Tris(2-benzimidazolyl)amine (NTB)-Modified Metal-Organic Framework: Preparation, Characterization, and Mercury Ion Removal Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
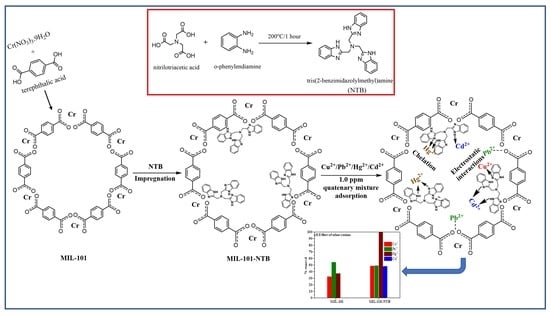
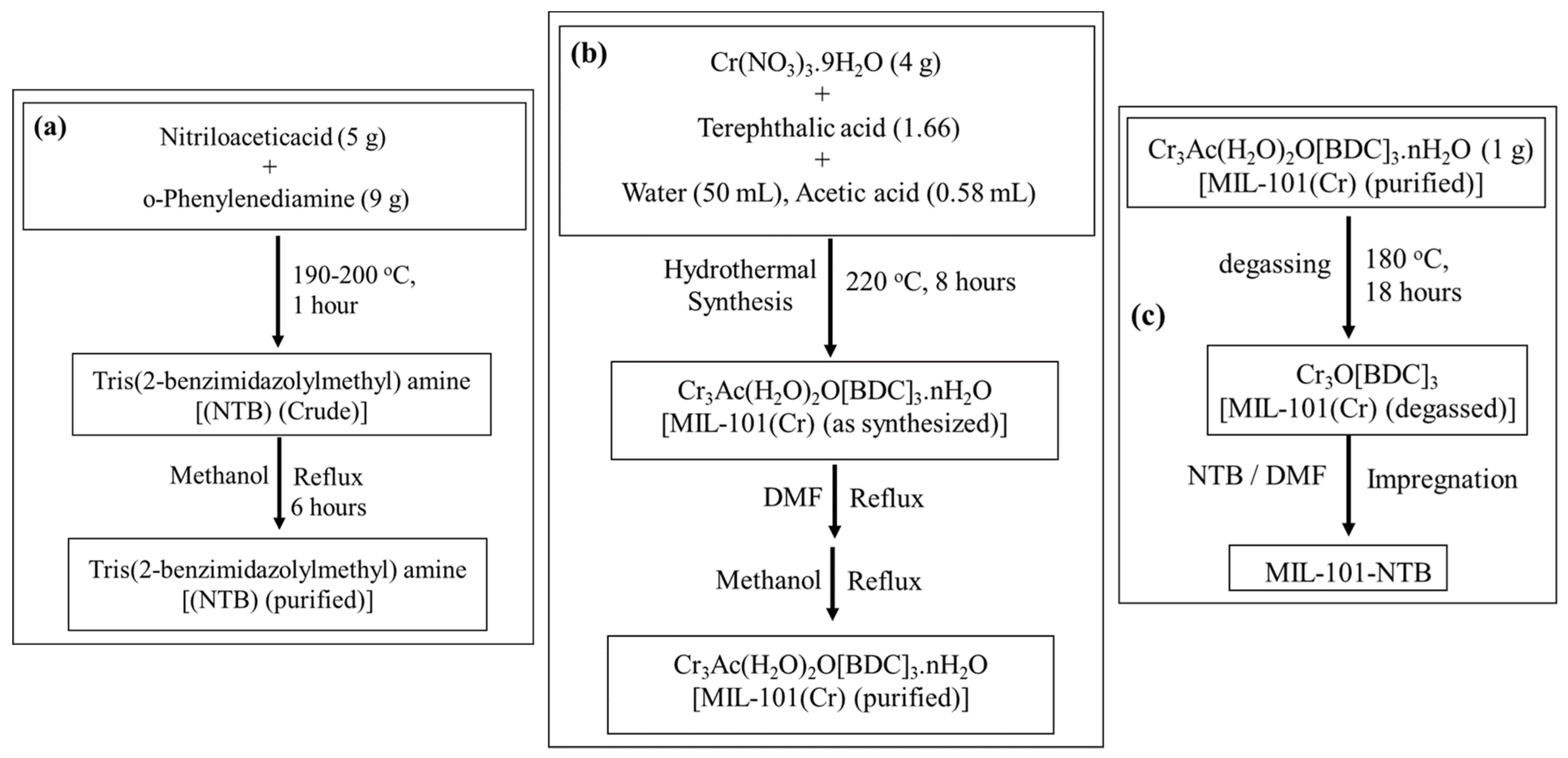
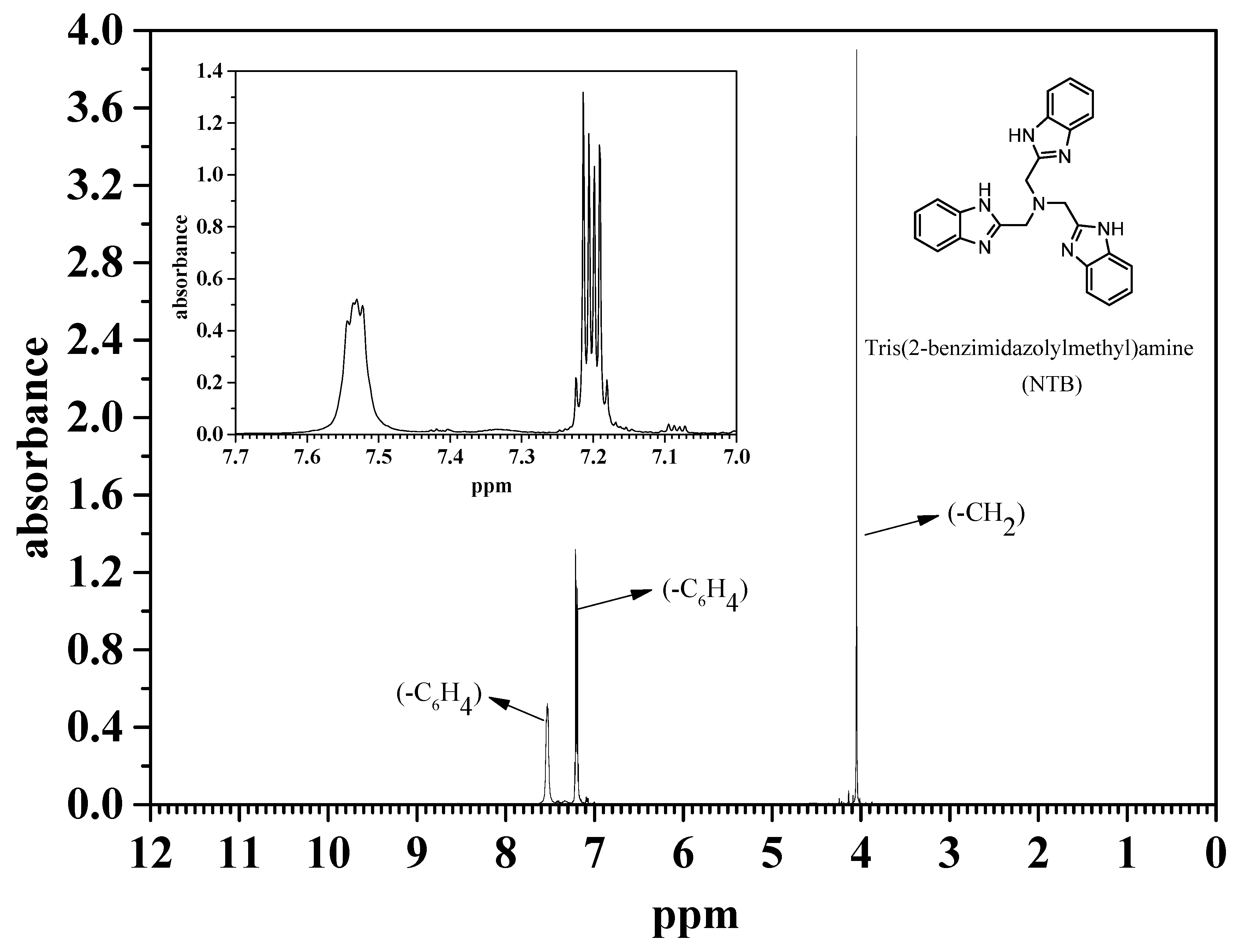
2.2.1. Preparation of Tris(2-benzimidazolylmethyl)amine (NTB)
2.2.2. Preparation of MIL-101(Cr)
2.2.3. Preparation of MIL-101-NTB
2.3. Batch Adsorption Experiments and Adsorbent Regeneration
2.4. Instrumentation
3. Results and Discussion
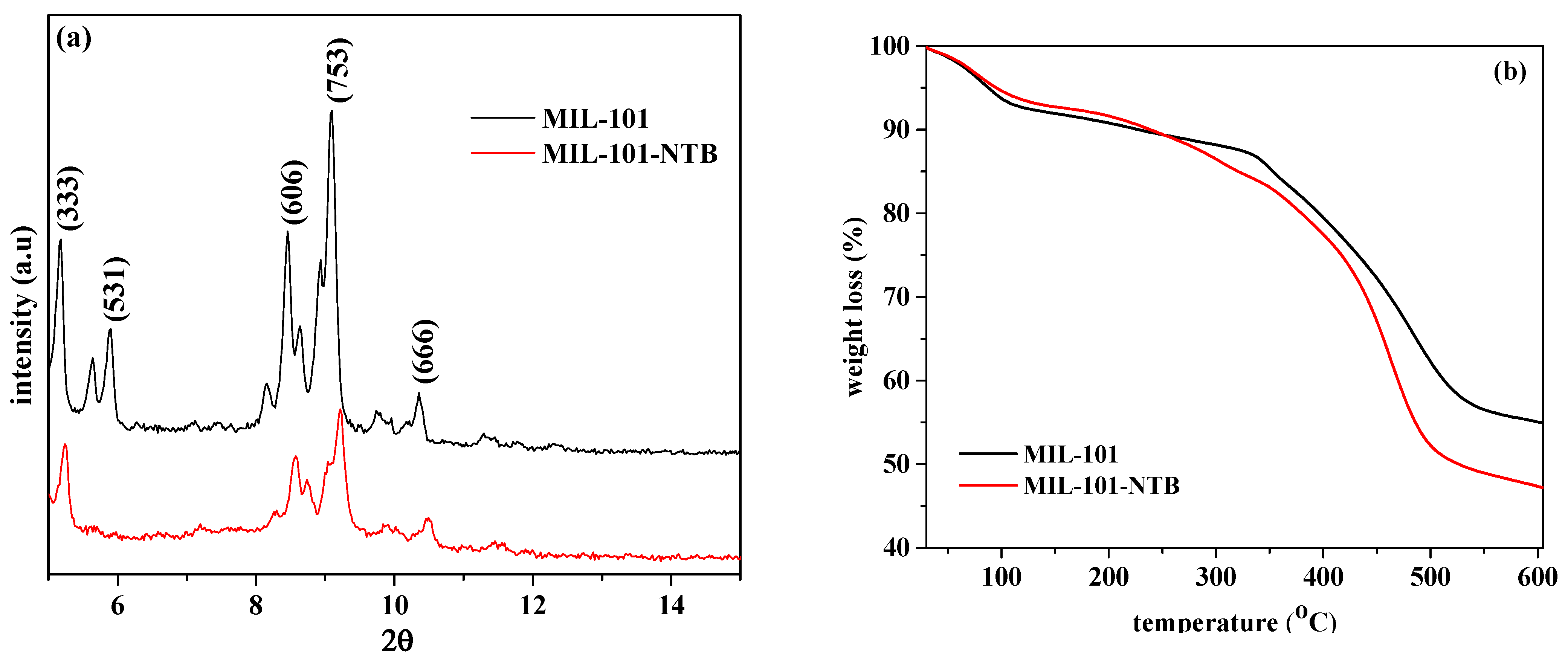
3.1. Characterization
3.2. Adsorption Experiments
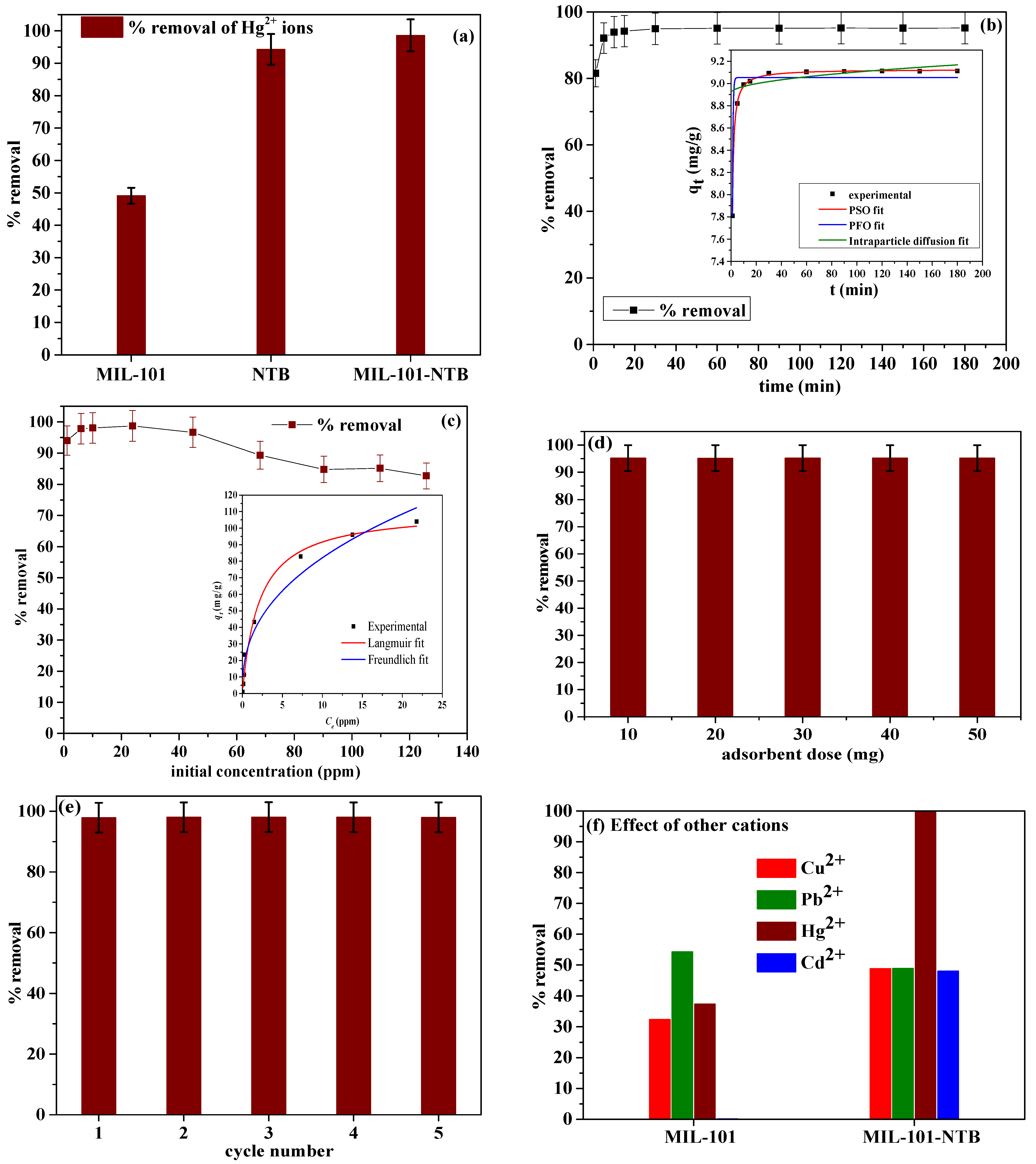
3.2.1. Preliminary Screening
3.2.2. Effect of Contact Time
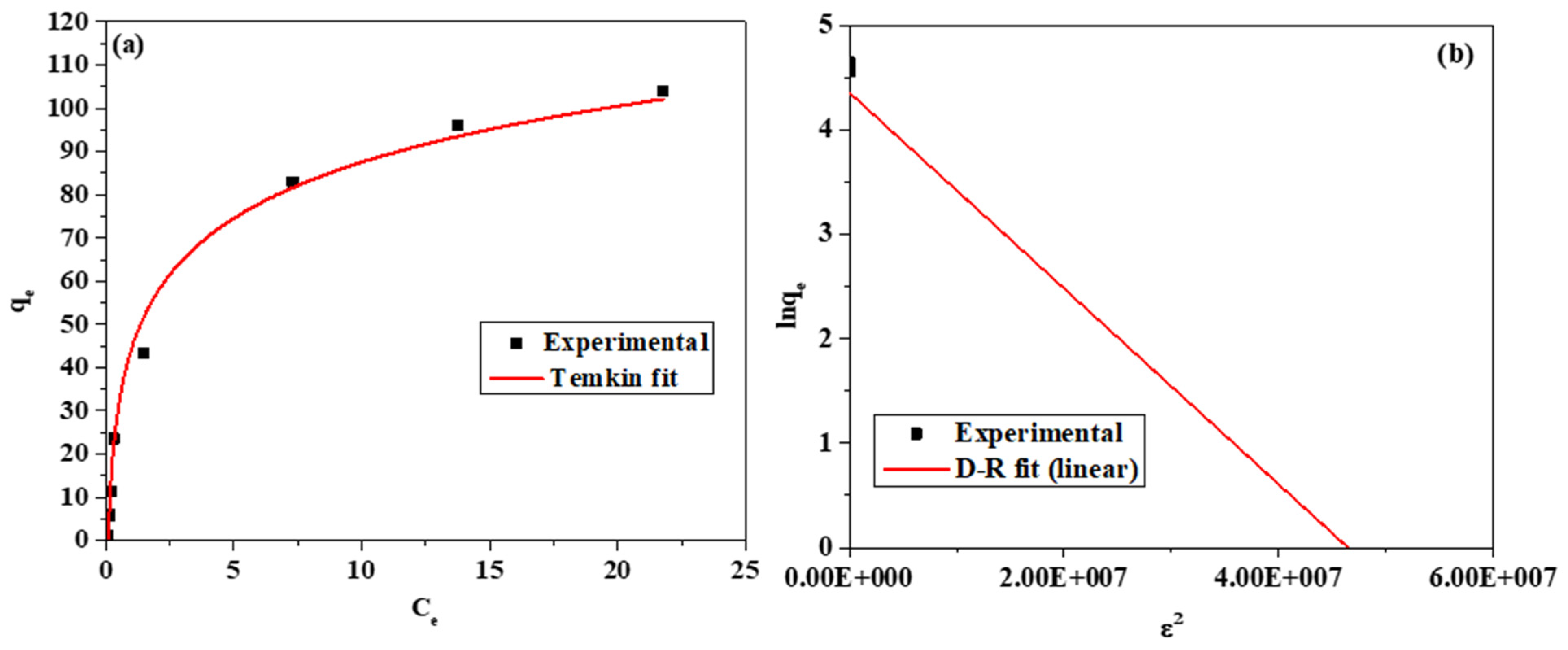
3.2.3. Effect of Initial Concentration
3.2.4. Effect of Adsorbent Dosage
3.2.5. Adsorbent Regeneration
3.2.6. Effect of Other Cations
3.2.7. Mechanism behind Adsorption of HMIs Using MIL-101-NTB
| Serial Number | MOF | Maximum Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| 1 | Zr-MSA-MA | 714.8 | [68] |
| 2 | UIO-66-DMTD | 670.5 | [69] |
| 3 | LMOF-263 | 380 | [70] |
| 4 | DUT-67-SH | 372 | [71] |
| 5 | FJI-H12 | 440 | [72] |
| 6 | MIL-101-NTB | 111.07 | This work |
| 7 | AMOF-1 | 78 | [73] |
| 8 | MOF-74-Zn | 63 | [74] |
| 9 | MIL-101-Thymine | 52 | [75] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Costa, F.G.; Pereira, M.D.L. Heavy metals and human health. In Environmental Health–Emerging Issues and Practice; Oosthuizen, J., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 227–245. [Google Scholar]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Norton-Brandão, D.; Scherrenberg, S.M.; Lier, J.B.V. Reclamation of used urban waters for irrigation purposes-a review of treatment technologies. J. Environ. Manag. 2013, 122, 85–98. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Jha, M.K.; Joshi, S.; Sharma, R.K.; Kim, A.A.; Pant, B.; Park, M.; Pant, H.R. Surface modified activated carbons: Sustainable bio-based materials for environmental remediation. Nanomaterials 2021, 11, 3140. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Ye, Z. Recent progress in heavy metal ion decontamination based on metal-organic frameworks. Nanomaterials 2020, 11, 1481. [Google Scholar] [CrossRef]
- Yuna, Z. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Mao, S.; Gao, M. Functional organoclays for removal of heavy metal ions from water: A review. J. Mol. Liq. 2021, 334, 116143. [Google Scholar] [CrossRef]
- Xu, G.-R.; An, Z.-H.; Xu, K.; Liu, Q.; Das, R.; Zhao, H.-L. Metal organic framework (MOF)-based micro/nanoscaled materials for heavy metal ions removal: The cutting-edge study on designs, synthesis, and applications. Coord. Chem. Rev. 2021, 427, 213554. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Q. Bimetallic metal-organic frameworks for gas storage and separation. Cryst. Growth Des. 2017, 17, 1450–1455. [Google Scholar] [CrossRef]
