Differential Molecular Responses of Zebrafish Larvae to Fluoxetine and Norfluoxetine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Chemicals
2.2. Zebrafish Rearing and Reproduction
2.3. Zebrafish Embryo Toxicity Test
2.4. RNA Extraction and cDNA Synthesis
2.5. Gene Expression
2.6. Statistical Analysis
3. Results
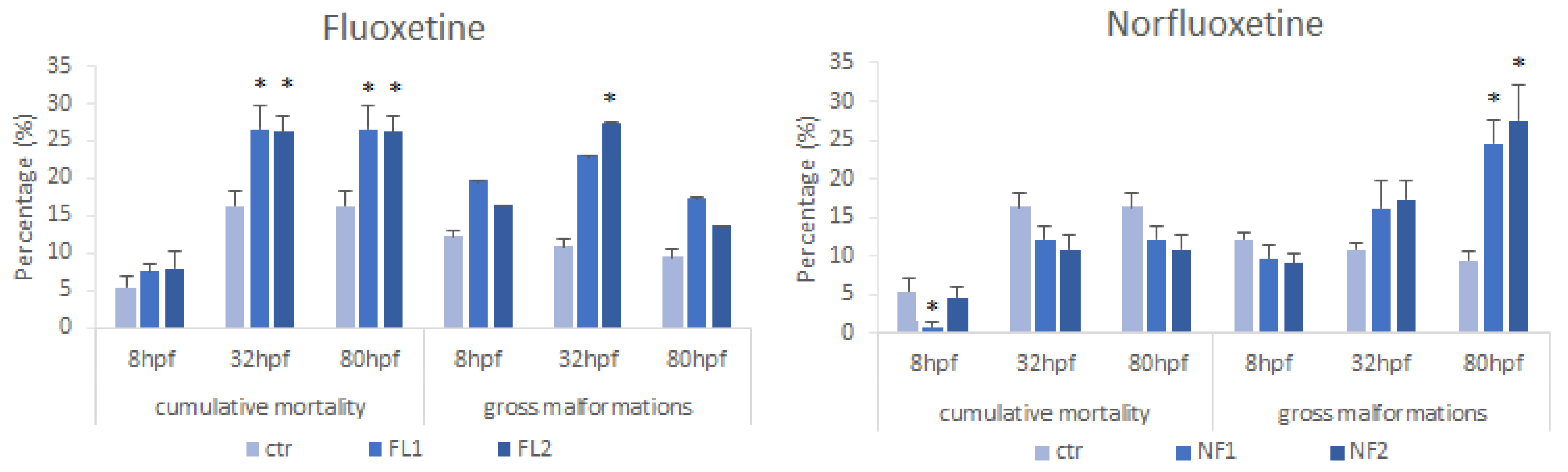
3.1. Zebrafish Embryo Toxicity Test
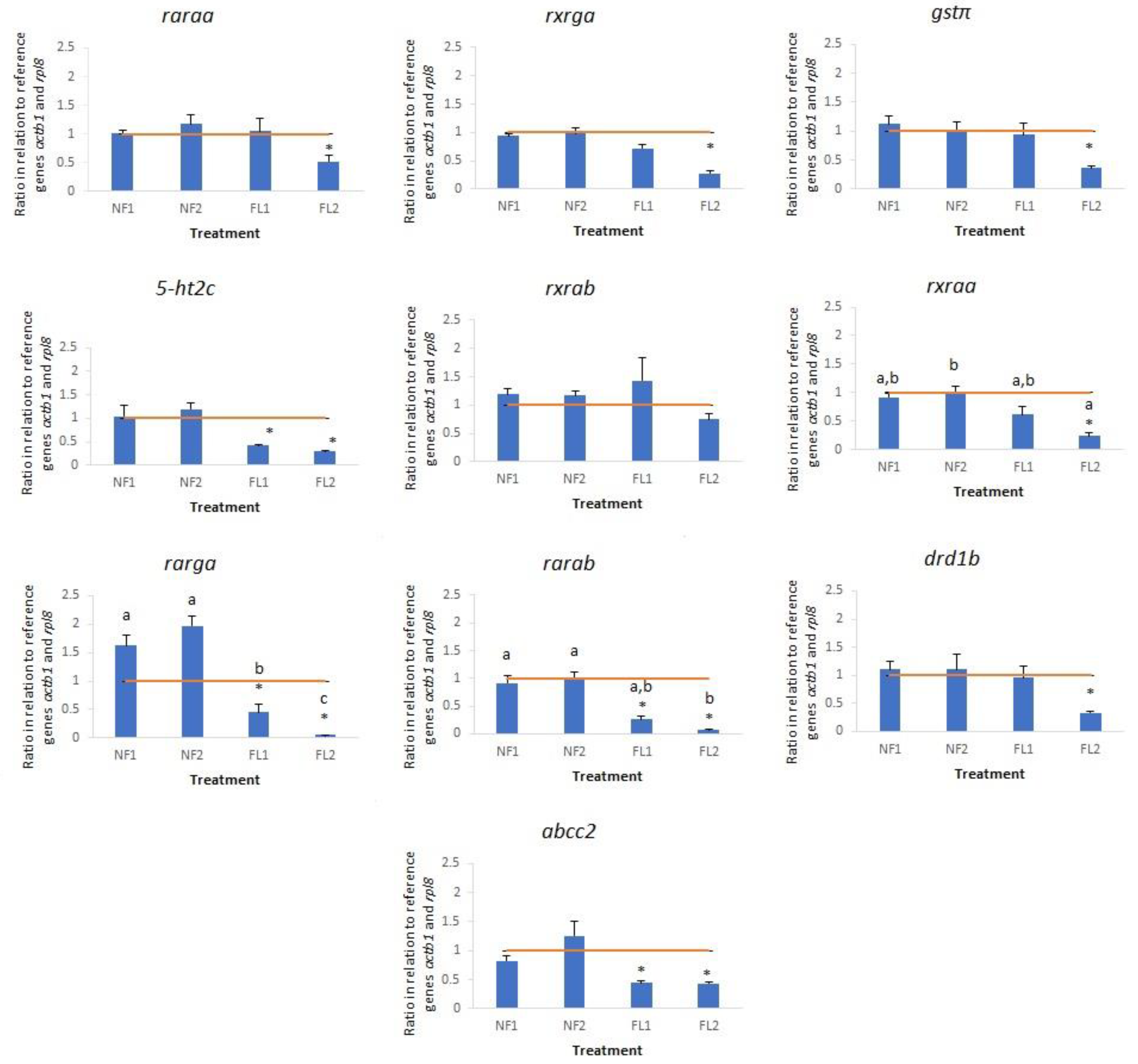
3.2. Gene Expression
4. Discussion
4.1. Zebrafish Embryo Toxicity Test
4.2. Gene Expression
4.3. Molecular Biomarkers of Exposure to Psychopharmaceuticals in Fish
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Identification of Amplicons Procedure
Appendix B. qPCR Reaction Volumes
References
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Salahinejad, A.; Attaran, A.; Meuthen, D.; Chivers, D.P.; Niyogi, S. Proximate causes and ultimate effects of common antidepressants, fluoxetine and venlafaxine, on fish behavior. Sci. Total Environ. 2021, 807, 150846. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, J.A.; Stroud, P.; Zamora, J.M.; Moon, T.W.; Trudeau, V.L. Pharmaceuticals as neuroendocrine disruptors: Lessons learned from fish on Prozac. J. Toxicol. Environ. Health Part B 2011, 14, 387–412. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Holick, K.A.; Gundersen, B.; Hen, R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 2004, 29, 1321–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunotte, L.; Zheng, S.; Mecate-Zambrano, A.; Tang, J.; Ludwig, S.; Rescher, U.; Schloer, S. Combination therapy with fluoxetine and the nucleoside analog GS-441524 exerts synergistic antiviral effects against different SARS-CoV-2 variants in vitro. Pharmaceutics 2021, 13, 1400. [Google Scholar] [CrossRef] [PubMed]
- Zimniak, M.; Kirschner, L.; Hilpert, H.; Geiger, N.; Danov, O.; Oberwinkler, H.; Steinke, M.; Sewald, K.; Seibel, J.; Bodem, J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci. Rep. 2021, 11, 5890. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.-S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: Results from an observational study. Mol. Psychiatry 2021, 26, 5199–5212. [Google Scholar] [CrossRef]
- Baumann, P.; Zullino, D.F.; Eap, C.B. Enantiomers’ potential in psychopharmacology—A critical analysis with special emphasis on the antidepressant escitalopram. Eur. Neuropsychopharmacol. 2002, 12, 433–444. [Google Scholar] [CrossRef]
- Winder, V.L.; Pennington, P.L.; Hurd, M.W.; Wirth, E.F. Fluoxetine effects on sheepshead minnow (Cyprinodon variegatus) locomotor activity. J. Environ. Sci. Health Part B 2012, 47, 51–58. [Google Scholar] [CrossRef]
