A New Optical Method for Quantitative Detection of Microplastics in Water Based on Real-Time Fluorescence Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Setup
2.3. Instrument Calibration
2.4. Particle Staining
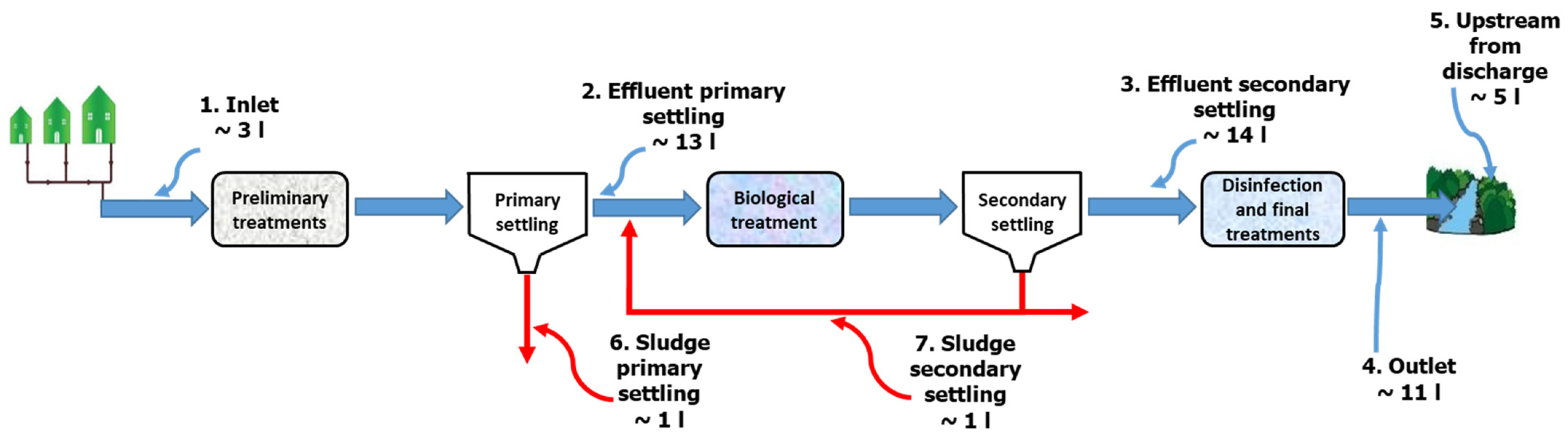
2.5. Sampling and Pre-Treatment of Real Samples from a WWTP
2.6. FTIR Analysis
3. Results and Discussion
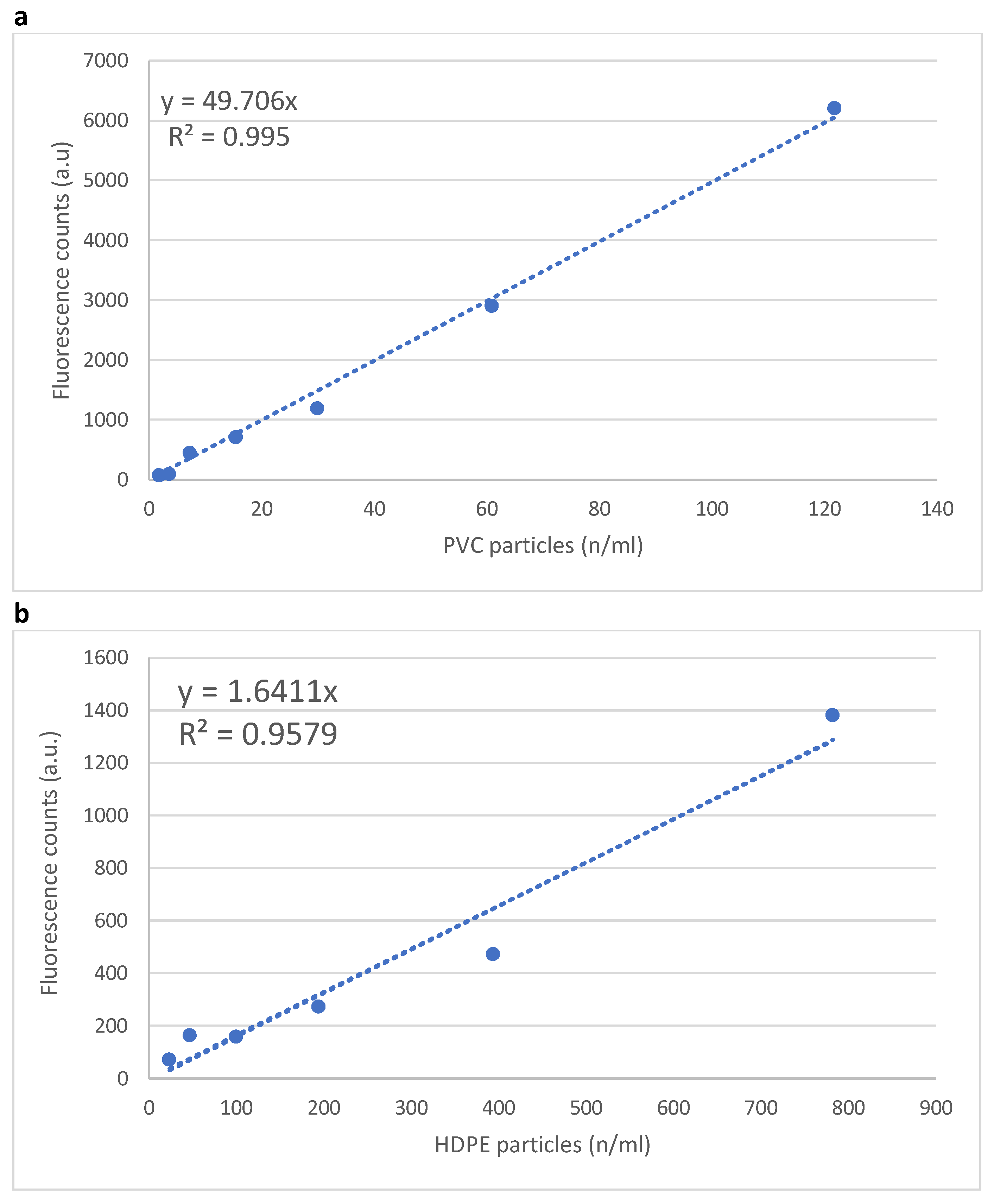
3.1. Instrument Calibration
3.2. Measurements on Wastewater Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- UNEP (United Nations Environment Programme). UNEA Resolution 5/14 Entitled “End Plastic Pollution: Towards an International Legally Binding Instrument”; United Nations: New York, NY, USA, 2022; pp. 1–5. [Google Scholar]
- UNEP (United Nations Environment Programme). What You Need to Know about the Plastic Pollution Resolution. Available online: https://www.unep.org/news-and-stories/story/what-you-need-know-about-plastic-pollution-resolution (accessed on 8 August 2022).
- UNEP (United Nations Environment Programme). From Pollution to Solution: A Global Assessment of Marine Litter and Plastic Pollution; United Nations: New York, NY, USA, 2021; Volume 237, ISBN 9789280738810. [Google Scholar]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008; NOAA Technical Memorandum NOS-OR&R-30; NOAA: Washington, DC, USA, 2009; p. 530.
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Jones, T.; Santosh, M.; Liu, P.; Zhang, M.; Xu, L.; Li, W.; Lu, J.; Yang, C.-X.; et al. Airborne Microplastics: A Review of Current Perspectives and Environmental Implications. J. Clean. Prod. 2022, 347, 959–6526. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Fowler, S.W.; Behbehani, M. Microplastics in the Atmosphere: A Review. J. Environ. Expo. Assess. 2022, 1, 6. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Sajid, S.; Dupont, S.; Behbehani, M. A Preliminary Assessment of Size-Fractionated Microplastics in Indoor Aerosol—Kuwait’s Baseline. Toxics 2022, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in Freshwater Systems: A Review on Occurrence, Environmental Effects, and Methods for Microplastics Detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of Microplastics in Freshwater Systems: A Review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in Freshwater Ecosystems: What We Know and What We Need to Know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Hashem, M.; Mostafa, Y.S.; Alhaithloul, H.A.S.; Alghanem, S.M. Linking Effects of Microplastics to Ecological Impacts in Marine Environments. Chemosphere 2021, 264, 128541. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Effect of Microplastics in Water and Aquatic Systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Cutroneo, L.; Reboa, A.; Besio, G.; Borgogno, F.; Canesi, L.; Canuto, S.; Dara, M.; Enrile, F.; Forioso, I.; Greco, G.; et al. Microplastics in Seawater: Sampling Strategies, Laboratory Methodologies, and Identification Techniques Applied to Port Environment. Environ. Sci. Pollut. Res. 2020, 27, 8938–8952. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Nie, P.; Feng, X.; Liu, J. An Effective Method for the Rapid Detection of Microplastics in Soil. Chemosphere 2021, 276, 128696. [Google Scholar] [CrossRef] [PubMed]
- Grause, G.; Kuniyasu, Y.; Chien, M.F.; Inoue, C. Separation of Microplastic from Soil by Centrifugation and Its Application to Agricultural Soil. Chemosphere 2022, 288, 132654. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and Environmental Pollutants: Key Interaction and Toxicology in Aquatic and Soil Environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [Green Version]
- Bakaraki Turan, N.; Sari Erkan, H.; Onkal Engin, G. Microplastics in Wastewater Treatment Plants: Occurrence, Fate and Identification. Process Saf. Environ. Prot. 2021, 146, 77–84. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A Review of the Removal of Microplastics in Global Wastewater Treatment Plants: Characteristics and Mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef]
- Naji, A.; Azadkhah, S.; Farahani, H.; Uddin, S.; Khan, F.R. Microplastics in Wastewater Outlets of Bandar Abbas City (Iran): A Potential Point Source of Microplastics into the Persian Gulf. Chemosphere 2021, 262, 128039. [Google Scholar] [CrossRef] [PubMed]
- Oanh, D.T.; Thuy, D.T.; Huong, N.T.N.; Quynh, H.T.; Hieu, P.D.; Vu, D.M.; Nguyet, V.T.; Quynh, L.T.P.; Van Cuong, B.; Thuong, B.H. Preliminary Investigation of Microplastics in Sediments from Industrial Manufacturing Waste Sources. VNU J. Sci. Nat. Sci. Technol. 2022, 38, 1–8. [Google Scholar] [CrossRef]
- Drummond, J.D.; Schneidewind, U.; Li, A.; Hoellein, T.J.; Krause, S.; Packman, A.I. Microplastic Accumulation in Riverbed Sediment via Hyporheic Exchange from Headwaters to Mainstems. Sci. Adv. 2022, 8, eabi9305. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in Freshwater Sediment: A Review on Methods, Occurrence, and Sources. Sci. Total Environ. 2021, 754, 141948. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in Sediments: A Review of Techniques, Occurrence and Effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Katare, Y. Microplastics in Aquatic Environments: Sources, Ecotoxicity, Detection & Remediation. Biointerface Res. Appl. Chem. 2021, 12, 3407–3428. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Elliott, M.; Almeida, C.M.R.; Ramos, S. Microplastics and Plankton: Knowledge from Laboratory and Field Studies to Distinguish Contamination from Pollution. J. Hazard. Mater. 2021, 417, 126057. [Google Scholar] [CrossRef]
