect of Different Nitrogen Rates on the Nitrification Potential and Abundance of Ammonia-oxidizer in Intercropping Maize Soils. Environ. Sci. 2016, 37, 3229–3236. [Google Scholar] [CrossRef]
- Zhu, Z. Research on soil nitrogen in china. Acta Pedol. Sin. 2008, 45, 778–783. [Google Scholar]
- Qin, Y.; He, F.; Tong, Z.; Xie, K.; Wang, D.; Gammal, Q.; Song, Q.; Wang, H.; Zhang, W.; Li, X. Influence of fertilizer use on nitrogen transformation in soils of the Leymus chinensis steppe. Acta Prataculturae Sin. 2016, 25, 48–55. [Google Scholar]
- Zeng, K.; Liu, L.; Cai, Y.; Chen, Y.; Chen, D.; Sun, F.; Pei, S.; Zhou, C.; Shen, X. The nitrogen cycle and factors affecting it in the belowground ecosystem. Pratacultural Sci. 2017, 34, 502–514. [Google Scholar]
- Wen, P. Study on Nitrogen Transformation and Effects of Nitrogen onWinter Wheat Seedlings Growth in Saline Soil in Yellow River Delta. Bachelor’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2018. [Google Scholar]
- Reddy, N.; Crohn, D.M. Effects of soil salinity and carbon availability from organic amendments on nitrous oxide emissions. Geoderma 2014, 235–236, 363–371. [Google Scholar] [CrossRef]
- Li, J.; Huang, G. Pilot Study of Salinity(NaCI)Affecting Nitrogen Transformation in Silt Loam Soil. Res. Environ. Sci. 2008, 21, 98–103. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, L.; Chen, D.; Chen, Y. Effects of bacterial manure on soil fertility and above-ground parts of Prunus persica (L.). J. Henan Agric. Univ. 2020, 54, 597–603. [Google Scholar] [CrossRef]
- Xuan, X.; Zhao, W.; Li, S.; Bao, S.; Pei, X. Study on the effect of microbial fertilizer on the reduction of nitrogen fertilization in saline sunflower. J. Shanxi Agric. Univ. 2023, 43, 102–111. [Google Scholar] [CrossRef]
- Zhang, L. Effects of Soil Conditioner on Physical and Chemical Properties of Saline-alkali Soil and Bacterial Community Structure. Bachelor’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2020. [Google Scholar]
- Meng, Q.; Yang, X.; Yao, L.; Zhang, M.; Qiu, L.; Wang, Z.; Ding, U.; Zhu, B. Effects of Combined Application of Straw and Microbial Fertilizer on SoilAggregates and Fungal Community Diversity of Rice Field Saline Alkali Soil. Heilongjiang Agric. Sci. 2022, 45, 25–30. [Google Scholar]
- Sun, B.; Bai, Z.; Bao, L.; Xue, L.; Zhang, S.; Wei, Y.; Zhang, Z.; Zhuang, G.; Zhuang, X. Bacillus subtilis biofertilizer mitigating agricultural ammonia emission and shifting soil nitrogen cycling microbiomes. Environ. Int. 2020, 144, 105989. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Li, D.; Peng, C. Nitrogen-fixing cyanobacteria have the potential to improve nitrogen use efficiency through the reduction of ammonia volatilization in red soil paddy fields. Soil Tillage Res. 2022, 217, 105274. [Google Scholar] [CrossRef]
- Cao, X. A preliminary study on the effect of biofertilizer application in maize fields. Farmers Consult. 2019, 37, 38. [Google Scholar]
- Fan, B. Advances in biofertilizer research and development in China. J. Plant Nutr. Fertil. 2017, 23, 1602–1613. [Google Scholar]
- LV, N.; Zhuang, H.; Cheng, W. Feasibility study on reduction of agricultural chemical fertilizer and substitution of bio-fertilizer: An empirical study of cotton survey data in Xinjiang. Geogr. Res. 2022, 41, 1459–1480. [Google Scholar]
- Li, J.; Chen, X. Study on determination method of soil ammonium nitrogen. World Nonferrous Met. 2017, 32, 211–212. [Google Scholar]
- Zhou, Y. Studies on automatic nitrogen determinators for the determination of total nitrogen in soils. Agric. Henan 2014, 25, 16–17. [Google Scholar]
