Acceleration of Aerobic Granulation in Sidestream Treatment with Exogenous Autoinducer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Batch Reactor Operation
2.2. Analytical Methods
2.2.1. Sludge Characteristic Test
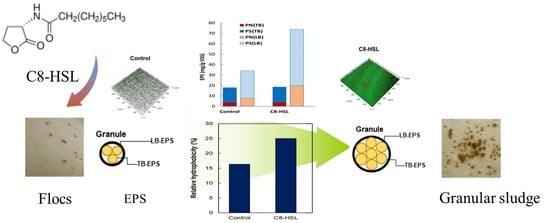
2.2.2. EPS Analysis
2.2.3. Relative Hydrophobicity
2.2.4. Biofilm Analysis
3. Results and Discussion
3.1. Effect of AHL on the Expression of EPS
3.1.1. Content of EPS
3.1.2. Composition of EPS
3.2. Effect of AHLs on the Relative Hydrophobicity and Biofilm Formation
3.2.1. Change in Relative Hydrophobicity of Sludge
3.2.2. Biofilm Formation
3.3. Formation of Granules
3.3.1. Change in the Size of Sludge Floc with the Addition of AHL
3.3.2. MLSS and Settleability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Q.; Song, J.; Zhang, W.; Gao, S.; Wang, H.; Yu, J. Enhanced simultaneous nitrification, denitrification and phosphorus removal through mixed carbon source by aerobic granular sludge. J. Hazard. Mater. 2020, 382, 121043. [Google Scholar] [CrossRef]
- Liu, X.; Pei, Q.; Han, H.; Yin, H.; Chen, M.; Guo, C.; Li, j.; Qiu, H. Functional analysis of extracellular polymeric substances (EPS) during the granulation of aerobic sludge: Relationship among EPS, granulation and nutrients removal. Environ. Res. 2022, 208, 112692. [Google Scholar] [CrossRef]
- Ran, X.; Zhou, M.; Wang, T.; Wang, W.; Kumari, S.; Wang, Y. Multidisciplinary characterization of nitrogen-removal granular sludge: A review of advances and technologies. Water Res. 2022, 214, 118214. [Google Scholar] [CrossRef]
- Ji, Y.; Yu, H.; Cao, R.; Xu, X.; Zhu, L. Promoting the granulation process of aerobic sludge via a sustainable strategy of effluent reflux in view of AHLs-mediated quorum sensing. J. Environ. Manag. 2022, 303, 114091. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, J.; Wang, J.; Zhu, H.; Xiong, J.; Nong, G.; Luo, M.; Wang, J. Direct start-up of aerobic granular sludge system with dewatered sludge granular particles as inoculant. J. Environ. Manag. 2023, 326, 116540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Liu, Y.; Tay, J.H. The effects of extracellular polymeric substances on the formation and stability of biogranules. Appl. Microbiol. Biotechnol. 2004, 65, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Qin, L.; Liu, X.; Li, B.; Chen, J.; You, J.; Shen, Y.; Chen, X. Effect of granular activated carbon on the aerobic granulation of sludge and its mechanism. Bioresour. Technol. 2017, 236, 60–67. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, B.; Zhang, Z.; Shi, W.; Zhang, R.; Cheng, J.; Li, W.; Cui, F. Enhanced aerobic granulation by applying the low-intensity direct current electric field via reactive iron anode. Water Res. 2019, 149, 159–168. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Zhong, C.; Li, Y.; Hao, W.; Zhu, J. The effect of quorum sensing and extracellular proteins on the microbial attachment of aerobic granular activated sludge. Bioresour. Technol. 2014, 152, 53–58. [Google Scholar] [CrossRef]
- Xiong, F.; Zhao, X.; Wen, D.; Li, Q. Effects of N-acyl-homoserine lactones-based quorum sensing on biofilm formation, sludge characteristics, and bacterial community during the start-up of bioaugmented reactors. Sci. Total Environ. 2020, 735, 139449. [Google Scholar] [CrossRef]
- Ren, T.T.; Li, X.Y.; Yu, H.Q. Effect of N-acy-l-homoserine lactones-like molecules from aerobic granules on biofilm formation by Escherichia coli K12. Bioresour. Technol. 2013, 129, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Hou, B.L.; Li, M.X. Cell adhesion, ammonia removal and granulation of autotrophic nitrifying sludge facilitated by N-acyl-homoserine lactones. Bioresour. Technol. 2015, 196, 550–558. [Google Scholar] [CrossRef]