- Li, X.R.; Yang, X.C.; Xue, H.G.; Pang, H.; Xu, Q. Metal-organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Li, H.; Li, L.B.; Lin, R.B.; Zhou, W.; Zhang, Z.J.; Xiang, S.C.; Chen, B.L. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Samanta, P.; Desai, A.V.; Sharma, S.; Chandra, P.; Ghosh, S.K. Selective recognition of Hg2+ ion in water by a functionalized metal–organic framework (MOF) based chemodosimeter. Inorg. Chem. 2018, 57, 2360–2364. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Yang, Y.W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Yi, F.Y.; Chen, D.; Wu, M.K.; Han, L.; Jiang, H.L. Chemical sensors based on metal-organic frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Castillo, J.; Thijs, V.; Sofia, C. Understanding water adsorption in Cu-BTC metal-organic frameworks. J. Phys. Chem. C 2008, 112, 15934–15939. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef]
- Tan, K.; Nijem, N.; Canepa, P.; Gong, Q.; Li, J.; Thonhauser, T.; Chabal, Y.J. Stability and hydrolyzation of metal organic frameworks with paddle-wheel SBUs upon hydration. Chem. Mater. 2012, 24, 3153–3167. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.H.; Feng, Y.; Zhang, X.F.; Jia, M.M.; Yao, J.F. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Zhao, X.; Liu, B.; Li, Z.; Wei, Y. Rationally Tailoring Pore and Surface Properties of Metal–Organic Frameworks for Boosting Adsorption of Dy3+. ACS Appl. Mater. Interfaces 2021, 13, 46763–46771. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Wang, X.; Wang, F.; Cui, X.; Du, X.; Lu, X. UiO series of metal-organic frameworks composites as advanced sorbents for the removal of heavy metal ions: Synthesis, applications and adsorption mechanism. Ecotoxicol. Environ. Saf. 2021, 208, 111577. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G.; Draznieks, C.M.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Dong, M.; Zhao, T. Advances in metal-organic frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef]
- Luo, X.; Ding, L.; Luo, J. Adsorptive removal of Pb(II) ions from aqueous samples with amino-functionalization of metal-organic frameworks MIL-101(Cr). J. Chem. Eng. Data 2015, 60, 1732–1743. [Google Scholar] [CrossRef]
- Rastkari, N.; Akbari, S.; Brahmand, M.B.; Takhvar, A.; Ahmadkhaniha, R. Synthesis and characterization of tetraethylenepentamine functionalized MIL-101(Cr) for removal of metals from water. J. Environ. Health Sci. Eng. 2021, 19, 1735–1742. [Google Scholar] [CrossRef]
- Thompson, L.K.; Ramaswamy, B.S.; Seymour, E.A. Cobalt (II) and zinc(II) complexes of the tripod’ ligand tris(2-benzimidazylmethy1)amine. Some five-coordinate derivatives and some with mixed stereochemistries. Can. J. Chem. 1977, 55, 878. [Google Scholar] [CrossRef] [Green Version]