- Kreke, N.; Dietrich, D.R. Physiological endpoints for potential SSRI interactions in fish. Crit. Rev. Toxicol. 2008, 38, 215–247. [Google Scholar] [CrossRef] [Green Version]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A.; Valenti, T.W.; Lawless, K.; Sackerman, J.; Onaivi, E.S.; Brooks, B.W.; Gould, G.G. Similar anxiolytic effects of agonists targeting serotonin 5-HT1A or cannabinoid CB receptors on zebrafish behavior in novel environments. Aquat. Toxicol. 2013, 151, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marty, M.S.; Blankinship, A.; Chambers, J.; Constantine, L.; Kloas, W.; Kumar, A.; Lagadic, L.; Meador, J.; Pickford, D.; Schwarz, T.; et al. Population-Relevant endpoints in the evaluation of endocrine-active substances (EAS) for ecotoxicological hazard and risk assessment. Integr. Environ. Assess. Manag. 2017, 13, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastenhuber, E.; Kratochwil, C.F.; Ryu, S.; Schweitzer, J.; Driever, W. Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J. Comp. Neurol. 2010, 518, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Huang, I.J.; Sirotkin, H.I.; McElroy, A.E. Varying the exposure period and duration of neuroactive pharmaceuticals and their metabolites modulates effects on the visual motor response in zebrafish (Danio rerio) larvae. Neurotoxicol. Teratol. 2019, 72, 39–48. [Google Scholar] [CrossRef]
- Atzei, A.; Jense, I.; Zwart, E.; Legradi, J.; Venhuis, B.J.; van der Ven, L.T.M.; Heusinkveld, H.J.; Hessel, E.V.S. Developmental neurotoxicity of environmentally relevant pharmaceuticals and mixtures thereof in a zebrafish embryo behavioural test. Int. J. Environ. Res. Public Health 2021, 18, 6717. [Google Scholar] [CrossRef]
- Vera-Chang, M.N.; St-Jacques, A.D.; Gagné, R.; Martyniuk, C.J.; Yauk, C.L.; Moon, T.W.; Trudeau, V.L. Transgenerational hypocortisolism and behavioral disruption are induced by the antidepressant fluoxetine in male zebrafish Danio rerio. Proc. Natl. Acad. Sci. USA 2018, 115, E12435–E12442. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.; Vera-Chang, M.N.; Haddad, M.; Zon, J.; Navarro-Martin, L.; Trudeau, V.L.; Mennigen, J.A. Developmental fluoxetine exposure in zebrafish reduces offspring basal cortisol concentration via life stage-dependent maternal transmission. PLoS ONE 2019, 14, e0212577. [Google Scholar] [CrossRef]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Danio rerio embryos on Prozac effects on the detoxification mechanism and embryo development. Aquat. Toxicol. 2016, 178, 182–189. [Google Scholar] [CrossRef]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Fluoxetine modulates the transcription of genes involved in serotonin, dopamine and adrenergic signalling in zebrafish embryos. Chemosphere 2018, 191, 954–961. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Multi-Residue determination of the sorption of illicit drugs and pharmaceuticals to wastewater suspended particulate matter using pressurised liquid extraction, solid phase extraction and liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 7901–7913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, M.J.; Paíga, P.; Silva, A.; Llaguno, C.P.; Carvalho, M.; Vázquez, F.M.; Delerue-Matos, C. Antibiotics and antidepressants occurrence in surface waters and sediments collected in the north of Portugal. Chemosphere 2020, 239, 124729. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.P.; Molnar, N. Norfluoxetine induces spawning and parturition in estuarine and freshwater bivalves. Bull. Environ. Contam. Toxicol. 2008, 81, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Ring, B.J.; Eckstein, J.A.; Gillespie, J.S.; Binkley, S.N.; VandenBranden, M.; Wrighton, S.A. Identification of the human cytochromes p450 responsible for in vitro formation of R- and S-norfluoxetine. J. Pharmacol. Exp. Ther. 2001, 297, 1044–1050. [Google Scholar] [CrossRef] [Green Version]