- Qian, S.; Wu, W. Discussion of methods for the determination of soil organic matter. Xinjiang Agric. Sci. Technol. 1997, 19, 19–20. [Google Scholar]
- You, Z. Comparative study of methods for the determination of quick-acting potassium in soils. Soil Fertil. Sci. China 1992, 29, 45–48. [Google Scholar]
- Shi, B.; Lai, X.; Li, J.; Tian, X.; Zhang, G. Effect of Interaction Between Manure and Urea on Transformation of Nitrogen in Brown Red Soil. J. Nucl. Agric. Sci. 2017, 31, 938–945. [Google Scholar]
- Tao, J.; Yang, J.; Yao, R.; Wang, X.; Liu, G.; Chen, Q. Effects of Soil Salinity on Nitrogen Transformation in Hetao Irrigation District of Inner Mongolia, China. Soils 2020, 52, 802–810. [Google Scholar] [CrossRef]
- Wang, J. The Effect of Manure and Chemical Fertilizingon Nitrogen Transformation and Functional Bacteria Diversity in Soil. Bachelor’s Thesis, Northeast Agricultural University, Haerbin, China, 2012. [Google Scholar]
- Gan, J.; Meng, Y.; Zheng, Z.; Sha, L.; Feng, Z. Effects of Fertilization on Mineralization and Nitrification of Nitrogen in Soil Grown Amomum UnderTropical Rainforest. J. Agro-Environ. Sci. 2003, 22, 174–177. [Google Scholar]
- Chen, Z.; Huang, Y. Effects Organic Manures with Different C-N ratio on Nitrogen in Tobacco-growing Yellow Soil. Guizhou Agric. Sci. 2016, 44, 75–78. [Google Scholar]
- Wen, J.; Yan, W.; Liu, Y.; Liang, X.; Gao, C. Effect of nitrogen application on soil nitrogen mineralization of Cinnamomum camphora plantation in subtropical area. J. Cent. South Univ. For. Technol. 2015, 35, 103–108. [Google Scholar] [CrossRef]
- Fu, X.; Yang, S.; Liu, D.; Liu, M.; Han, T. Responses of soil microbes to N fertilizers in two saline soils. J. Plant Nutr. Fertil. 2018, 24, 661–667. [Google Scholar]
- Pan, F.; Zhang, W.; Sun, Z.; Tang, J.; Chen, B.; Li, X. Effects of Exogenous Nitrogen Addition on Soil Mineral Nitrogen Content in Protected Vegetable Fields of Different Years. North. Hortic. 2022, 46, 79–86. [Google Scholar]
- Liu, Y.; Zhu, J.; Wu, Y.; Shu, L. Effects of biochar application on the abundance and structure of ammonia-oxidizer communities in coal-mining area. Chin. J. Appl. Ecol. 2017, 28, 3417–3423. [Google Scholar] [CrossRef]
- Jia, J.; Li, Z.; Liu, M.; Che, Y. Nitrification in Red Paddy Soils Different in Soil Fertility Under N Fertilization. J. Ecol. Rural. Environ. 2010, 26, 329–333. [Google Scholar]
- Yin, C.; Li, L.; Zhao, J.; Yang, J.; Zhao, H. Impacts of Returning Straw and Nitrogen Application on the Nitrification and Mineralization of Nitrogen in Saline Soil. Water 2023, 15, 564. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, W.; Zhang, W.; Li, Y.; Shen, B.; Wang, D.; Qiu, H. Effect of organic fertilizer partially replace chemical fertilizer on yield, Nmin’s level in soil and N fertilizer efficiency of potato in the Yellow River irrigation area of the central in Gansu. J. Gansu Agric. Univ. 2016, 51, 54–60+68. [Google Scholar] [CrossRef]
- Hu, M.; Tian, C.; Wang, L. Effects of nitrogen rate, applying time on cotton growth and soil mineral nitrogen content. J. Northwest A F Univ. 2011, 39, 103–109. [Google Scholar] [CrossRef]
- You, L.; Li, D.; Wu, Z.; Cui, L.; Yan, Z.; Zhang, J.; Cui, Y.; Liu, Y. Nitrogen transformation of stable ammonium fertilizer in black soil and cinnamon soil. Chin. J. Appl. Ecol. 2019, 30, 1079–1087. [Google Scholar] [CrossRef]


| Bulk Density/ kg/cm3 | pH | Conductivity/ dS/m | Organic Matter g/kg | Total N g/kg | Available Phosphorus mg/kg | Nitrate Nitrogen/ mg/kg | Ammonium Nitrogen/ mg/kg | Inorganic Nitrogen/ mg/kg |