- Nakata, K.; Shimomura, N.; Shiina, N.; Izumi, M.; Ichikawa, K.; Shiro, M. Kinetic study of catalytic CO2 hydration by water-soluble model compound of carbonic anhydrase and anion inhibition effect on CO2 hydration. J. Inorg. Biochem. 2002, 89, 255–266. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, F.; Hu, S.; Cao, Z.; Wu, Z.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part I: Gravimetric, electrochemical, SEM and XPS studies. Corros. Sci. 2013, 74, 271–282. [Google Scholar] [CrossRef]
- Phani, B.S.R.; Raj, M.C.; Senthilkumar, S.; Somani, R.S.; Bajaj, H.C. HF-free synthesis of MIL-101(Cr) and its hydrogen adsorption studies. Environ. Prog. Sustain. 2016, 32, 461–468. [Google Scholar]
- Kayal, S.; Sun, B.; Chakraborty, A. Study of metal-organic framework MIL-101(Cr) for natural gas (methane) storage and compare with other MOFs (metal-organic frameworks). Energy 2015, 91, 772–781. [Google Scholar] [CrossRef]
- Rana, A.K.; Bankar, P.; Kumar, Y.; More, M.A.; Late, D.J.; Shirage, P.M. Synthesis of Ni-doped ZnO nanostructures by low-temperature wet chemical method and their enhanced field emission properties. RSC Adv. 2016, 6, 104318–104324. [Google Scholar] [CrossRef] [Green Version]
- Prasanth, K.P.; Rallapalli, P.; Raj, M.C.; Bajaj, H.C.; Jasra, R.V. Enhanced hydrogen sorption in single walled carbon nanotube incorporated MIL-101 composite metal–organic framework. Int. J. Hydrogen Energy 2011, 36, 7594–7601. [Google Scholar] [CrossRef]
- Khodkar, A.; Khezri, S.M.; Pendashteh, A.; Khoramnejadian, S.; Mamani, L. Preparation and application of α-Fe2O3@MIL-101(Cr)@TiO2 based on metal–organic framework for photocatalytic degradation of paraquat. Toxicol. Ind. Health 2018, 34, 842–859. [Google Scholar] [CrossRef]
- Jhung, S.H.; Lee, J.H.; Yoon, J.W.; Serre, C.; Ferey, G.; Chang, J.S. Microwave synthesis of chromium terephthalate MIL-101 and its benzene sorption ability. Adv. Mater. 2007, 19, 121–124. [Google Scholar] [CrossRef]
- Jafar, S.; Sedigheh, Z.; Maryam, T. Synthesis of a chromium terephthalate metal organic framework and use as nanoporous adsorbent for removal of diazinon organophosphorus insecticide from aqueous media. J. Dispers. Sci. Technol. 2019, 40, 1423–1440. [Google Scholar]
- Yurdakul, S.; Yilmaz, C. Vibrational spectroscopic investigations of M(benzimidazole)2Ni(CN)4 and Cd(benzimidazole)Cl2 complexes. Vib. Spectrosc. 1999, 21, 127–132. [Google Scholar] [CrossRef]
- Pashchevskaya, N.V.; Nazarenko, M.A.; Bolotin, S.N.; Oflidi, A.I.; Panyushkin, V.T. Effect of the condition of synthesis on the composition and structure of copper(II) complexes with benzimidazole. Russ. J. Inorg. Chem. 2010, 55, 1425–1432. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Segakweng, T.; North, B.C.; Mathe, M.; Kang, X. Modulated synthesis of chromium-based metal-organic framework (MIL-101) with enhanced hydrogen uptake. Int. J. Hydrogen Energy 2014, 39, 12018–12023. [Google Scholar] [CrossRef]
- Quantachrome Instruments. Pore Size Analysis by Gas Adsorption and the Density Functional Theory. 2010, pp. 1–13. Available online: https://www.azom.com/article.aspx?ArticleID=5189 (accessed on 15 January 2020).