- Stanley, J.K.; Ramirez, A.J.; Chambliss, C.K.; Brooks, B.W. Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere 2007, 69, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Aluisio, L.; Lord, B.; Boggs, J.; Hoey, K.; Mazur, C.; Lovenberg, T. Pharmacokinetics and pharmacodynamics of norfluoxetine in rats: Increasing extracellular serotonin level in the frontal cortex. Pharmacol. Biochem. Behav. 2009, 92, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Cunha, V.; Oliva-Teles, L.; Ferreira, M.; Guimarães, L. Norfluoxetine and venlafaxine in zebrafish larvae: Single and combined toxicity of two pharmaceutical products relevant for risk assessment. J. Hazard. Mater. 2020, 400, 123171. [Google Scholar] [CrossRef]
- Zindler, F.; Stoll, S.; Baumann, L.; Knoll, S.; Huhn, C.; Braunbeck, T. Do environmentally relevant concentrations of fluoxetine and citalopram impair stress-related behavior in zebrafish (Danio rerio) embryos? Chemosphere 2020, 261, 127753. [Google Scholar] [CrossRef]
- Chai, T.; Cui, F.; Di, S.; Wu, S.; Zhang, Y.; Wang, X. New insights into cardiotoxicity induced by chiral fluoxetine at environmental-level: Enantioselective arrhythmia in developmental zebrafish (Danio rerio). Environ. Pollut. 2020, 270, 116182. [Google Scholar] [CrossRef]
- Zindler, F.; Tisler, S.; Loerracher, A.-K.; Zwiener, C.; Braunbeck, T. Norfluoxetine is the only metabolite of fluoxetine in zebrafish (Danio rerio) embryos that accumulates at environmentally relevant exposure scenarios. Environ. Sci. Technol. 2020, 54, 4200–4209. [Google Scholar] [CrossRef]
- Chen, F.; Gong, Z.; Kelly, B.C. Bioaccumulation behavior of pharmaceuticals and personal care products in adult zebrafish (Danio rerio): Influence of physical-chemical properties and biotransformation. Environ. Sci. Technol. 2017, 51, 11085–11095. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Reis-Henriques, M.A.; Castro, L.F.C.; Ferreira, M. Gene expression analysis of ABC efflux transporters, CYP1A and GSTα in Nile tilapia after exposure to benzo(a)pyrene. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Urbatzka, R.; Galante-Oliveira, S.; Rocha, E.; Castro, L.F.C.; Cunha, I. Normalization strategies for gene expression studies by real-time PCR in a marine fish species, Scophthalmus maximus. Mar. Genom. 2013, 10, 17–25. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Rodrigues, P.; Oliva-Teles, L.; Carvalho, A.P.; Guimarães, L. NorFluox. Mendeley Data 2021, 1. [Google Scholar] [CrossRef]
- Santos, L.H.; Ramalhosa, M.J.; Ferreira, M.; Delerue-Matos, C. Development of a modified acetonitrile-based extraction procedure followed by ultra-high performance liquid chromatography–tandem mass spectrometry for the analysis of psychiatric drugs in sediments. J. Chromatogr. A 2016, 1437, 37–48. [Google Scholar] [CrossRef]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef]
