Using Wavelet Analysis to Examine Long-Term Variability of Phytoplankton Biomass in the Tropical, Saline Lake Alchichica, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Physical, Chemical, and Chl-a Characterisation
2.3. Wavelet Analysis
3. Results
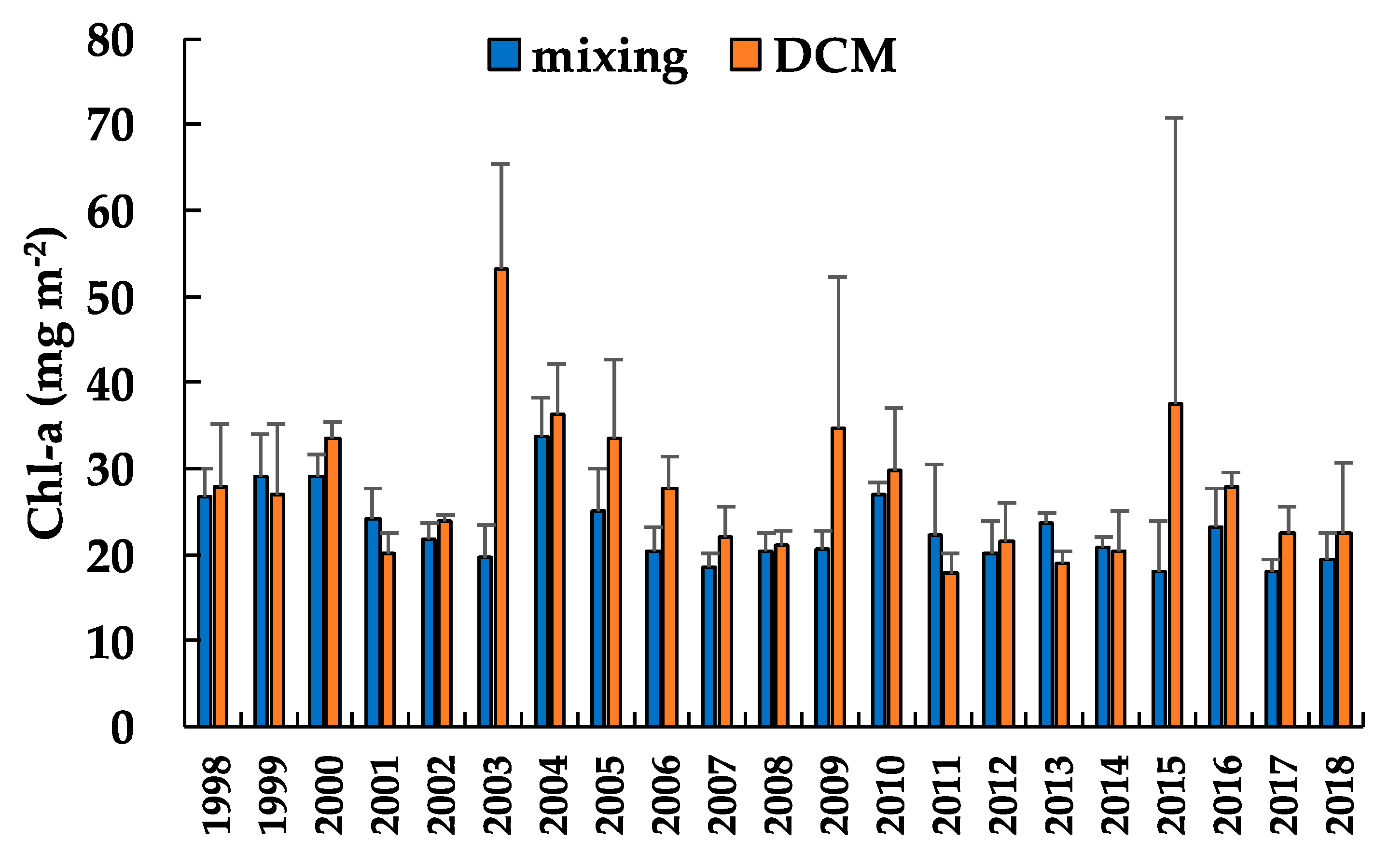
3.1. Chlorophyll-a
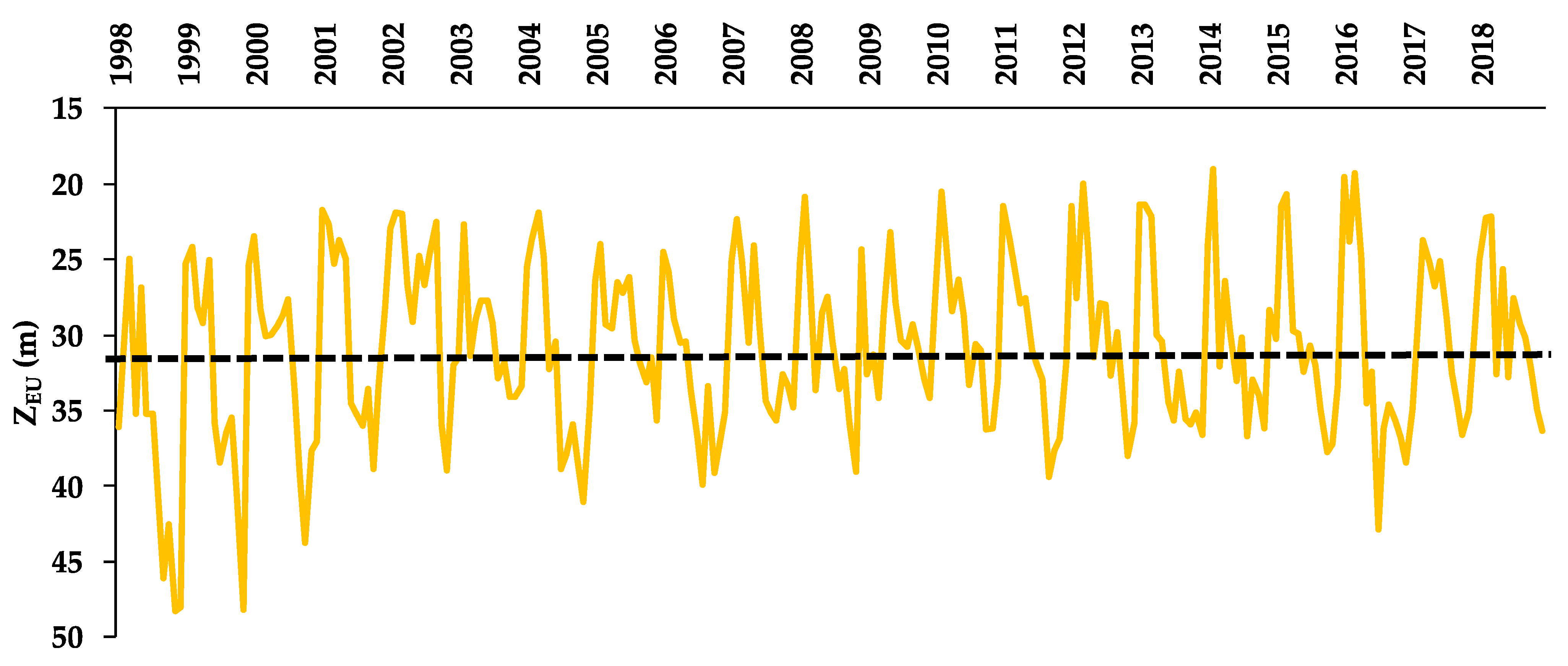
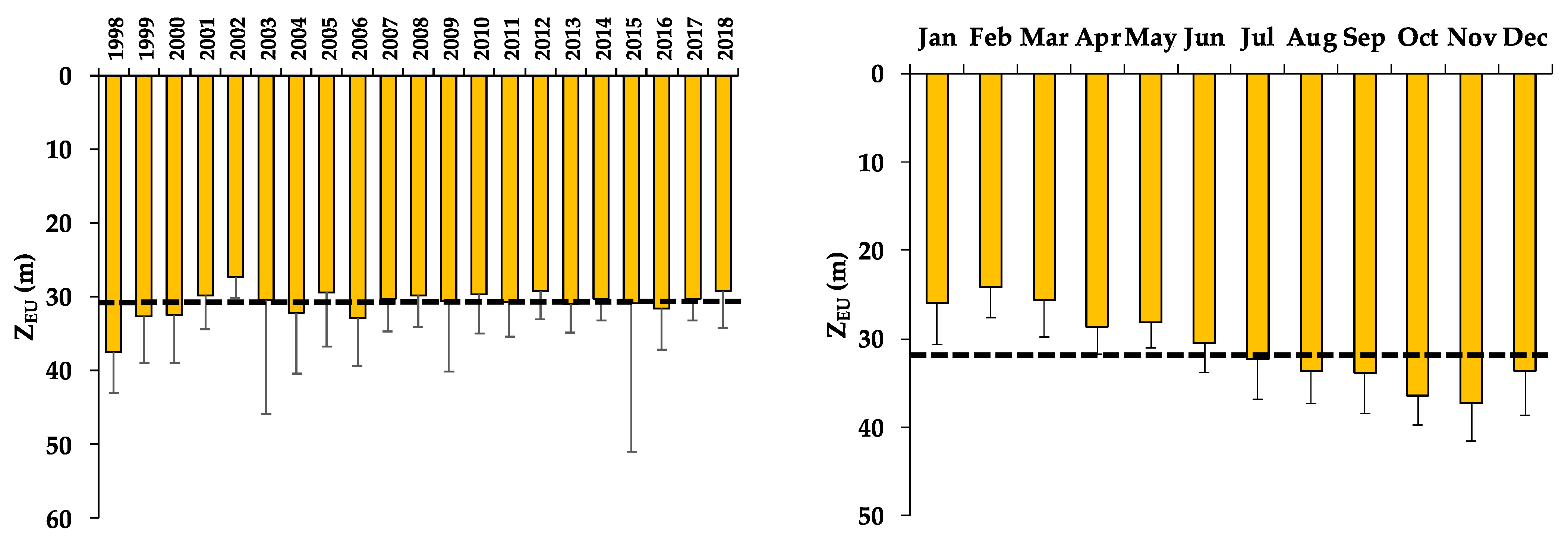
3.2. Euphotic Zone (ZEU)
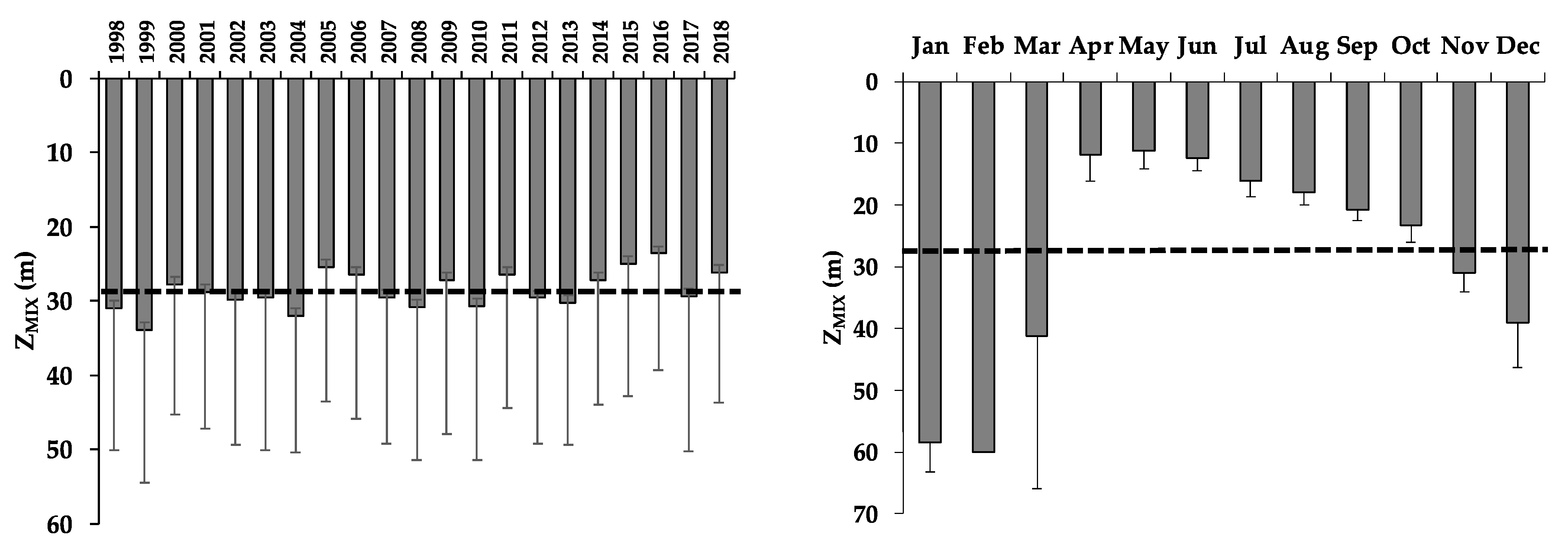
3.3. Mixed Layer Depth (ZMIX)
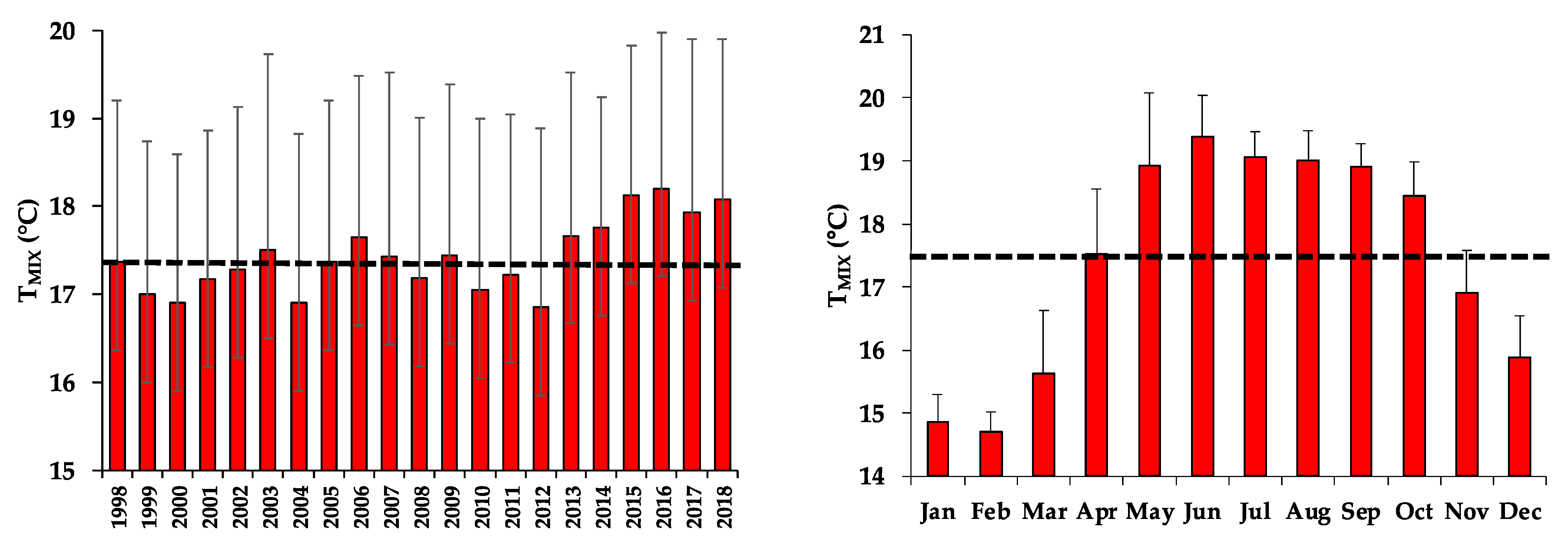
3.4. Temperature of the Mixed Layer (TMIX)
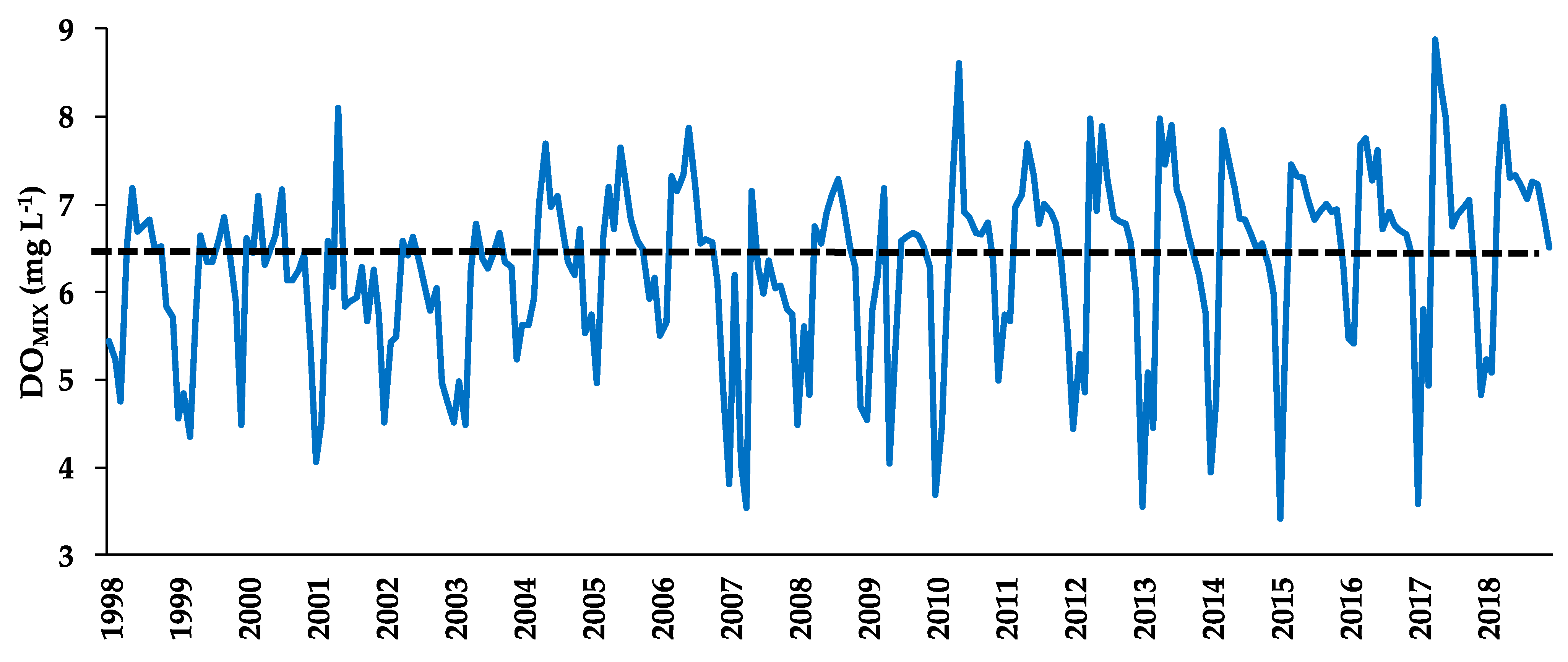
3.5. Dissolved Oxygen of the Mixed Layer (DOMIX)
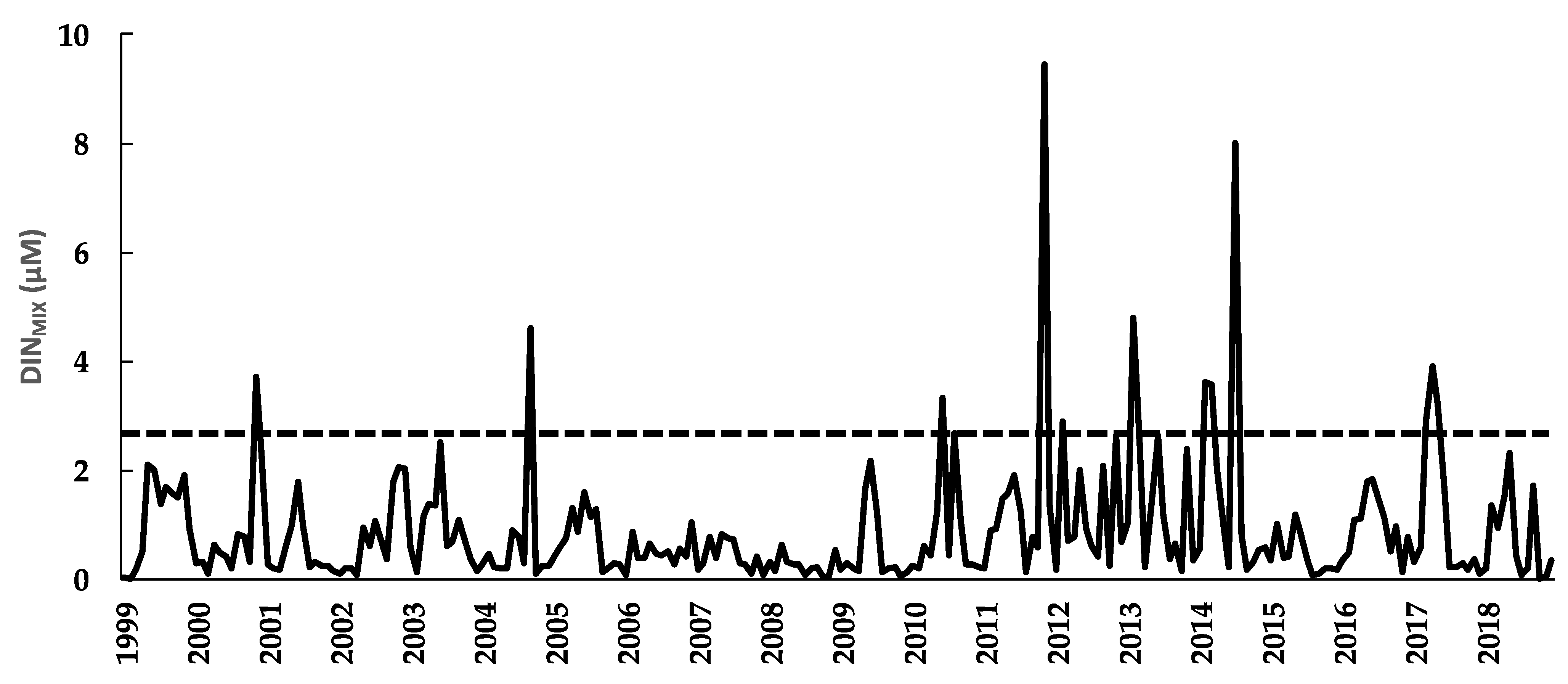
3.6. Dissolved Inorganic Nitrogen of the Mixed Layer (DINMIX)
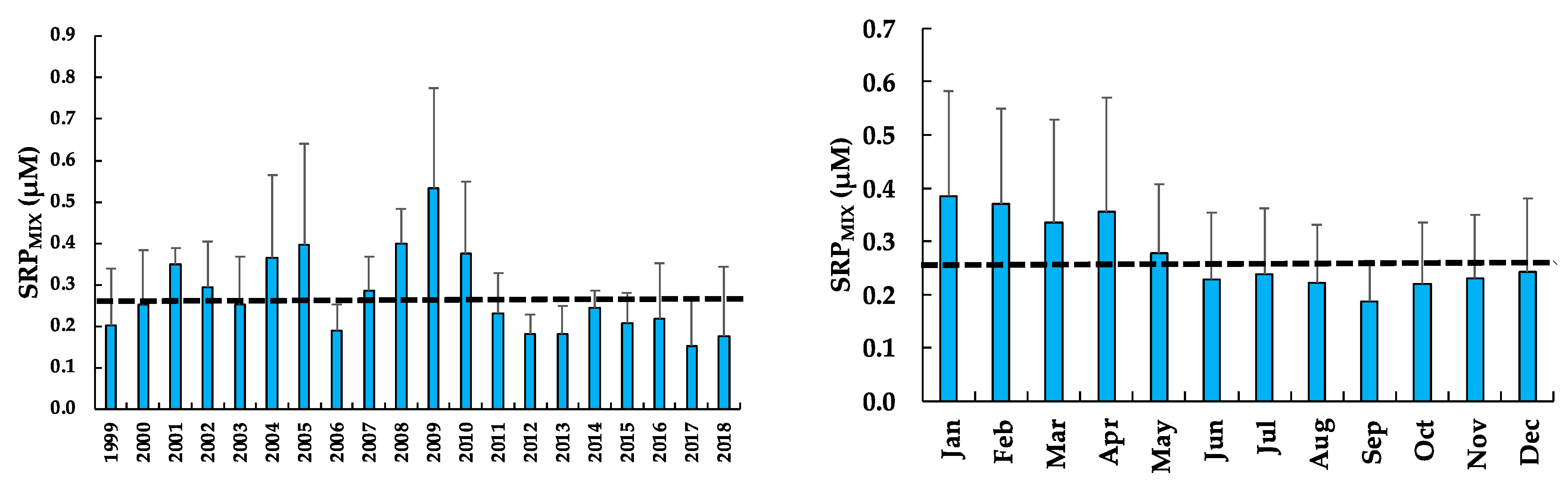
3.7. Soluble Reactive Phosphorus of the Mixed Layer (SRPMIX)
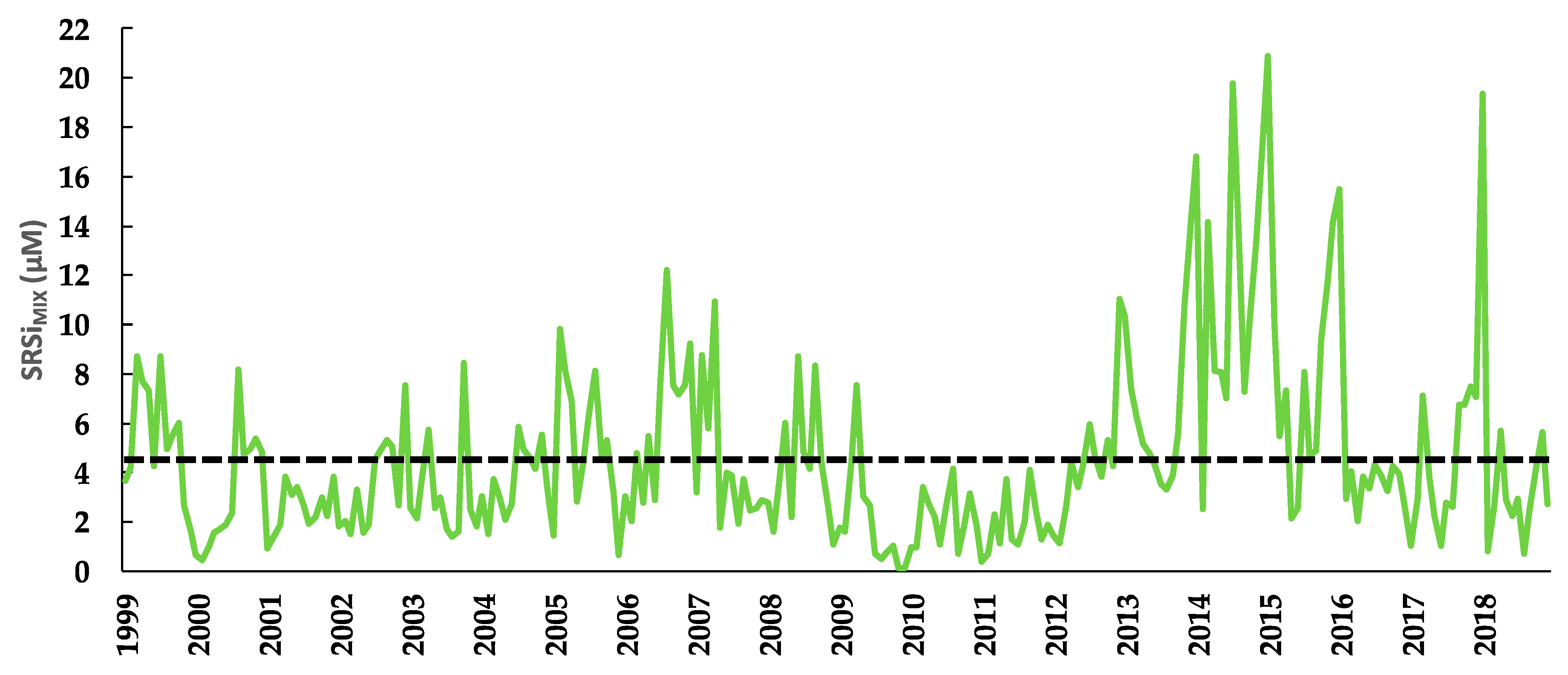
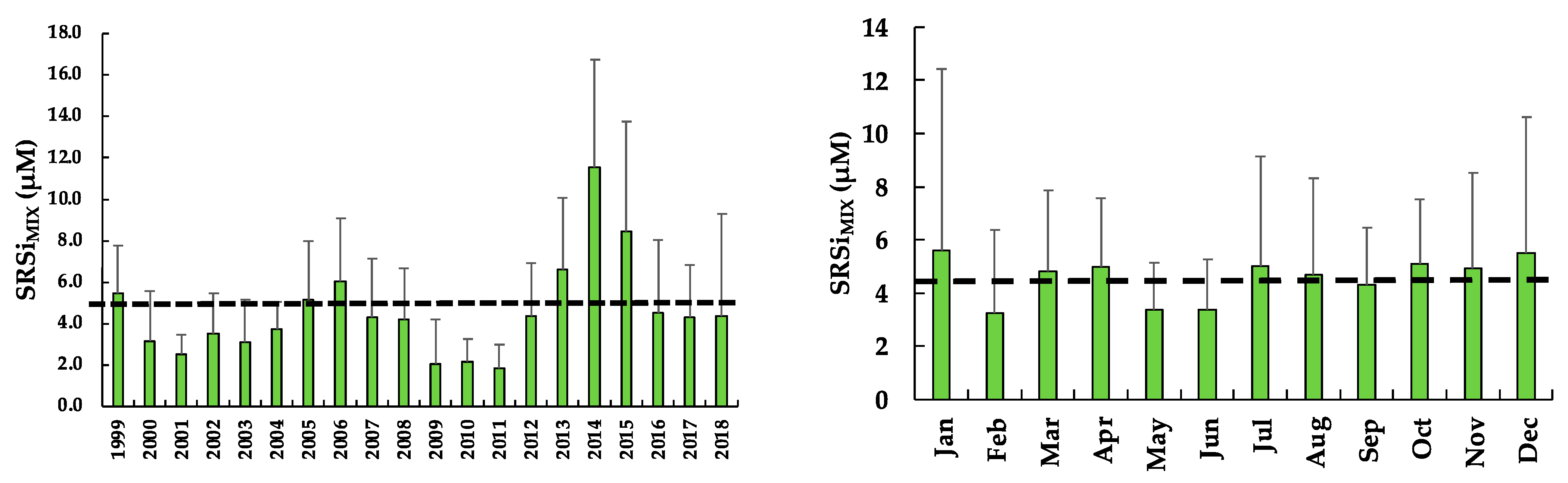
3.8. Soluble Reactive Silica of the Mixed Layer (SRSiMIX)
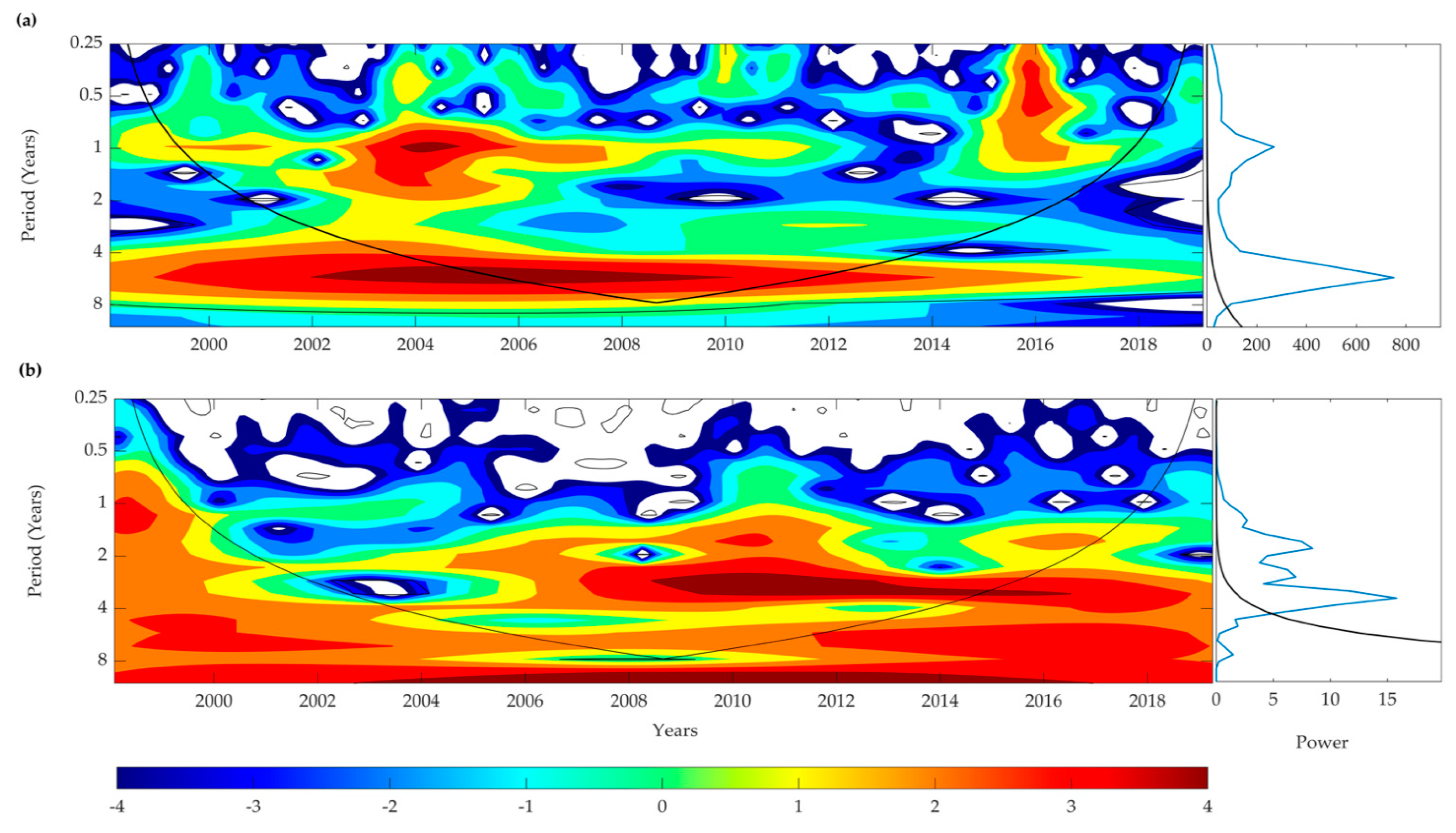
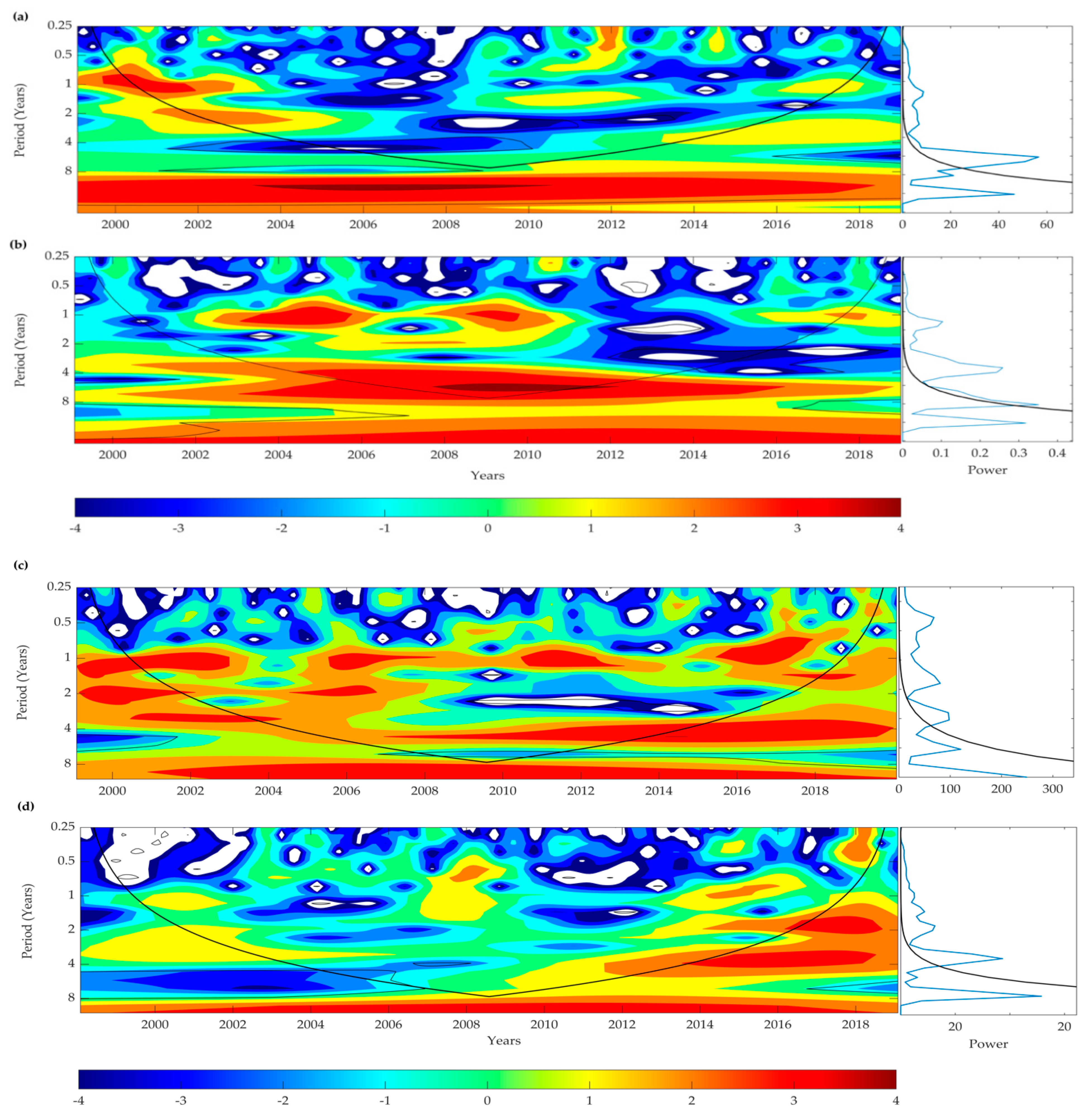
3.9. Wavelet Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melack, J.M. Primary productivity and fish yields in tropical lakes. Trans. Am. Fish. Soc. 1976, 105, 575–580. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Adame, M.F.; Alcocer, J.; Escobar, E. Size-fractionated phytoplankton biomass and its implications for the dynamics of an oligotrophic tropical lake. Freshw. Biol. 2008, 53, 22–31. [Google Scholar] [CrossRef]