- Burkhardt-Holm, P.; N’Guyen, A. Ingestion of Microplastics by Fish and Other Prey Organisms of Cetaceans, Exemplified for Two Large Baleen Whale Species. Mar. Pollut. Bull. 2019, 144, 224–234. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Shahadat Hossain, M.; Li, Q.; Kolandhasamy, P.; et al. Using Mussel as a Global Bioindicator of Coastal Microplastic Pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Thomas, M.; Jon, B.; Craig, S.; Edward, R.; Ruth, H.; John, B.; Dick, V.A.; Heather, L.A.; Matthew, S. The World Is Your Oyster: Low-Dose, Long-Term Microplastic Exposure of Juvenile Oysters. Heliyon 2020, 6, e03103. [Google Scholar] [CrossRef]
- Jinhui, S.; Sudong, X.; Yan, N.; Xia, P.; Jiahao, Q.; Yongjian, X. Effects of Microplastics and Attached Heavy Metals on Growth, Immunity, and Heavy Metal Accumulation in the Yellow Seahorse, Hippocampus Kuda Bleeker. Mar. Pollut. Bull. 2019, 149, 110510. [Google Scholar] [CrossRef] [PubMed]
- Meaza, I.; Toyoda, J.H.; Wise Sr, J.P. Microplastics in Sea Turtles, Marine Mammals and Humans: A One Environmental Health Perspective. Front. Environ. Sci. 2021, 8, 575614. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, A.; Saavedra, C.; Gago, J.; Covelo, P.; Santos, M.B.; Pierce, G.J. Microplastics in the Stomach Contents of Common Dolphin (Delphinus delphis) Stranded on the Galician Coasts (NW Spain, 2005–2010). Mar. Pollut. Bull. 2018, 137, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Loseto, L.; Noel, M.; Etemadifar, A.; Brewster, J.D.; MacPhee, S.; Bendell, L.; Ross, P.S. Microplastics in Beluga Whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar. Pollut. Bull. 2020, 150, 110723. [Google Scholar] [CrossRef] [PubMed]
- Provencher, J.F.; Vermaire, J.C.; Avery-Gomm, S.; Braune, B.M.; Mallory, M.L. Garbage in Guano? Microplastic Debris Found in Faecal Precursors of Seabirds Known to Ingest Plastics. Sci. Total Environ. 2018, 644, 1477–1484. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic Contamination of Tap Water, Beer, and Sea Salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, H.J.; Kim, S.K.; Kim, H.J. Global Pattern of Microplastics (MPs) in Commercial Food-Grade Salts: Sea Salt as an Indicator of Seawater MP Pollution. Environ. Sci. Technol. 2018, 52, 12819–12828. [Google Scholar] [CrossRef]