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical metabolites in the environment: Analytical challenges and ecological risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef]
- Kosma, C.I.; Kapsi, M.G.; Konstas, P.-S.G.; Trantopoulos, E.P.; Boti, V.I.; Konstantinou, I.K.; Albanis, T.A. Assessment of multiclass pharmaceutical active compounds (PhACs) in hospital WWTP influent and effluent samples by UHPLC-Orbitrap MS: Temporal variation, removals and environmental risk assessment. Environ. Res. 2020, 191, 110152. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Armbrust, K.L. Laboratory persistence and fate of fluoxetine in aquatic environments. Environ. Toxicol. Chem. 2006, 25, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Merlob, P.; Schaefer, C. 4.9-Psychotropic drugs. In Drugs During Pregnancy and Lactation, 3rd ed.; Schaefer, C., Peters, P., Miller, R.K., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 743–774. [Google Scholar] [CrossRef]
- Pan, C.; Yang, M.; Xu, H.; Xu, B.; Jiang, L.; Wu, M. Tissue bioconcentration and effects of fluoxetine in zebrafish (Danio rerio) and red Crucian cap (Carassius auratus) after short-term and long-term exposure. Chemosphere 2018, 205, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lu, G.; Sun, H.; Bao, X.; Jiang, R.; Liu, J.; Ji, Y. Comparison of the accumulation and metabolite of fluoxetine in zebrafish larva under different environmental conditions with or without carbon nanotubes. Ecotoxicol. Environ. Saf. 2019, 172, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Maia, A.S.; Moreira, I.S.; Afonso, C.M.; Castro, P.M.; Tiritan, M.E. Enantioselective quantification of fluoxetine and norfluoxetine by HPLC in wastewater effluents. Chemosphere 2014, 95, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Airhart, M.J.; Lee, D.H.; Wilson, T.D.; Miller, B.E.; Miller, M.N.; Skalko, R.G.; Monaco, P.J. Adverse effects of serotonin depletion in developing zebrafish. Neurotoxicol. Teratol. 2012, 34, 152–160. [Google Scholar] [CrossRef]
- Xu, J.; Xie, F.-K. α- and β-Adrenoceptors of zebrafish in melanosome movement: A comparative study between embryo and adult melanophores. Biochem. Biophys. Res. Commun. 2011, 405, 250–255. [Google Scholar] [CrossRef]
- Svetic, V.; Hollway, G.E.; Elworthy, S.; Chipperfield, T.R.; Davison, C.; Adams, R.J.; Eisen, J.S.; Ingham, P.W.; Currie, P.D.; Kelsh, R.N. Sdf1a patterns zebrafish melanophores and links the somite and melanophore pattern defects inchokermutants. Development 2007, 134, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Hiemke, C.; Härtter, S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol. Ther. 1999, 85, 11–28. [Google Scholar] [CrossRef]
- Chu, S.; Metcalfe, C.D. Analysis of paroxetine, fluoxetine and norfluoxetine in fish tissues using pressurized liquid extraction, mixed mode solid phase extraction cleanup and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2007, 1163, 112–118. [Google Scholar] [CrossRef]