- Talling, J.F.; Lemoalle, J. Ecological Dynamics of Tropical Inland Waters; Cambridge University Press: Cambridge, UK, 1998; p. 452. [Google Scholar]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in freshwaters. Arch. Für Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- Sommer, U.; Adrian, R.; De Senerpont Domis, L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lürling, M.; Molinero, J.C.; Mooij, W.M.; et al. Beyond the Plankton Ecology Group (PEG) model: Mechanisms driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Carey, C.C.; Hanson, P.C.; Lathrop, R.C.; St. Amand, A.L. Using wavelet analyses to examine variability in phytoplankton seasonal succession and annual periodicity. J. Plankton Res. 2016, 38, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Sarmento, H. New paradigms in tropical limnology: The importance of the microbial food web. Hydrobiologia 2012, 686, 1–14. [Google Scholar] [CrossRef]
- Plisnier, P.D.; Nshombo, M.; Mgana, H.; Ntakimazi, G. Monitoring climate change and anthropogenic pressure at Lake Tanganyika. J. Great Lakes Res. 2018, 44, 1194–1208. [Google Scholar] [CrossRef]
- Talling, J.F. The seasonality of phytoplankton in African lakes. Hydrobiologia 1986, 138, 138–160. [Google Scholar] [CrossRef]
- Lewis, W.M.J. Phytoplankton succession in Lake Valencia, Venezuela. Hydrobiologia 1986, 138, 189–203. [Google Scholar] [CrossRef]
- Darchambeau, F.; Sarmento, H.; Descy, J.-P. Primary production in a tropical large lake: The role of phytoplankton composition. Sci. Total Environ. 2014, 473–474, 178–188. [Google Scholar] [CrossRef]
- Kilham, P.; Kilham, S.S. Endless summer: Internal loading processes dominate nutrient cycling in tropical lakes. Freswater Biol. 1990, 23, 379–389. [Google Scholar] [CrossRef]