- Afrin, S.; Rahman, M.M.; Hossain, M.N.; Uddin, M.K.; Malafaia, G. Are There Plastic Particles in My Sugar? A Pioneering Study on the Identification/Characterization of Microplastics in Commercial Sugars and Risk Assessment. Sci. Total Environ. 2022, 837, 155849. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Naghshbandi, M.; Ghaderzadeh, K.; Mirzabeigi, M.; Yazdanbakhsh, A.; Hossini, H. Micro-Plastic Occurrence in Bottled Vinegar: Qualification, Quantification and Human Risk Exposure. Process Saf. Environ. Prot. 2021, 152, 404–413. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First Study of Its Kind on the Microplastic Contamination of Soft Drinks, Cold Tea and Energy Drinks—Future Research and Environmental Considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic Contamination of Packaged Meat: Occurrence and Associated Risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First Evidence of Microplastics in Human Placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiriccò, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Han, Q.; Wei, Z.; Wang, Y.; Xie, J.; Chen, M. Polystyrene Microplastics Affect Learning and Memory in Mice by Inducing Oxidative Stress and Decreasing the Level of Acetylcholine. Food Chem. Toxicol. 2022, 162, 278–6915. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Behbehani, M. An Assessment of Microplastic Inputs into the Aquatic Environment from Wastewater Streams. Mar. Pollut. Bull. 2020, 160, 111538. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; Swedish Environmental Protection Agency: Stockholm, Sweden, 2014; Volume C55. [Google Scholar]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to Microplastic Pollution—Removal of Microplastics from Wastewater Effluent with Advanced Wastewater Treatment Technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papa, M.; Foladori, P.; Guglielmi, L.; Bertanza, G. How Far Are We from Closing the Loop of Sewage Resource Recovery? A Real Picture of Municipal Wastewater Treatment Plants in Italy. J. Environ. Manag. 2017, 198, 9–15. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Saeed, T.; Naji, A.; Al-Jandal, N. Standardized Protocols for Microplastics Determinations in Environmental Samples from the Gulf and Marginal Seas. Mar. Pollut. Bull. 2020, 158, 111374. [Google Scholar] [CrossRef]
- IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Guidelines for the Monitoring and Assessment of Plastic Litter in the Ocean: GESAMP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. Rep. Stud. GESAMP 2019, 99, 138.