- Tan, C.H.; Koh, K.S.; Xie, C.; Tay, M.; Zhou, Y.; Williams, R.; Ng, W.J.; Rice, S.A.; Kjelleberg, S. The role of quorum sensing signalling in EPS production and the assembly of a sludge community into aerobic granules. ISME J. 2014, 8, 1186–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Zheng, S.; Huo, Z.; Shi, B.; Huang, J.; Yang, J.; Ma, J.; Han, Y.; Wang, Y.; Cheng, K.; et al. Effects of exogenous N-acyl-homoserine lactones on nutrient removal, sludge properties and microbial community structures during activated sludge process. Chemosphere 2020, 255, 126945. [Google Scholar] [CrossRef]
- Ma, H.; Ma, S.; Hu, H.; Ding, L.; Ren, H. The biological role of N-acyl-homoserine lactone-based quorum sensing (QS) in EPS production and microbial community assembly during anaerobic granulation process. Sci. Rep. 2018, 8, 15793. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Deng, C.; Chen, J.; Lü, J.; Chen, S.; Zhou, S. Accelerating the start-up of the cathodic biofilm by adding acyl-homoserine lactone signaling molecules. Bioresour. Technol. 2018, 266, 548–554. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Ji, Y.; Cao, R.; Zhou, J.; Li, M.; Zhu, L.; Xu, X. Understanding the N-acylated homoserine lactones(AHLs)-based quorum sensing for the stability of aerobic granular sludge in the aspect of substrate hydrolysis enhancement. Sci. Total Environ. 2023, 858, 159581. [Google Scholar] [CrossRef]
- Tay, J.H.; Liu, Q.S.; Liu, Y. Aerobic granulation in sequential sludge blanket reactor. Water Sci. Technol. 2002, 46, 13–18. [Google Scholar] [CrossRef]
- Tay, J.H.; Liu, Q.S.; Liu, Y. The effects of shear force on the formation, structure and metabolism of aerobic granules. Appl. Microbiol. Biotechnol. 2001, 57, 227–233. [Google Scholar] [CrossRef]
- Chang, I.; Lee, C. Membrane filtration characteristics in membrane-coupled activated sludge system—the effect of physiological states of activated sludge on membrane fouling. Desalination 1998, 120, 221–233. [Google Scholar] [CrossRef]
- An, Q.; Chen, Y.; Tang, M.; Zhao, B.; Deng, S.; Li, Z. The mechanism of extracellular polymeric substances in the formation of activated sludge flocs. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131009. [Google Scholar] [CrossRef]
- Domínguez, L.; Rodríguez, M.; Prats, D. Effect of different extraction methods on bound EPS from MBR sludges. Part I: Influence of extraction methods over three-dimensional EEM fluorescence spectroscopy fingerprint. Desalination 2010, 261, 19–26. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Park, K.Y.; Jung, J.; Song, W.; Kim, J.; Ryu, J.; Lade, H.; Kweon, J. Monitoring biofouling based on aerobic respiration in reverse osmosis system. J. Environ. Sci. 2019, 78, 247–256. [Google Scholar] [CrossRef]
- Arabi, S.; Nakhla, G. Impact of protein/carbohydrate ratio in the feed wastewater on the membrane fouling in membrane bioreactors. J. Membr. Sci. 2008, 324, 142–150. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, T.; Samaranayake, Y.H.; Fang, H.H.P.; Yip, H.K.; Samaranayake, L.P. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia 2005, 159, 353–360. [Google Scholar] [CrossRef]
- Shailaja, A.; Bruce, T.F.; Gerard, P.; Powell, R.R.; Pettigrew, C.A.; Kerrigan, J.L. Comparison of cell viability assessment and visualization of Aspergillus niger biofilm with two fluorescent probe staining methods. Biofilm 2022, 4, 100090. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lyu, W.; Song, Q.; Zhou, D.; Hu, X.; Wang, B.; Chen, R. Role of weak magnetic strength in the operation of aerobic granular reactor for wastewater treatment containing ammonia nitrogen concentration gradient. Bioresour. Technol. 2021, 322, 124570. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Du, R.; Zhang, H.; Peng, Y. Understanding the granulation of partial denitrification sludge for nitrite production. Chemosphere 2019, 236, 124389. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Han, Z.; Shao, X.; Zhao, Z.; Zhang, H.; Lin, T.; Yang, S.; Zhou, C. Response of aerobic granular sludge under polyethylene microplastics stress: Physicochemical properties, decontamination performance, and microbial community. J. Environ. Manag. 2022, 323, 116215. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Bourven, I.; Simon, S.; Bordas, F.; van Hullebusch, E.D.; Rossano, S.; Lens, P.N.L.; Guibaud, G. Fluorescence detection to determine proteins and humic-like substances fingerprints of exopolymeric substances (EPS) from biological sludges performed by size exclusion chromatography (SEC). Bioresour. Technol. 2013, 131, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.M.; Cheong, W.S.; Oh, H.S.; Lee, W.N.; Hwang, B.K.; Lee, C.H.; Beyenal, H.; Lewandowski, Z. Quorum Sensing: A New Biofouling Control Paradigm in a Membrane Bioreactor for Advanced Wastewater Treatment. Environ. Sci. Technol. 2009, 43, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, J.; Lv, M.; Yu, H.; Zhao, H.; Xu, X. Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE. Chemosphere 2015, 121, 26–32. [Google Scholar] [CrossRef]
- Da Costa, N.P.A.V.; Libardi, N.; Da Costa, R.H.R. How can the addition of extracellular polymeric substances (EPS)-based bioflocculant affect aerobic granular sludge (AGS)? J. Environ. Manag. 2022, 310, 114807. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Liang, H.; He, J.; Ma, J.; Wang, Z.; Yu, H.; Li, G. Characterization of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) of Microcystis aeruginosa and their impacts on UF membrane fouling. Water Res. 2012, 46, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Qi, H.Y.; Lv, M.L.; Kong, Y.; Yu, Y.W.; Xu, X.Y. Component analysis of extracellular polymeric substances (EPS) during aerobic sludge granulation using FTIR and 3D-EEM technologies. Bioresour. Technol. 2012, 124, 455–459. [Google Scholar] [CrossRef]
- Liu, Y.; Tay, J.H. State of the art of biogranulation technology for wastewater treatment. Biotechnol. Adv. 2004, 22, 533–563. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Yao, J.; Cai, W. Effects of extracellular polymeric substances on aerobic granulation in sequencing batch reactors. Chemosphere 2006, 63, 1728–1735. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L.; Lv, Z.; Xin, F.; Dong, W.; Liu, G.; Li, Y.; Jia, H. N-acyl-homoserine lactones in extracellular polymeric substances from sludge for enhanced chloramphenicol-degrading anode biofilm formation in microbial fuel cells. Environ. Res. 2022, 207, 112649. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Li, X.; Ma, S.; Hu, H.; Wu, B.; Zhang, X.X.; Ren, H. In-situ monitoring AHL-mediated quorum-sensing regulation of the initial phase of wastewater biofilm formation. Environ. Int. 2020, 135, 105326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. A sustainable strategy for effective regulation of aerobic granulation: Augmentation of the signaling molecule content by cultivating AHL-producing strains. Water Res. 2020, 169, 115193. [Google Scholar] [CrossRef] [PubMed]
- Shuai, J.; Hu, X.; Wang, B.; Lyu, W.; Chen, R.; Guo, W.; Wang, H.; Zhou, D. Response of aerobic sludge to AHL-mediated QS: Granulation, simultaneous nitrogen and phosphorus removal performance. Chin. Chem. Lett. 2021, 32, 3402–3409. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.; Min, K.J.; Lee, E.; Choi, H.; Park, K.Y. Acceleration of Aerobic Granulation in Sidestream Treatment with Exogenous Autoinducer. Water 2023, 15, 2173. https://doi.org/10.3390/w15122173
Jang E, Min KJ, Lee E, Choi H, Park KY. Acceleration of Aerobic Granulation in Sidestream Treatment with Exogenous Autoinducer. Water. 2023; 15(12):2173. https://doi.org/10.3390/w15122173
Chicago/Turabian StyleJang, Eunae, Kyung Jin Min, Eunyoung Lee, Hanna Choi, and Ki Young Park. 2023. "Acceleration of Aerobic Granulation in Sidestream Treatment with Exogenous Autoinducer" Water 15, no. 12: 2173. https://doi.org/10.3390/w15122173