- Rugema, E.; Darchambeau, F.; Sarmento, H.; Stoyneva-Gärtner, M.; Leitao, M.; Thiery, W.; Latli, A.; Descy, J.P. Long-term change of phytoplankton in Lake Kivu: The rise of the greens. Freshw. Biol. 2019, 64, 1940–1955. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D. Complex seasonal patterns of primary producers at the land–sea interface. Ecol. Lett. 2008, 11, 1294–1303. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Soto, C.; Pingree, R.D. Spring and summer blooms of phytoplankton (SeaWiFS/MODIS) along a ferry line in the Bay of Biscay and western English Channel. Cont. Shelf Res. 2009, 29, 111–1122. [Google Scholar] [CrossRef]
- Cloern, J.E.; Jassby, A.D. Patterns and scales of phytoplankton variability in estuarine–coastal ecosystems. Estuaries Coasts 2010, 33, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Smayda, T.J. Patterns of variability characterizing marine phytoplankton, with examples from Narragansett Bay. ICES J. Mar. Sci. 1998, 55, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Winder, M.; Cloern, J.E. The annual cycles of phytoplankton biomass. Philos. Trans. R. Soc. B 2010, 365, 230–241. [Google Scholar] [CrossRef]
- Mallat, S. A Wavelet Tout of Signal Processing; Academic Press: San Diego, CA, USA, 1998; p. 832. [Google Scholar]
- Alcocer, J.; Lugo, A.; Escobar, E.; Sánchez, M.R.; Vilaclara, G. Water column stratification and its implications in the tropical warm monomictic Lake Alchichica, Puebla, Mexico. Verh. des Int. Ver. Limnol. 2000, 27, 3166–3169. [Google Scholar] [CrossRef]
- Filonov, A.; Tereshchenko, I.; Barba-Lopez, M.R.; Alcocer, J.; Ladah, L. Meteorological regime, local climate, and hydrodynamics of Lake Alchichica. In Lake Alchichica Limnology—The Uniqueness of a Tropical Maar Lake; Alcocer, J., Ed.; Springer: Cham, Switzerland, 2022; pp. 75–99. [Google Scholar]