- Baruah, A.; Sharma, A.; Sharma, S.; Nagraik, R. An Insight into Different Microplastic Detection Methods. Int. J. Environ. Sci. Technol. 2021, 19, 5721–5730. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Edson, E.C.; Patterson, M.R. MantaRay: A Novel Autonomous Sampling Instrument for in Situ Measurements of Environmental Microplastic Particle Concentrations. In Proceedings of the OCEANS 2015-MTS/IEEE Washington, Washington, DC, USA, 19–22 October 2015. [Google Scholar] [CrossRef]
- Asamoah, B.O.; Kanyathare, B.; Roussey, M.; Peiponen, K.E. A Prototype of a Portable Optical Sensor for the Detection of Transparent and Translucent Microplastics in Freshwater. Chemosphere 2019, 231, 161–167. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, D.G.; Jung, H.Y.; Kim, S.H. Study on Real Time Sensor Monitoring Systems Based on Pulsed Laser for Microplastic Detection in Tap Water. J. Sens. Sci. Technol. 2019, 28, 294–298. [Google Scholar] [CrossRef]
- Nicolai, E.; Garau, S.; Favalli, C.; D’Agostini, C.; Gratton, E.; Motolese, G.; Rosato, N. Evaluation of BiesseBioscreen as a New Methodology for Bacteriuria Screening. New Microbiol. 2014, 37, 495–501. [Google Scholar] [PubMed]
- Toosky, M.N.; Grunwald, J.T.; Pala, D.; Shen, B.; Zhao, W.; D’agostini, C.; Coghe, F.; Angioni, G.; Motolese, G.; Abram, T.J.; et al. A Rapid, Point-of-Care Antibiotic Susceptibility Test for Urinary Tract Infections. J. Med. Microbiol. 2020, 69, 52–62. [Google Scholar] [CrossRef]
- Nicolai, E.; Pieri, M.; Gratton, E.; Motolese, G.; Bernardini, S. Bacterial Infection Diagnosis and Antibiotic Prescription in 3 h as an Answer to Antibiotic Resistance: The Case of Urinary Tract Infections. Antibiotics 2021, 10, 1168. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, Identification and Removal of Microplastic Particles and Fibers in Conventional Activated Sludge Process and Advanced MBR Technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hurley, R.R.; Vogelsang, C.; Nizzetto, L.; Olsen, M. Mapping Microplastics in Sludge; Norsk Institutt for Vannforskning: Oslo, Norway, 2017. [Google Scholar]
- Fernández-González, V.; Andrade-Garda, J.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Misidentification of PVC Microplastics in Marine Environmental Samples. TrAC Trends Anal. Chem. 2022, 153, 116649. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Çevik, C.; Güzel, E.; Kilercioğlu, S. Microplastics in Municipal Wastewater Treatment Plants in Turkey: A Comparison of the Influent and Secondary Effluent Concentrations. Environ. Monit. Assess. 2018, 190, 626. [Google Scholar] [CrossRef] [PubMed]
- Okoffo, E.D.; O’Brien, S.; O’Brien, J.W.; Tscharke, B.J.; Thomas, K.V. Wastewater Treatment Plants as a Source of Plastics in the Environment: A Review of Occurrence, Methods for Identification, Quantification and Fate. Environ. Sci. Water Res. Technol. 2019, 5, 1908–1931. [Google Scholar] [CrossRef]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of Microplastics in a Major Secondary Wastewater Treatment Plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Carbonaro, M.; Di Venere, A.; Filabozzi, A.; Maselli, P.; Minicozzi, V.; Morante, S.; Nicolai, E.; Nucara, A.; Placidi, E.; Stellato, F. Role of Dietary Antioxidant (−)-Epicatechin in the Development of β-Lactoglobulin Fibrils. Biochim. Biophys. Acta -Proteins Proteom. 2016, 1864, 766–772. [Google Scholar] [CrossRef]
- Zięba-Palus, J. The Usefulness of Infrared Spectroscopy in Examinations of Adhesive Tapes for Forensic Purposes. Forensic Sci. Criminol. 2017, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Colman, S.M.; Brown, E.T.; Minor, E.C.; Li, H. Estimation of Carbonate, Total Organic Carbon, and Biogenic Silica Content by FTIR and XRF Techniques in Lacustrine Sediments. J. Paleolimnol. 2013, 50, 387–398. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolai, E.; Pizzoferrato, R.; Li, Y.; Frattegiani, S.; Nucara, A.; Costa, G. A New Optical Method for Quantitative Detection of Microplastics in Water Based on Real-Time Fluorescence Analysis. Water 2022, 14, 3235. https://doi.org/10.3390/w14203235
Nicolai E, Pizzoferrato R, Li Y, Frattegiani S, Nucara A, Costa G. A New Optical Method for Quantitative Detection of Microplastics in Water Based on Real-Time Fluorescence Analysis. Water. 2022; 14(20):3235. https://doi.org/10.3390/w14203235
Chicago/Turabian StyleNicolai, Eleonora, Roberto Pizzoferrato, Yuliu Li, Simona Frattegiani, Alessandro Nucara, and Giulia Costa. 2022. "A New Optical Method for Quantitative Detection of Microplastics in Water Based on Real-Time Fluorescence Analysis" Water 14, no. 20: 3235. https://doi.org/10.3390/w14203235