- Yoon, H.C.; Rallapalli, P.B.S.; Beum, H.T.; Han, S.S.; Kim, J.N. Hybrid postsynthetic functionalization of tetraethylenepentamine onto MIL-101(Cr) for separation of CO2 from CH4. Energy Fuels 2018, 32, 1365–1373. [Google Scholar] [CrossRef]
- Diephuis, W.R.; Molloy, A.L.; Boltz, L.L.; Porter, T.B.; Aragon Orozco, A.; Duron, R.; Crespo, D.; George, L.J.; Reiffer, A.D.; Escalera, G. The effect of agglomeration on arsenic adsorption using iron oxide nanoparticles. Nanomaterials 2022, 12, 1598. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.; Newbury, D.; Joy, D.; Lyman, C.; Echlin, P.; Lifshin, E. Scanning Electron Microscopy and X-ray Microanalysis, 3rd ed.; Springer: New York, NY, USA, 2003. [Google Scholar]
- Stevie, F.A.; Donley, C.L. Introduction to X-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 063204. [Google Scholar] [CrossRef]
- Quan, X.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. Surface functionalization of MIL-101(Cr) by aminated mesoporous silica and improved adsorption selectivity toward special metal ions. Dalton Trans. 2019, 48, 5384–5396. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Du, T.; Zhang, W.; Zhu, W.; Yang, C.; Yue, T.; Sun, J.; Li, T.; Wang, J. NH2-MIL-53(Al) metal-organic framework as the smart platform for simultaneous high-performance detection and removal of Hg2+. Inorg. Chem. 2019, 58, 12573–12581. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Hong, D.Y.; Chang, J.-S.; Jhung, S.H.; Seo, Y.K.; Kim, J.H.; Vimont, A.; Daturi, M.; Serre, C.; Ferey, G. Amine grafting on coordinatively unsaturated metal centers of MOFs: Consequences for catalysis and metal encapsulation. Agnew. Chem. Int. Ed. 2008, 47, 4144–4148. [Google Scholar] [CrossRef]
- Bui, N.T.; Kang, H.; Teat, S.J. A nature-inspired hydrogen-bonded supramolecular complex for selective copper ion removal from water. Nat. Commun. 2020, 11, 3947. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Zhang, K.; Liu, T.; Hu, Y.; Chen, X.; Wang, C.; Huang, X.; Kong, L.; Liua, J. Super rapid removal of copper, cadmium and lead ions from water by NTA-silica gel. RSC Adv. 2019, 9, 397–407. [Google Scholar] [CrossRef]