- Silva-Aguilera, R.A.; Vilaclara, G.; Armienta, M.A.; Escolero, O. Hydrogeology and hydrochemistry of the Serdán-Oriental basin and the Lake Alchichica. In Lake Alchichica Limnology—The Uniqueness of a Tropical Maar Lake; Alcocer, J., Ed.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Ramírez-Olvera, M.A.; Alcocer, J.; Merino-Ibarra, M.; Lugo, A. Nutrient limitation in a tropical saline lake: A microcosm experiment. Hydrobiologia 2009, 626, 5–13. [Google Scholar] [CrossRef]
- Oliva, M.G.; Lugo, A.; Alcocer, J.; Peralta, L.; Sánchez, M.R. Phytoplankton dynamics in a deep, tropical, hyposaline lake. Hydrobiologia 2001, 446, 299–306. [Google Scholar] [CrossRef]
- Vilaclara, G.; Oliva-Martínez, M.G.; Macek, M.; Ortega-Mayagoitia, E.; Alcántara-Hernández, R.J.; López-Vázquez, C. Phytoplankton of Alchichica: A unique community for an oligotrophic lake. In Lake Alchichica Limnology—The Uniqueness of a Tropical Maar Lake; Alcocer, J., Ed.; Springer: Cham, Switzerland, 2022; pp. 192–211. [Google Scholar]
- Oliva, M.G.; Lugo, A.; Alcocer, J.; Peralta, L.; Oseguera, L.A. Planktonic bloom-forming Nodularia in the saline Lake Alchichica, Mexico. In Saline Lakes Around the World: Unique Systems with Unique Values; Oren, A., Naftz, D.L., Wurtsbaugh, W.A., Eds.; The S.J. and Jessie E. Quinney Natural Resources Research Library, College Of Natural Resources, Utah State University: Logan, UT, USA, 2009; pp. 121–126. [Google Scholar]
- Keifer, D.A.; Chamberlain, W.S.; Booth, C.R. Natural fluorescence of chlorophyll a: Relationship to photosynthesis and chlorophyll concentration in the western South Pacific gyre. Limnol. Oceanogr. 1989, 34, 868–881. [Google Scholar] [CrossRef]