|---|---|---|---|---|---|---|---|---|
| 1.45 | 8.0 | 2.40 | 17.2 | 0.78 | 21.35 | 18.88 | 13.79 | 32.67 |
| Indicator | Incubation Time (d) | A0N1 | A0N2 | A1N1 | A1N2 |
|---|---|---|---|---|---|
| Apparent ammonification rate | 1 | 132.18 | 166.74 | 212.71 | 174.44 |
| 3 | −22.16 | 4.93 | −23.59 | 6.85 | |
| 7 | −11.20 | −10.85 | −16.92 | 1.58 | |
| 15 | −6.64 | −8.63 | −10.70 | −8.48 | |
| 25 | −0.23 | −7.41 | −2.51 | −13.89 | |
| 35 | −0.12 | −0.35 | −0.09 | −0.15 | |
| Net ammonification rate | 1 | 140.62 | 175.18 | 214.54 | 176.27 |
| 3 | −20.99 | 6.10 | −21.46 | 8.99 | |
| 7 | −10.54 | −10.19 | −16.50 | 2.01 | |
| 15 | −6.63 | −8.61 | −10.24 | −8.03 | |
| 25 | −0.50 | −7.68 | −2.32 | −13.71 | |
| 35 | −0.05 | −0.28 | −0.05 | −0.11 |
| Indicator | Incubation Time (d) | A0N1 | A0N2 | A1N1 | A1N2 |
|---|---|---|---|---|---|
| Apparent nitrification rate | 1 | 81.81 | 79.07 | 83.44 | 97.32 |
| 3 | 3.97 | 10.32 | 18.74 | 3.37 | |
| 7 | 8.91 | 7.01 | 2.74 | 8.99 | |
| 15 | 3.57 | 4.19 | 2.82 | 3.26 | |
| 25 | −0.62 | −0.92 | −0.46 | −1.44 | |
| 35 | −0.82 | −1.19 | −1.03 | −0.48 | |
| Net nitrification rate | 1 | −19.55 | −22.28 | −52.03 | −38.15 |
| 3 | 1.67 | 8.02 | 23.19 | 7.82 | |
| 7 | 10.85 | 8.96 | 4.77 | 11.02 | |
| 15 | 2.14 | 2.76 | 4.11 | 4.56 | |
| 25 | −1.94 | −2.25 | −0.05 | 1.03 | |
| 35 | −0.05 | −0.42 | 0.16 | 0.71 |
| Treatment | 1 d | 3 d | 7 d | 15 d | 25 d | 35 d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| A | 191.001 | <0.001 * | 338.419 | <0.001 * | 306.779 | <0.001 * | 209.350 | <0.001 * | 15.421 | 0.002 * | 1.611 | 0.228 |
| N | 2650.629 | <0.001 * | 4438.005 | <0.001 * | 1886.159 | <0.001 * | 1154.125 | <0.001 * | 3.923 | 0.049 * | 0.981 | 0.403 |
| A × N | 114.332 | <0.001 * | 187.127 | <0.001 * | 57.575 | <0.001 * | 78.798 | <0.001 * | 2.113 | 0.164 | 0.815 | 0.466 |
| Treatment | 1 d | 3 d | 7 d | 15 d | 25 d | 35 d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| A | 0.289 | 0.600 | 56.671 | <0.001 * | 22.332 | <0.001 * | 0.289 | 0.600 | 7.392 | 0.019 * | 10.245 | 0.008 * |
| N | 208.775 | <0.001 * | 12.525 | 0.001 * | 32.013 | <0.001 * | 208.775 | <0.001 * | 137.461 | <0.001 * | 219.571 | <0.001 * |
| A × N | 0.631 | 0.549 | 10.248 | 0.003 * | 2.091 | 0.166 | 0.631 | 0.549 | 9.886 | 0.003 * | 27.776 | <0.001 * |
| Treatment | 1 d | 3 d | 7 d | 15 d | 25 d | 35 d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| A | 101.409 | <0.001 * | 207.978 | <0.001 * | 206.777 | <0.001 * | 101.409 | <0.001 * | 13.006 | 0.004 * | 8.420 | 0.013 * |
| N | 1009.699 | <0.001 * | 913.651 | <0.001 * | 957.147 | <0.001 * | 1009.699 | <0.001 * | 143.508 | <0.001 * | 127.711 | <0.001 * |
| A × N | 42.794 | <0.001 * | 73.259 | <0.001 * | 14.696 | <0.001 * | 42.794 | <0.001 * | 11.421 | 0.002 * | 19.337 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zhao, J.; Li, L.; Yin, C.; Chen, Q.; Nie, X.; Pang, J.; Wang, L.; Li, E. Effect of Nitrogen Application and Microbial Fertilizer on Nitrogen Conversion Processes in Saline Farmland. Water 2023, 15, 2748. https://doi.org/10.3390/w15152748
Zhao H, Zhao J, Li L, Yin C, Chen Q, Nie X, Pang J, Wang L, Li E. Effect of Nitrogen Application and Microbial Fertilizer on Nitrogen Conversion Processes in Saline Farmland. Water. 2023; 15(15):2748. https://doi.org/10.3390/w15152748
Chicago/Turabian StyleZhao, Haogeng, Ju Zhao, Lijun Li, Chunyan Yin, Qiang Chen, Xiaoxue Nie, Jiahe Pang, Lixia Wang, and Erzhen Li. 2023. "Effect of Nitrogen Application and Microbial Fertilizer on Nitrogen Conversion Processes in Saline Farmland" Water 15, no. 15: 2748. https://doi.org/10.3390/w15152748