- Ai, W.; Zhou, W.; Du, Z.; Du, Y.; Zhang, H.; Jia, X.; Xie, L.; Yi, M.; Yu, T.; Huang, W. Benzoxazole and benzimidazole heterocycle-grafted graphene for high-performance supercapacitor electrodes. J. Mater. Chem. 2012, 22, 23439–23446. [Google Scholar] [CrossRef]
- Chen, Y.; An, D.; Sun, S.; Gao, J.; Qian, L. Reduction and removal of chromium VI in water by powdered activated carbon. Materials 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, T.K.; Kim, J.-H.; Kwon, H.T.; Kim, J. Cost-effective and eco-friendly synthesis of MIL-101(Cr) from waste hexavalent chromium and its application for carbon monoxide separation. Ind. Eng. Chem. Res. 2019, 80, 345–351. [Google Scholar] [CrossRef]
- Huang, X.; Hu, Q.; Gao, L.; Hao, Q.; Wang, P.; Qin, D. Adsorption characteristics of metal–organic framework MIL-101(Cr) towards sulfamethoxazole and its persulfate oxidation regeneration. RSC Adv. 2018, 8, 27623–27630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jin, Y.; Fu, Y.; Zhong, Y.; Zhu, W.; Ibrahim, A.A.; El-Shall, M.S. Palladium Nanoparticles Incorporated within Sulfonic Acid-Functionalized MIL–101(Cr) for Efficient Catalytic Conversion of Vanillin. J. Mater. Chem. A 2015, 3, 17008–17015. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Z.; Wang, Z.; Feng, X.; Wang, Y.; Wu, A. Unveiling the adsorption mechanism of zeolitic imidazolate framework-8 with high removal efficiency on copper ions from aqueous solutions. Dalton Trans. 2016, 45, 12653–12660. [Google Scholar] [CrossRef] [PubMed]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal-organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Rahmani, A.; Shabanloo, A.; Zabihollahi, S.; Mehdi, S.; Mostafa, L.; Mohammad, K.; Saber, A.; Davood, N. Facile fabrication of amino-functionalized MIL-68(Al) metal–organic framework for effective adsorption of arsenate (As(V)). Sci. Rep. 2022, 12, 11865. [Google Scholar] [CrossRef]
- Dariush, R. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 55–60. [Google Scholar]