- Chamberlain, W.S.; Booth, C.R.; Keifer, D.A.; Morrow, J.H.; Murphy, R.C. Evidence for a simple relationship between natural fluorescence, photosynthesis and chlorophyll in the sea. Deep-Sea Res. 1990, 37, 951–973. [Google Scholar] [CrossRef]
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley-Verlag: Weinheim, Germany, 1999; pp. 159–228. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 6 August 2021).
- Percival, D.B.; Walden, A.T. Wavelet Methods for Time Series Analysis; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Ruffinatti, F.A.; Lovisolo, D.; Distasi, C.; Ariano, P.; Erriquez, J.; Ferraro, M. Calcium signals: Analysis in time and frequency domains. J. Neurosci. Methods 2011, 199, 310–320. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Cushing, D.H. The seasonal variation in oceanic production as a problem in population dynamics. ICES J. Mar. Sci. 1959, 24, 455–464. [Google Scholar] [CrossRef]
- Lewis, W.M., Jr. Tropical lakes: How latitude makes a difference. In Perspectives in Tropical Limnology; Schiemer, F., Boland, K.T., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1996; pp. 43–64. [Google Scholar]
- Cuevas-Lara, J.D.; Alcocer, J.; Oseguera, L.A.; Quiroz-Martínez, B. Dinámica Largo Plazo (1999–2014) de la Productividad Primaria Fitoplanctónica en el Lago Alchichica, Puebla. In Estado Actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2016; Paz Pellat, F., Wong González, J., Torres Alamilla, R., Eds.; Serie Síntesis Nacionales; Programa Mexicano del Carbono en colaboración con la Universidad Autónoma del Estado de Hidalgo: Texcoco, México, 2016; p. 732. [Google Scholar]