- Andrés-Costa, M.J.; Proctor, K.; Sabatini, M.; Gee, A.P.; Lewis, S.; Pico, Y.; Kasprzyk-Hordern, B. Enantioselective transformation of fluoxetine in water and its ecotoxicological relevance. Sci. Rep. 2017, 7, 15777. [Google Scholar] [CrossRef]
- Nalecz-Jawecki, G. Evaluation of the in vitro biotransformation of fluoxetine with HPLC, mass spectrometry and ecotoxicological tests. Chemosphere 2007, 70, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.P.; Santos, L.H.M.L.M.; Oliva-Teles, M.T.; Delerue-Matos, C.; Guimarães, L. Joint effects of salinity and the antidepressant sertraline on the estuarine decapod Carcinus maenas. Aquat. Toxicol. 2014, 156, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.P.; Santos, L.H.M.L.M.; Ramalhosa, M.J.; Delerue-Matos, C.; Guimarães, L. Sertraline accumulation and effects in the estuarine decapod Carcinus maenas: Importance of the history of exposure to chemical stress. J. Hazard. Mater. 2015, 283, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzella, C.; Singhal, M.; Alrefai, W.A.; Saksena, S.; Dudeja, P.K.; Gill, R.K. Serotonin is an endogenous regulator of intestinal CYP1A1 via AhR. Sci. Rep. 2018, 8, 6103. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Li, C.Y.-T.; Kong, A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharmacal. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Parolini, M.; Ghilardi, A.; DE Felice, B.; Del Giacco, L. Environmental concentration of fluoxetine disturbs larvae behavior and increases the defense response at molecular level in zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 34943–34952. [Google Scholar] [CrossRef] [PubMed]
- Lewin, D.A.; Weiner, M.P. Molecular biomarkers in drug development. Drug Discov. Today 2004, 9, 976–983. [Google Scholar] [CrossRef]
- Fonseka, T.M.; Wen, X.-Y.; Foster, J.A.; Kennedy, S.H. Zebrafish models of major depressive disorders. J. Neurosci. Res. 2015, 94, 3–14. [Google Scholar] [CrossRef]
- van der Ven, K.; Keil, D.; Moens, L.N.; Van Hummelen, P.; van Remortel, P.; Maras, M.; De Coen, W. Effects of the antidepressant mianserin in zebrafish: Molecular markers of endocrine disruption. Chemosphere 2006, 65, 1836–1845. [Google Scholar] [CrossRef]
- Park, J.-W.; Heah, T.P.; Gouffon, J.S.; Henry, T.B.; Sayler, G.S. Global gene expression in larval zebrafish (Danio rerio) exposed to selective serotonin reuptake inhibitors (fluoxetine and sertraline) reveals unique expression profiles and potential biomarkers of exposure. Environ. Pollut. 2012, 167, 163–170. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, W.; Chen, J.; Zhan, J.; Pan, C.; Lei, X.; Wu, M. Growth inhibition and coordinated physiological regulation of zebrafish (Danio rerio) embryos upon sublethal exposure to antidepressant amitriptyline. Aquat. Toxicol. 2014, 151, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, S.; Hu, L.; Qu, H.; Pan, C.; Lei, P.; Shen, Y.; Yang, M. Global transcriptomic analysis of zebrafish in response to embryonic exposure to three antidepressants, amitriptyline, fluoxetine and mianserin. Aquat. Toxicol. 