- Ragheb, S.M. Phosphate removal from aqueous solution using slag and fly ash. Hbrc J. 2013, 9, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Salman, N.; Vijay, B.; Jiri, M.; Jakub, W.; Promoda, B.; Azeem, A. Sorption properties of iron impregnated activated carbon web for removal of methylene blue from aqueous media. Fibers Polym. 2016, 17, 1245–1255. [Google Scholar]
- Aboutorabi, L.; Morsali, A.; Tahmasebi, E.; Buyukgungor, O. Metal-organic framework based on isonicotinate N-oxide for fast and highly efficient aqueous phase Cr (VI) adsorption. Inorg. Chem. 2016, 55, 5507–5513. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Y.; Xiao, X.F.; Wang, D.; Han, B.; Gao, Y.; Xue, J.L. Adsorption of Cr(VI) from aqueous solution by ethylenediaminetetraacetic acid-chitosan modified metal-organic framework. J. Nanosci. Nanotechnol. 2020, 20, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Joseph, L.; Saha, M.; Kim, S.; Jun, B.-M.; Heo, J.; Park, C.M.; Jang, M.; Flora, J.R.V.; Yoon, Y. Removal of Cu2+, Cd2+, and Pb2+ from aqueous solution by fabricated MIL-100(Fe) and MIL-101(Cr): Experimental and molecular modeling study. J. Environ. Chem. Eng. 2021, 9, 106663. [Google Scholar] [CrossRef]
- Gao, X.; Liu, B.; Zhao, X. Thiol-decorated defective metal-organic framework for effective removal of mercury(II) ion. Chemosphere 2023, 317, 137891. [Google Scholar] [CrossRef]
- Fu, L.; Wang, S.; Lin, G.; Zhang, L.; Liu, Q.; Fang, J.; Wei, C.; Liu, G. Post-functionalization of UiO-66-NH2 by 2,5-dimercapto-1,3,4-thiadiazole for the high efficient removal of Hg(II) in water. J. Hazard. Mater. 2019, 368, 42–51. [Google Scholar] [CrossRef]
- Rudd, N.D.; Wang, H.; Fuentes-Fernandez, E.M.A.; Teat, S.J.; Chen, F.; Hall, G.; Chabal, Y.J.; Li, J. Highly efficient luminescent metal-organic framework for the simultaneous detection and removal of heavy metals from water. ACS Appl. Mater. Interfaces 2016, 8, 30294–30303. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Gao, X.; Zhang, Y.-N.; Wang, M.; Gao, X.; Liu, B. Construction of dual sulfur sites in metal–organic framework for enhanced mercury(II) removal. J. Colloid Interface Sci. 2023, 631, 191–201. [Google Scholar] [CrossRef]