- González Contreras, C.G.; Alcocer, J.; Oseguera, L.A. Clorofila a fitoplanctónica en el lago tropical profundo Alchichica: Un registro de largo plazo (1999–2010). Hidrobiológica 2015, 25, 347–356. [Google Scholar]
- Alcocer, J.; Lugo, A.; Fernández, R.; Vilaclara, G.; Oliva, M.G.; Oseguera, L.A.; Silva-Aguilera, R.A.; Escolero, Ó. 20 years of global change on the limnology and plankton of a tropical, high-altitude lake. Diversity 2022, 14, 190. [Google Scholar] [CrossRef]
- Fernández, R.; Alcocer, J.; Lugo, A.; Oseguera, L.A.; Guadarrama-Hernández, S. Seasonal and interannual dynamics of pelagic rotifers in a tropical, saline, deep lake. Diversity 2022, 14, 113. [Google Scholar] [CrossRef]
- Ardiles, V.; Alcocer, J.; Vilaclara, G.; Oseguera, L.A.; Velasco, L. Diatom fluxes in a tropical, oligotrophic lake dominated by large-sized phytoplankton. Hydrobiologia 2012, 679, 77–90. [Google Scholar] [CrossRef]
- Alcocer, J.; Ruíz-Fernández, R.; Escobar, E.; Pérez-Bernal, L.H.; Oseguera, L.A.; Ardiles-Gloria, V. Deposition, burial and sequestration of carbon in an oligotrophic, tropical lake. J. Limnol. 2014, 72, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Lewis, W.M., Jr. Comparisons of phytoplankton biomass in temperate and tropical lakes. Limnol. Oceanogr. 1990, 35, 1838–1845. [Google Scholar] [CrossRef] [Green Version]
- Kalff, J.; Watson, S. Phytoplankton and its dynamics in two tropical lakes: A tropical and temperate zone comparison. Hydrobiologia 1986, 138, 161–176. [Google Scholar] [CrossRef]