2017, 192, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.J.; Dheilly, N.M.; Sirotkin, H.I.; McElroy, A.E. Comparative transcriptomics implicate mitochondrial and neurodevelopmental impairments in larval zebrafish (Danio rerio) exposed to two selective serotonin reuptake inhibitors (SSRIs). Ecotoxicol. Environ. Saf. 2020, 203, 110934. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Semedo, M.; Machado, S.P.; Cunha, V.; Ferreira, M.; Urbatzka, R. Transcriptional analyses reveal different mechanism of toxicity for a chronic exposure to fluoxetine and venlafaxine on the brain of the marine fish Dicentrarchrus labrax. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 250, 109170. [Google Scholar] [CrossRef] [PubMed]




| Endpoint | 8 hpf | 32 hpf | 80 hpf |
|---|---|---|---|
| Cumulative mortality | X | X | X |
| Delay/pause in development | X | X | X |
| Abnormal growth | X | X | X |
| 75% epiboly | X | ||
| Anomalies in the eyes | X | X | |
| Anomalies in the head | X | X | |
| Anomalies in the spine/tail | X | X | |
| Anomalies in the yolk sac | X | X | |
| Anomalies in pigmentation | X | X | |
| Pericardium oedema | X | X | |
| Unhatched embryos | X |
| PPharm | Fish Species | Stage/Duration of Exposure | Exposure Concentration (µg/L−1) | Relevant Altered Genes or Biochemical Parameters | Reference |
|---|---|---|---|---|---|
| Mianserin | Danio rerio | Adults/ 14 days | 25 and 250 |
| [60] |
| Fluoxetine Sertraline | Danio rerio | Larvae/ 96 h | 25 and 250 |
| [61] |
| Amitriptyline | Danio rerio | Larvae/ 120 h | 0.001, 0.01, 0.1, 1, 10, 100 and 1000 |
| [62] |
| Amitriptyline Fluoxetine Mianserin | Danio rerio | Larvae/ 120 h | 0.1, 1 and 10 |
| [63] |
| Venlafaxine | Danio rerio | Larvae/ 80 h | 0.016, 0.08, 0.4, 2.0 and 10 |
| [27] |
| Fluoxetine | Danio rerio | Larvae/96 h | 0.05 and 0.5 |
| [58] |
| Fluoxetine | Danio rerio | Larvae/144 h | 0.54 and 54 |
| [16] |
| Fluoxetine Paroxetine | Danio rerio | Larvae/144 h | 100 |
| [64] |
| Fluoxetine Norfluoxetine | Danio rerio | Larvae/ 9 days | 0.1 |
| [29] |
| Fluoxetine Sertraline | Dicentrarchrus labrax | juveniles/21 days | 0.5 and 50 0.01 and 1 |
| [65] |
| Fluoxetine | Danio rerio | Larvae/ 80 h | 0.5 and 17 |
| Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, P.; Cunha, V.; Ferreira, M.; Reis-Henriques, M.A.; Oliva-Teles, L.; Guimarães, L.; Carvalho, A.P. Differential Molecular Responses of Zebrafish Larvae to Fluoxetine and Norfluoxetine. Water 2022, 14, 417. https://doi.org/10.3390/w14030417
Rodrigues P, Cunha V, Ferreira M, Reis-Henriques MA, Oliva-Teles L, Guimarães L, Carvalho AP. Differential Molecular Responses of Zebrafish Larvae to Fluoxetine and Norfluoxetine. Water. 2022; 14(3):417. https://doi.org/10.3390/w14030417
Chicago/Turabian StyleRodrigues, Pedro, Virgínia Cunha, Marta Ferreira, Maria Armanda Reis-Henriques, Luís Oliva-Teles, Laura Guimarães, and António Paulo Carvalho. 2022. "Differential Molecular Responses of Zebrafish Larvae to Fluoxetine and Norfluoxetine" Water 14, no. 3: 417. https://doi.org/10.3390/w14030417