- Liang, L.F.; Chen, Q.; Jiang, F.; Yuan, D.; Qian, J.; Lv, G.; Xue, H.; Liu, L.; Jiang, H.L.; Hong, M. In situ large-scale construction of sulfur-functionalized metal-organic framework and its efficient removal of Hg(II) from water. J. Mater. Chem. A 2016, 4, 15370–15374. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.R.; Yuan, D.Q.; Sculley, J.; Li, J.R.; Han, Z.B.; Zhou, H.C. Functional mesoporous metal-organic frameworks for the capture of heavy metal ions and size-selective catalysis. Inorg. Chem. 2010, 49, 11637–11642. [Google Scholar] [CrossRef]
- Xiong, Y.Y.; Li, J.Q.; Gong, L.L.; Feng, X.F.; Meng, L.N.; Zhang, L.; Meng, P.P.; Luo, M.B.; Luo, F. Using MOF-74 for Hg2+ removal from ultra-low concentration aqueous solution. J. Solid State Chem. 2017, 246, 16–22. [Google Scholar] [CrossRef]
- Luo, X.B.; Shen, T.T.; Ding, L.; Zhong, W.P.; Luo, J.F.; Luo, S.L. Novel thymine-functionalized MIL-101 prepared by post-synthesis and enhanced removal of Hg2+ from water. J. Hazard. Mater. 2016, 306, 313–322. [Google Scholar] [CrossRef] [PubMed]

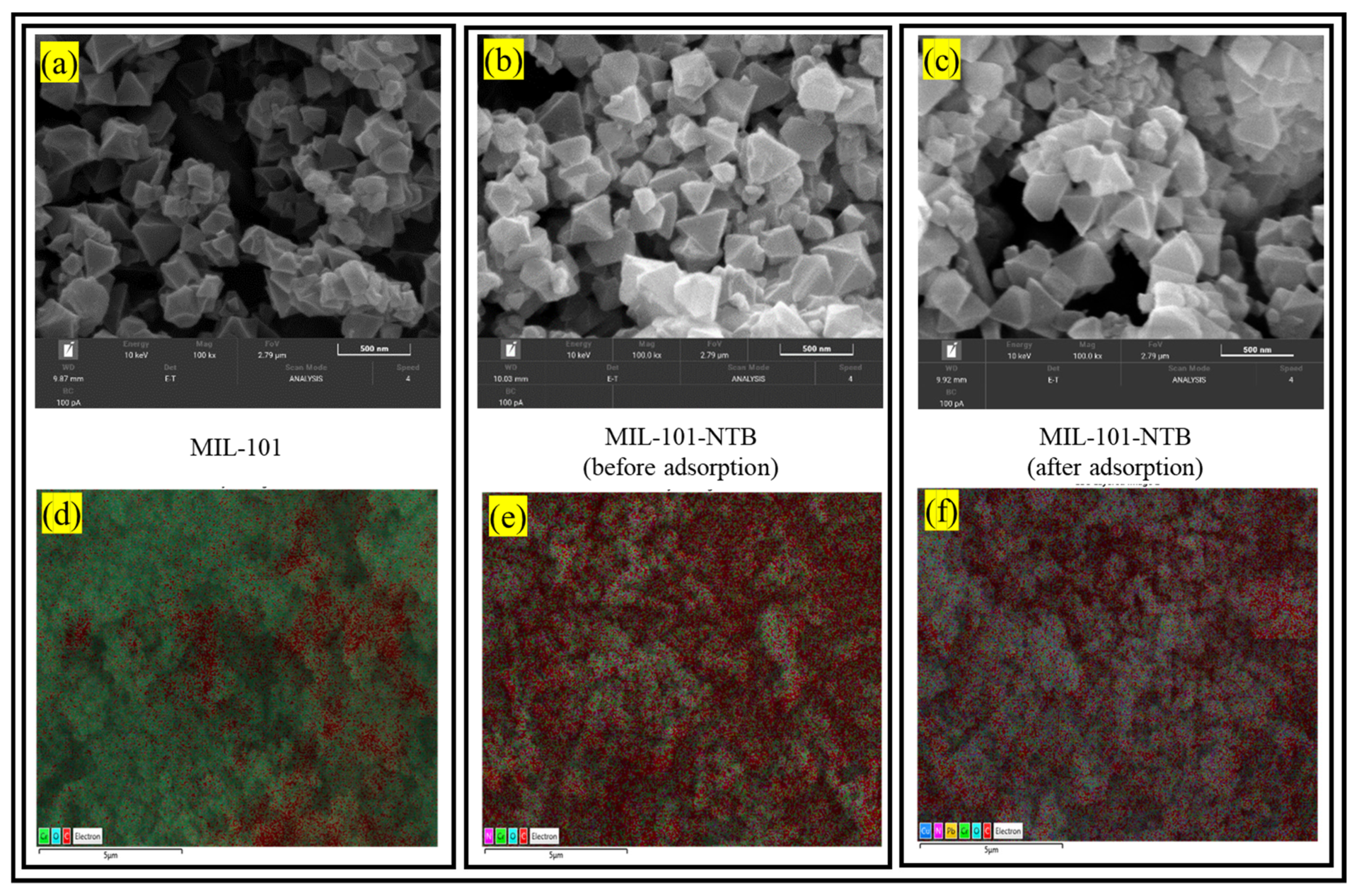
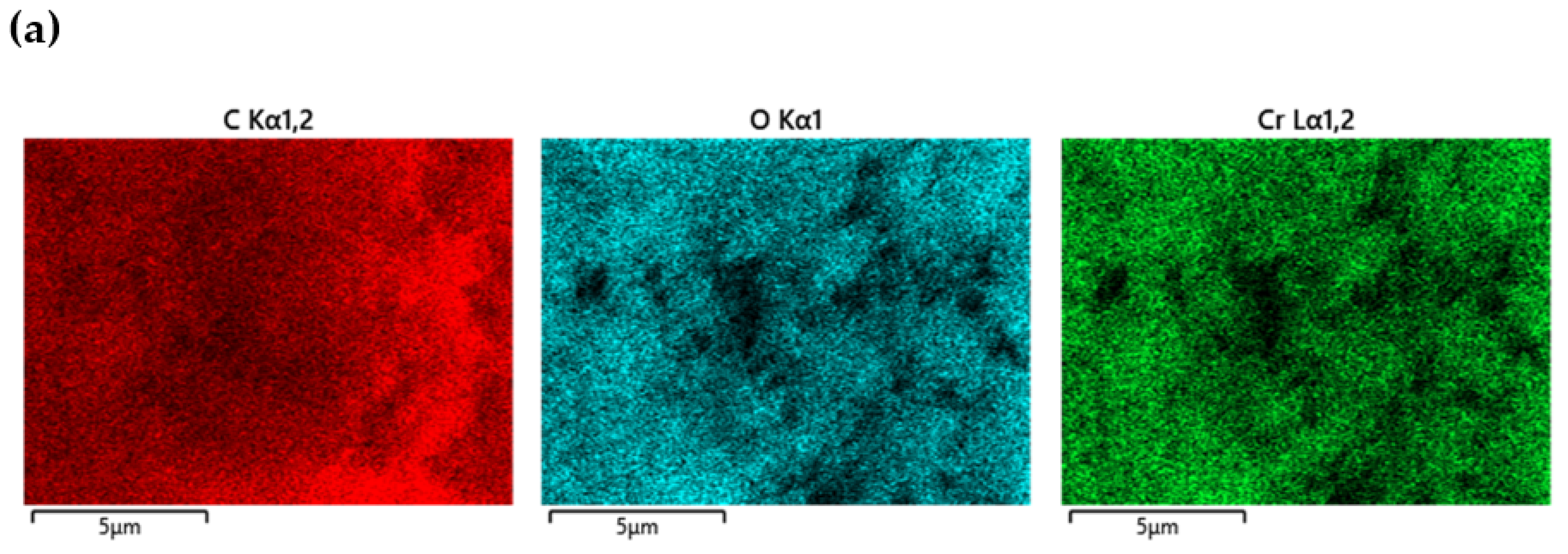
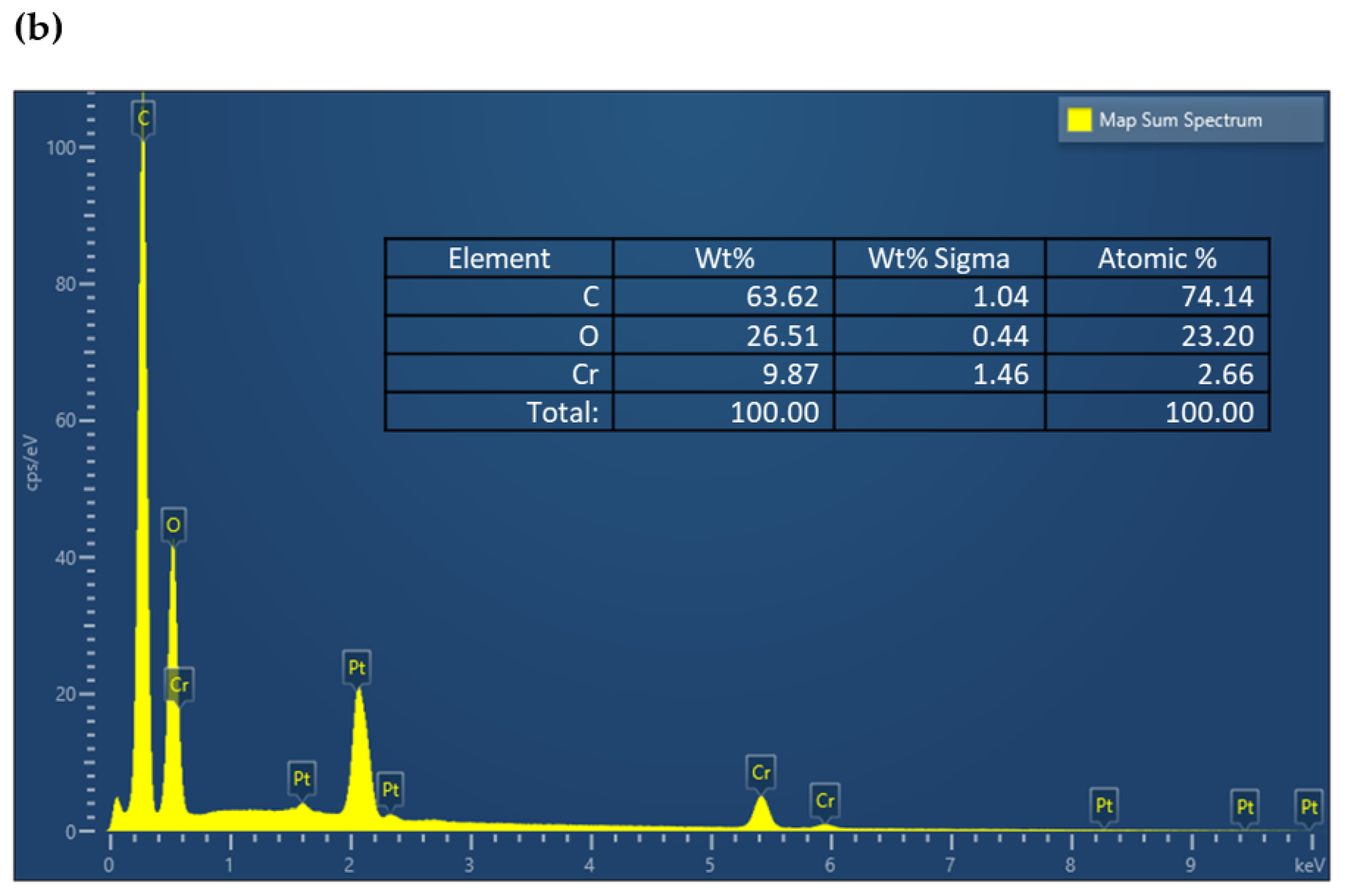
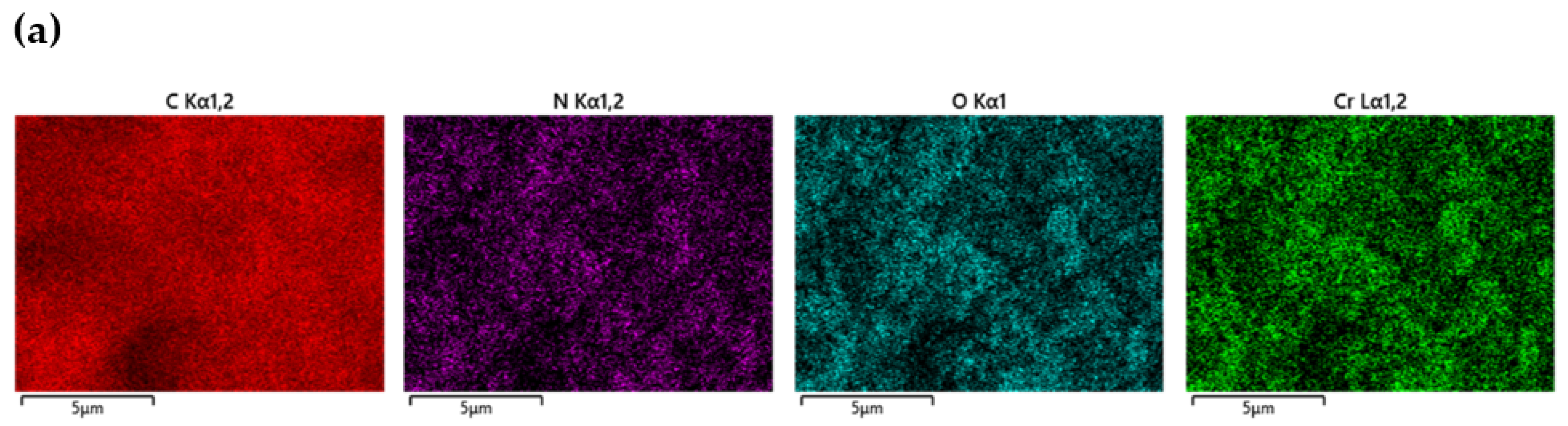
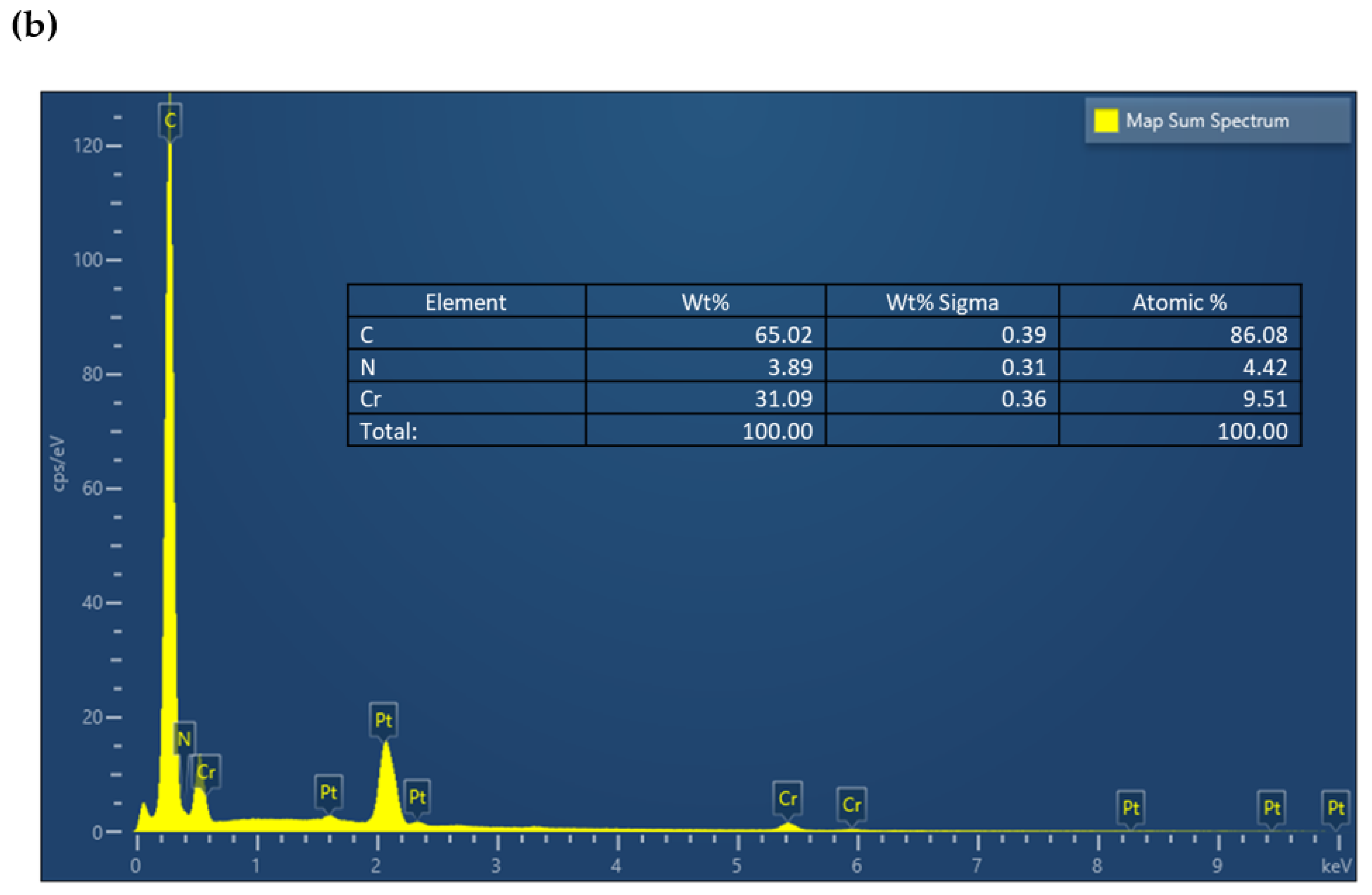
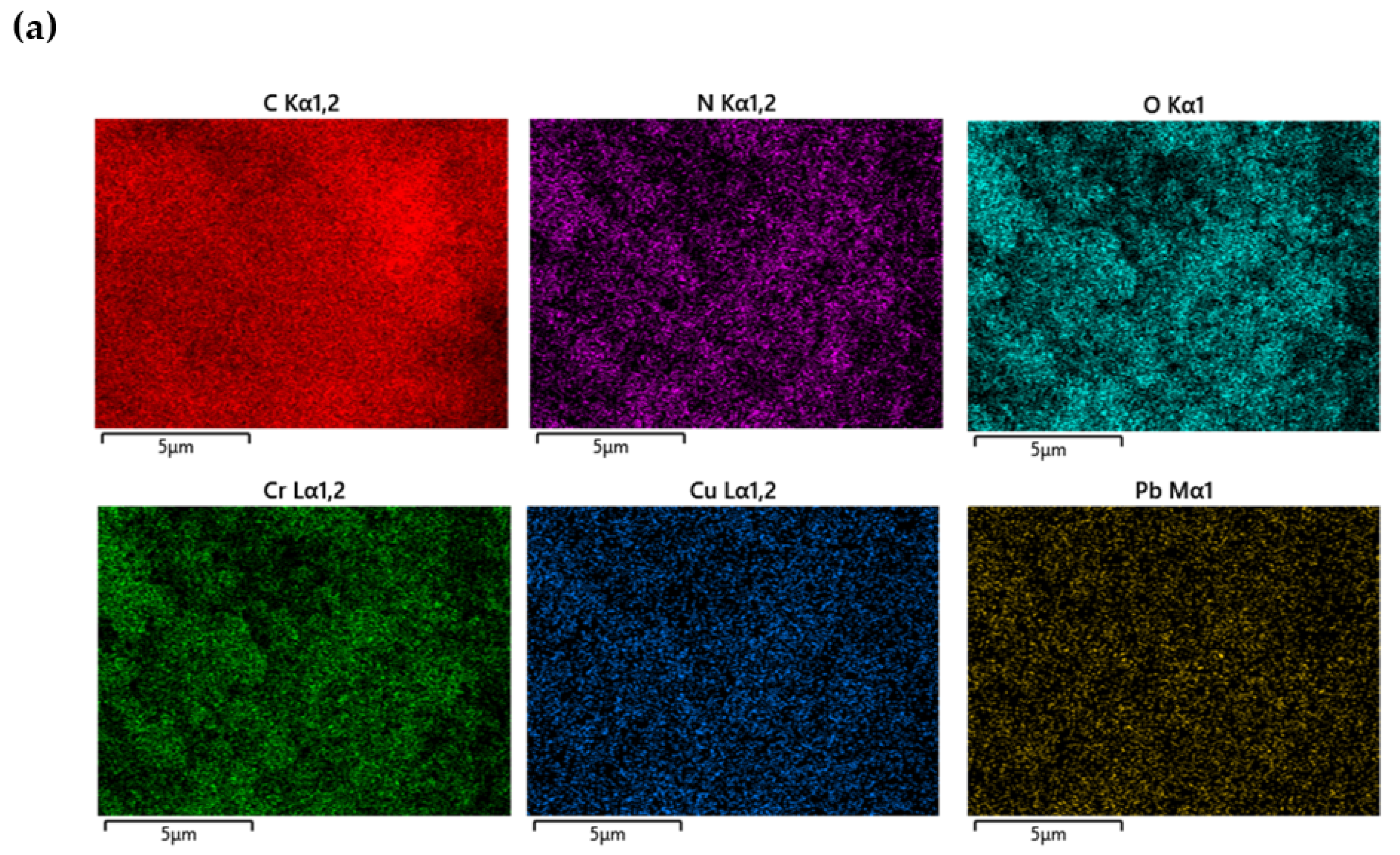
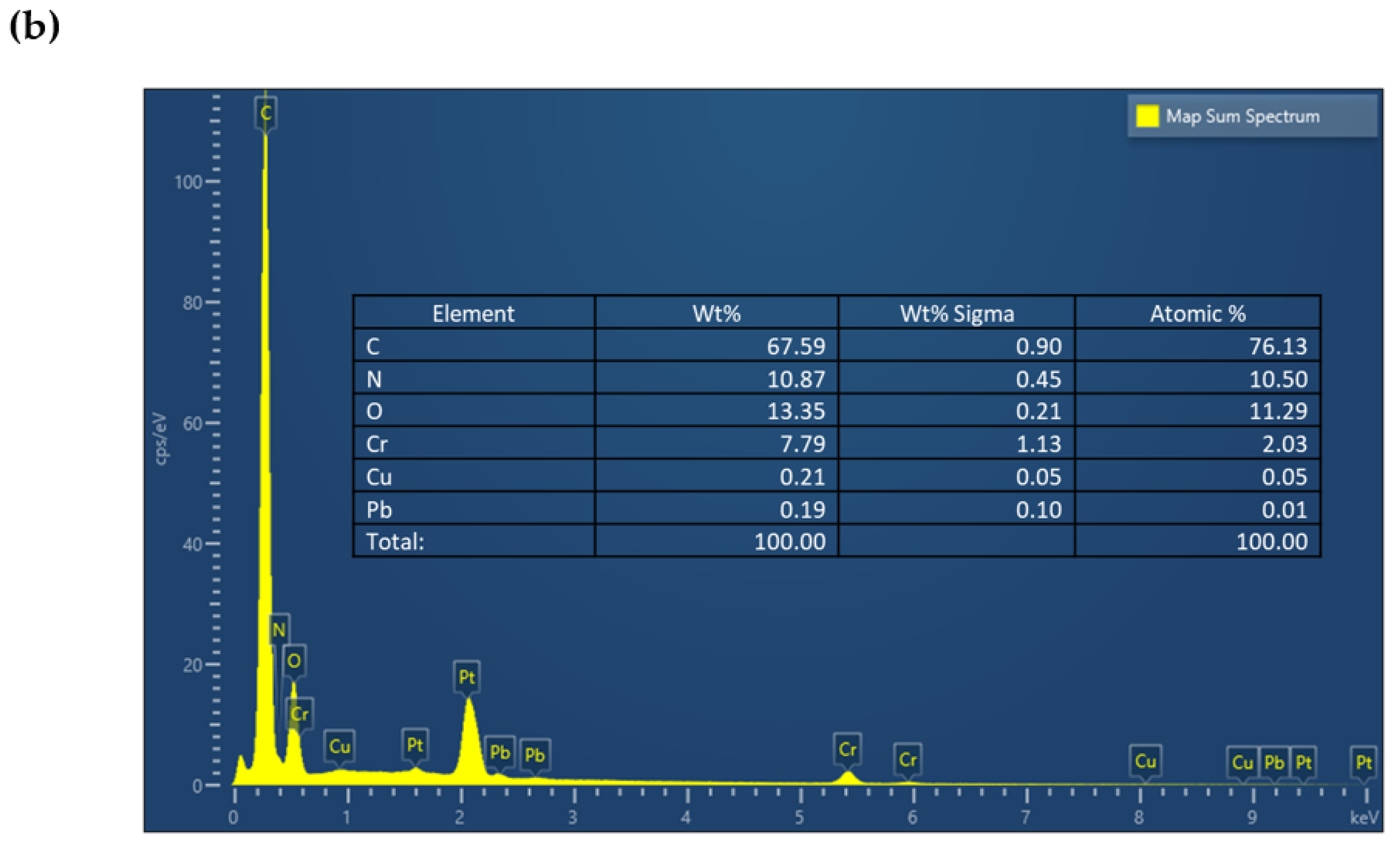
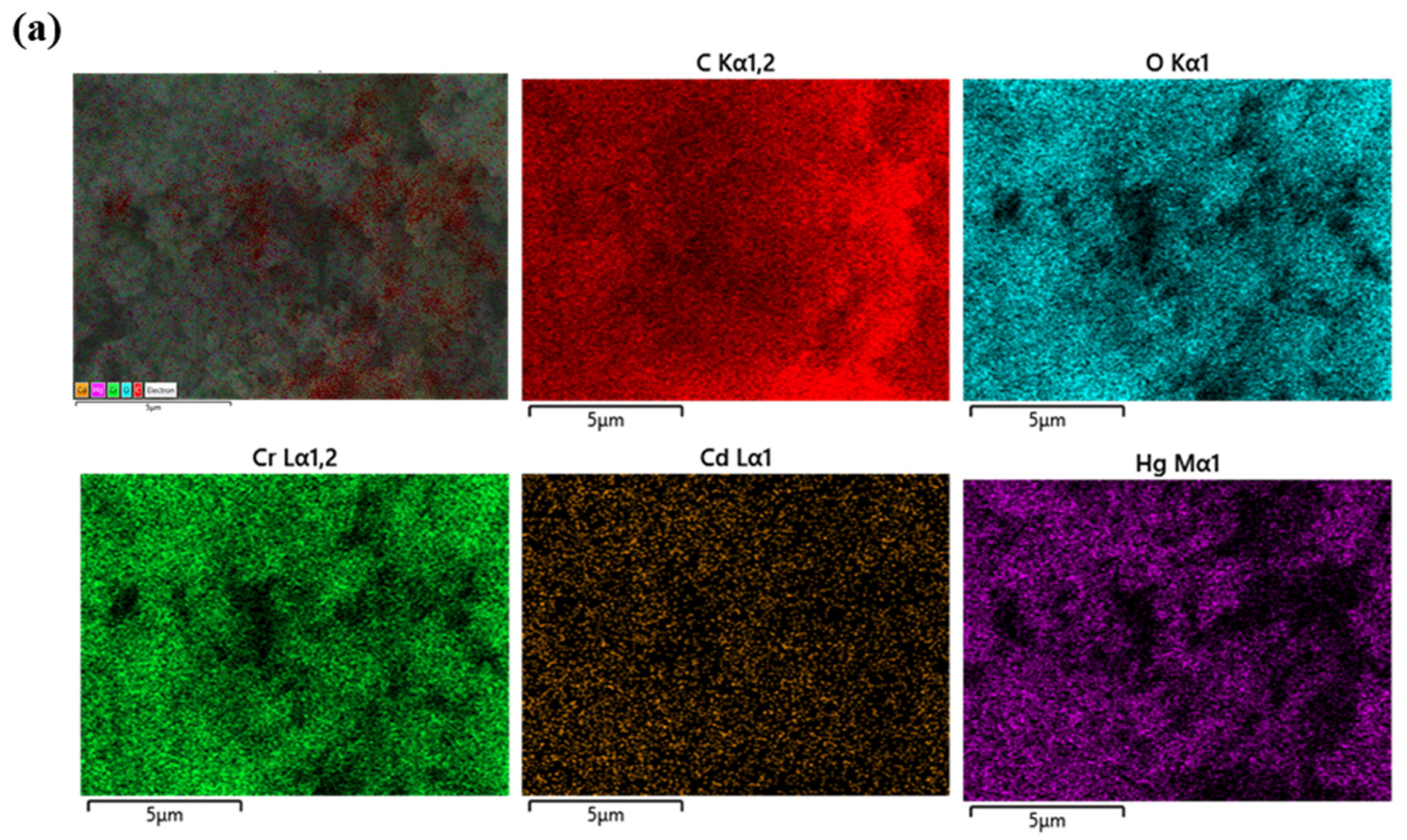
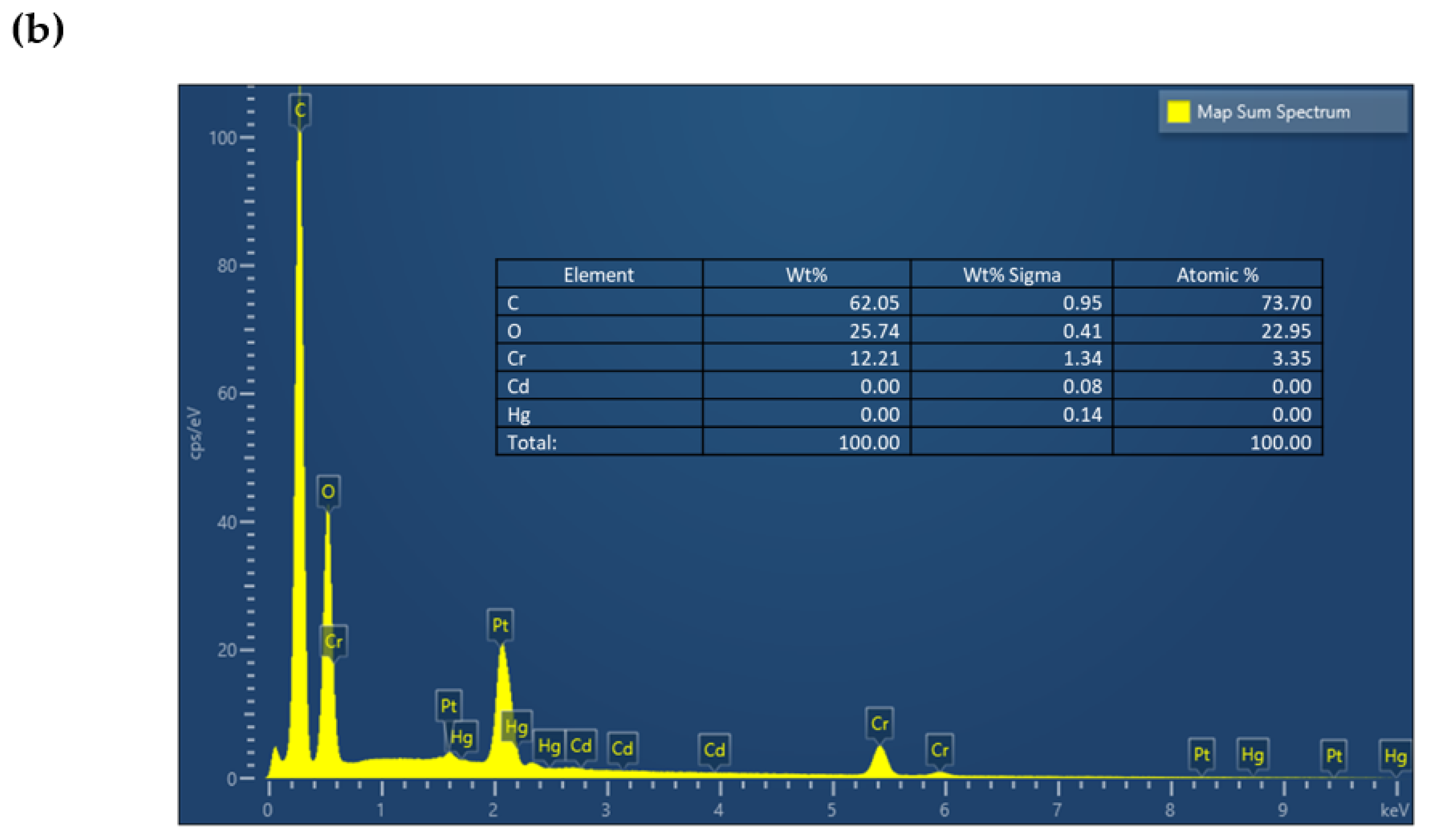
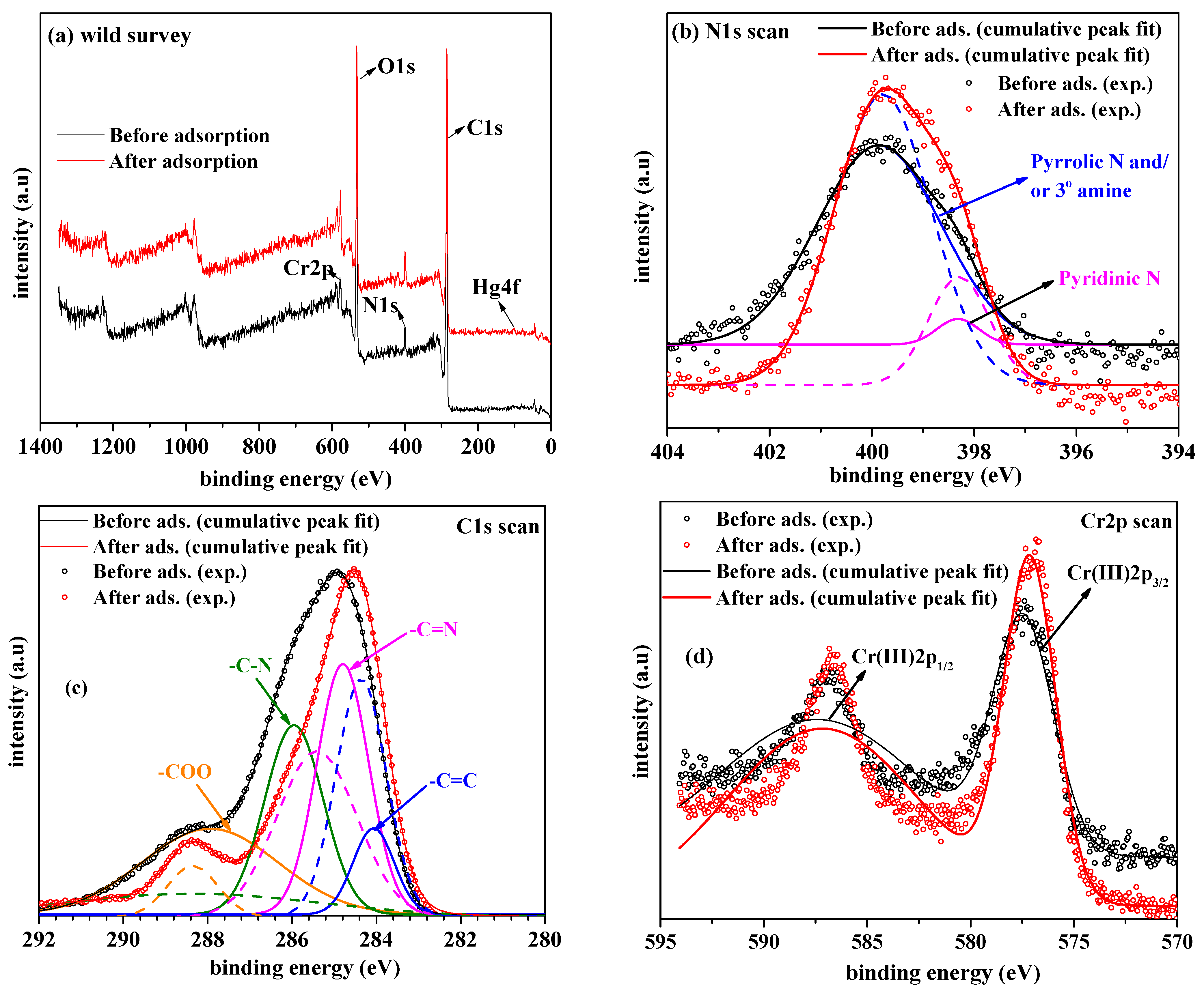
| Serial Number | Sample | %C | %H | %N |
|---|---|---|---|---|
| 1 | MIL-101 | 34.95 | 6.69 | 0.95 |
| 2 | MIL-101-NTB (Before adsorption) | 48.47 | 4.99 | 8.95 |
| 3 | MIL-101-NTB (After adsorption) | 48.22 | 5.77 | 8.18 |
| Serial Number | Sample | C1s | Cr2p | N1s | O1s | Hg4f |
|---|---|---|---|---|---|---|
| 1 | MIL-101-NTB (Before adsorption) | 48.47 | 1.57 | 4.04 | 23.55 | --- |