- Descy, J.P.; Sarmento, H. Microorganisms of the East African Great Lakes and their response to environmental changes. Freshw. Rev. 2008, 1, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, B.M.; Pilla, R.M.; Woolway, R.I.; Anneville, O.; Ban, S.; Colom-Montero, W.; Devlin, S.P.; Dokulil, M.T.; Gaiser, E.E.; Hambright, K.D.; et al. Climate change drives widespread shifts in lake thermal habitat. Nat. Clim. Change 2021, 11, 521–529. [Google Scholar] [CrossRef]
- Oseguera, L.A.; Alcocer, J.; Vilaclara, G. Relative importance of dust inputs and aquatic biological production as sources of lake sediments in an oligotrophic lake in a semi-arid area. Earth Surf. Process. Landf. 2011, 3, 419–426. [Google Scholar] [CrossRef]
- Lewis, W.M., Jr. Causes for the high frequency of nitrogen limitation in tropical lakes. Verh. des Int. Ver. Limnol. 2002, 28, 210–213. [Google Scholar] [CrossRef]
- Ramos-Higuera, E.; Alcocer, J.; Ortega-Mayagoitia, E.; Camacho, A. Nitrógeno: Elemento limitante para el crecimiento fitoplanctónico en un lago oligotrófico tropical. Hidrobiológica 2008, 18, 105–113. [Google Scholar]
- Falcón, L.I.; Escobar-Briones, E.; Romero, D. Nitrogen fixation patterns displayed by cyanobacterial consortia in Alchichica crater-lake, Mexico. Hydrobiologia 2002, 467, 71–78. [Google Scholar] [CrossRef]
- Butcher, J.B.; Nover, D.; Johnson, T.E.; Clark, C.M. Sensitivity of lake thermal and mixing dynamics to climate change. Clim. Chang. 2015, 129, 295–305. [Google Scholar] [CrossRef] [Green Version]





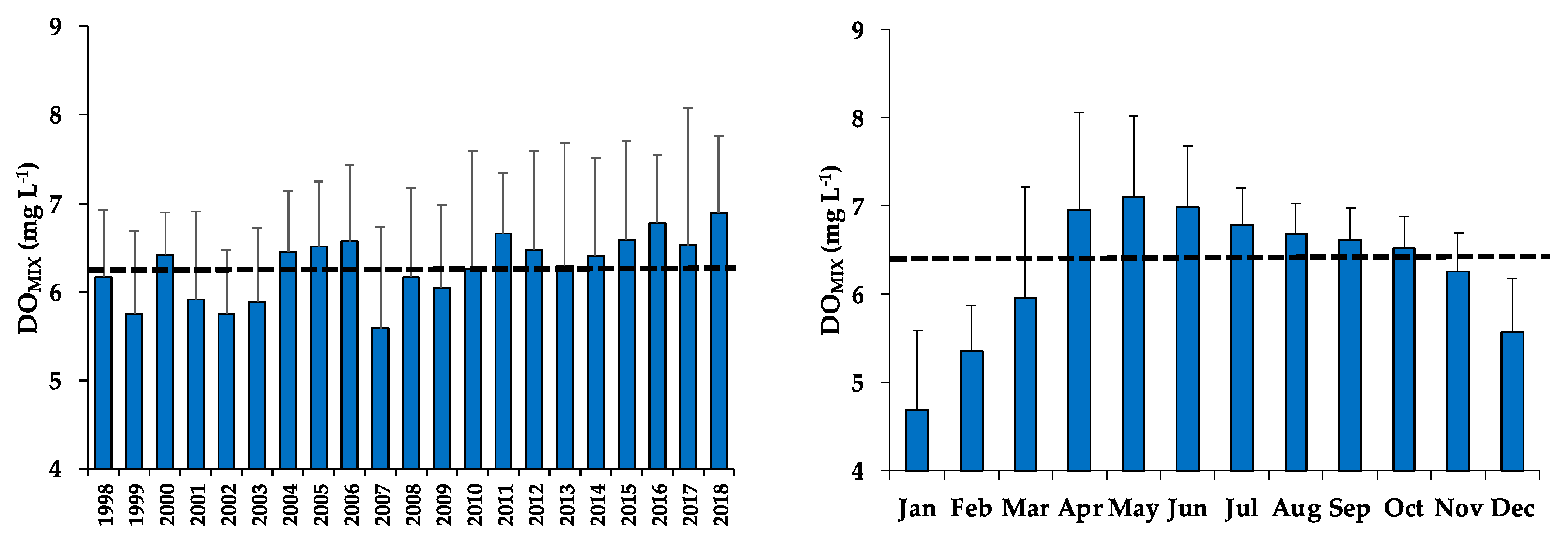



| Variable | Mixing (Dry, Cold) | Stratification (Wet, Warm) |
|---|---|---|
| Chl-a | High The phytoplankton distributes throughout the water column | High The phytoplankton concentrates at the metalimnion (DCM) |
| ZEU | Lower Turbid water phase Biogenic turbidity | Higher Clear water phase DCM at the bottom of ZEU |
| ZMIX | High | Low |
| TMIX | Colder | Warmer |
| ODMIX | Lower | Higher |
| DINMIX | Higher | Lower |
| SRPMIX | Higher | Lower |
| SRSiMIX | Higher and lower | Higher and lower |
| Variable | S | Z | P | Trend |
|---|---|---|---|---|
| Chl-a | −4045 | −3.4669 | 0.00052653 | Decreasing |
| ZEU | −1397 | −1.0255 | 0.30513 | Non-Significant |
| ZMIX | −1977 | −1.4539 | 0.14599 | Non-Significant |
| TMIX | 4341 | 3.1881 | 0.001432 | Increasing |
| ODMIX | 6063 | 4.4531 | 8.4643 × 10−6 | Increasing |
| DINMIX | −5525 | −4.4426 | 8.8884 × 10−6 | Decreasing |
| SRPMIX | −5570 | −4.4796 | 7.4786 × 10−6 | Decreasing |
| SRSiMIX | 2751 | 2.212 | 0.026964 | Increasing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcocer, J.; Quiroz-Martínez, B.; Merino-Ibarra, M.; Oseguera, L.A.; Macek, M. Using Wavelet Analysis to Examine Long-Term Variability of Phytoplankton Biomass in the Tropical, Saline Lake Alchichica, Mexico. Water 2022, 14, 1346. https://doi.org/10.3390/w14091346
Alcocer J, Quiroz-Martínez B, Merino-Ibarra M, Oseguera LA, Macek M. Using Wavelet Analysis to Examine Long-Term Variability of Phytoplankton Biomass in the Tropical, Saline Lake Alchichica, Mexico. Water. 2022; 14(9):1346. https://doi.org/10.3390/w14091346
Chicago/Turabian StyleAlcocer, Javier, Benjamín Quiroz-Martínez, Martín Merino-Ibarra, Luis A. Oseguera, and Miroslav Macek. 2022. "Using Wavelet Analysis to Examine Long-Term Variability of Phytoplankton Biomass in the Tropical, Saline Lake Alchichica, Mexico" Water 14, no. 9: 1346. https://doi.org/10.3390/w14091346