| 2 | MIL-101-NTB (After adsorption) | 48.22 | 2.41 | 6.29 | 23.81 | 0.06 |
| Serial Number | Kinetic Model | Kinetics Parameters | Parameter Values | Standard Error |
|---|---|---|---|---|
| 1 | Pseudo-first-order model | qe (mg/g) | 9.0525 | 0.0327 |
| K1 (min−1) | 1.9856 | 0.08202 | ||
| R2 | 0.9411 | --- | ||
| 2 | Pseudo-second-order model | qe (mg/g) | 9.1276 | 0.00324 |
| K2 (g mg−1 min−1) | 0.6491 | 0.0057 | ||
| R2 | 0.9995 | --- | ||
| 3 | Intraparticle diffusion model | C | 8.9134 | 0.00795 |
| Ki (mg/g min0.5) | 0.0189 | 8.3534 × 10−4 | ||
| R2 | 0.3393 | --- |
| Serial Number | Adsorption Isotherms | Isotherm Parameters | Parameter Values | Standard Error |
|---|---|---|---|---|
| 1 | Langmuir | qmax (mg/g) | 111.03 | 4.5919 |
| b (L/mg) | 0.4725 | 0.0829 | ||
| R2 | 0.9888 | --- | ||
| 2 | Freundlich | k (mg/g) | 32.43 | 4.2249 |
| n | 2.4791 | 0.3049 | ||
| R2 | 0.9594 | --- | ||
| 3 | Temkin | Kt (mg/g) | 10.67 | 1.6221 |
| b (Jol/mol) | 132.20 | 0.8786 | ||
| R2 | 0.9848 | --- | ||
| 4 | D–R | Intercept | 4.3593 | 0.1206 |
| qmax (mg/g) | 78.20 | --- | ||
| β (mol2/kJ2) (slope) | −9.385 × 10−8 | 5.80712 × 10−9 | ||
| E | 2.24 | --- | ||
| R2 | 0.9738 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rallapalli, P.B.S.; Choi, S.S.; Moradi, H.; Yang, J.-K.; Lee, J.-H.; Ha, J.H. Tris(2-benzimidazolyl)amine (NTB)-Modified Metal-Organic Framework: Preparation, Characterization, and Mercury Ion Removal Studies. Water 2023, 15, 2559. https://doi.org/10.3390/w15142559
Rallapalli PBS, Choi SS, Moradi H, Yang J-K, Lee J-H, Ha JH. Tris(2-benzimidazolyl)amine (NTB)-Modified Metal-Organic Framework: Preparation, Characterization, and Mercury Ion Removal Studies. Water. 2023; 15(14):2559. https://doi.org/10.3390/w15142559
Chicago/Turabian StyleRallapalli, Phani Brahma Somayajulu, Suk Soon Choi, Hiresh Moradi, Jae-Kyu Yang, Jae-Hoon Lee, and Jeong Hyub Ha. 2023. "Tris(2-benzimidazolyl)amine (NTB)-Modified Metal-Organic Framework: Preparation, Characterization, and Mercury Ion Removal Studies" Water 15, no. 14: 2559. https://doi.org/10.3390/w15142559