Effective Desalination of Acid Mine Drainage Using an Advanced Oxidation Process: Sodium Ferrate (VI) Salt
Abstract
:1. Introduction
2. Methodology
2.1. Water Sampling Process and Guidelines
2.2. Chemicals and Reagents
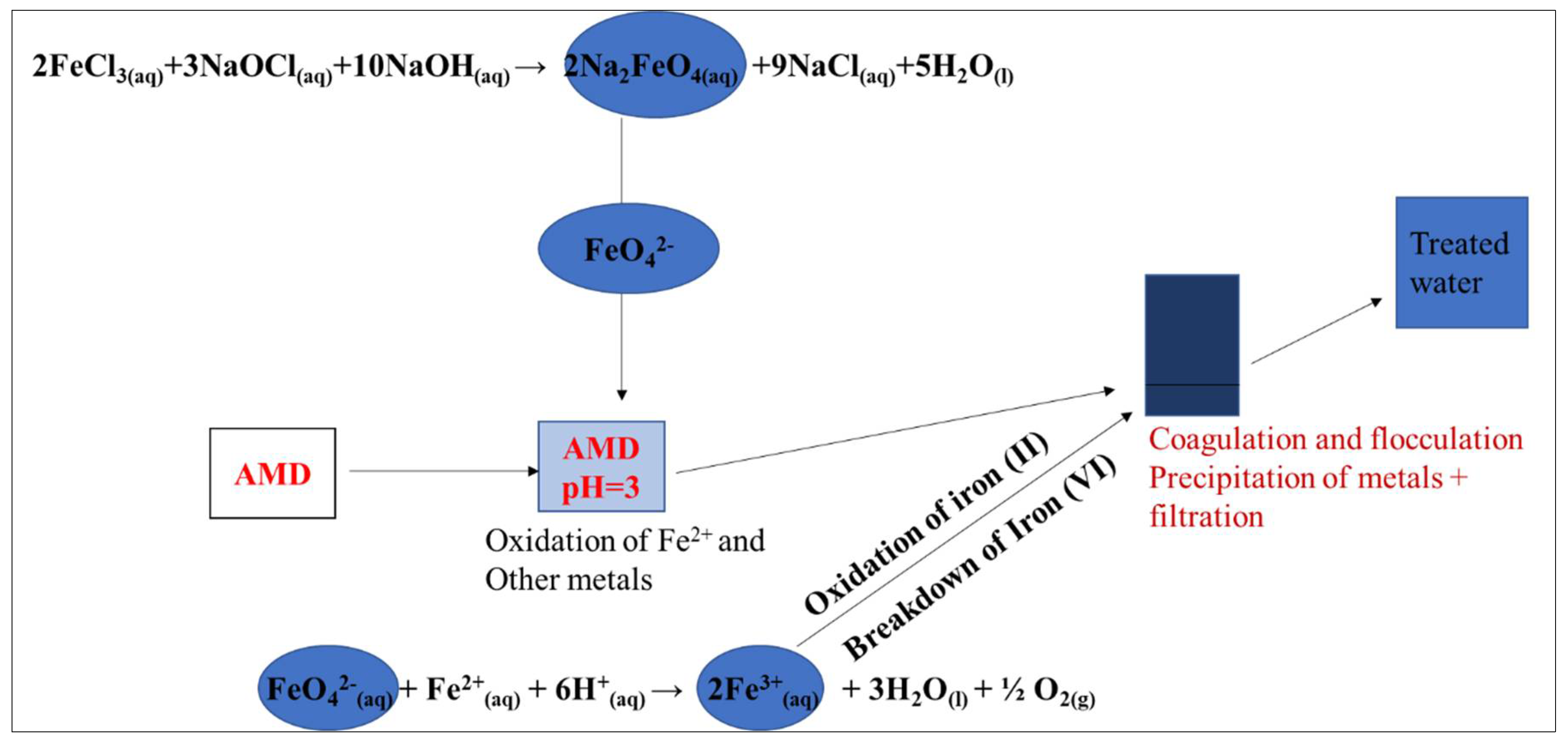
2.3. Preparation of Liquid Sodium Ferrate (VI)
2.4. Characterization and Quantification of Sodium Ferrate (VI) Using UV-Vis Spectroscopy
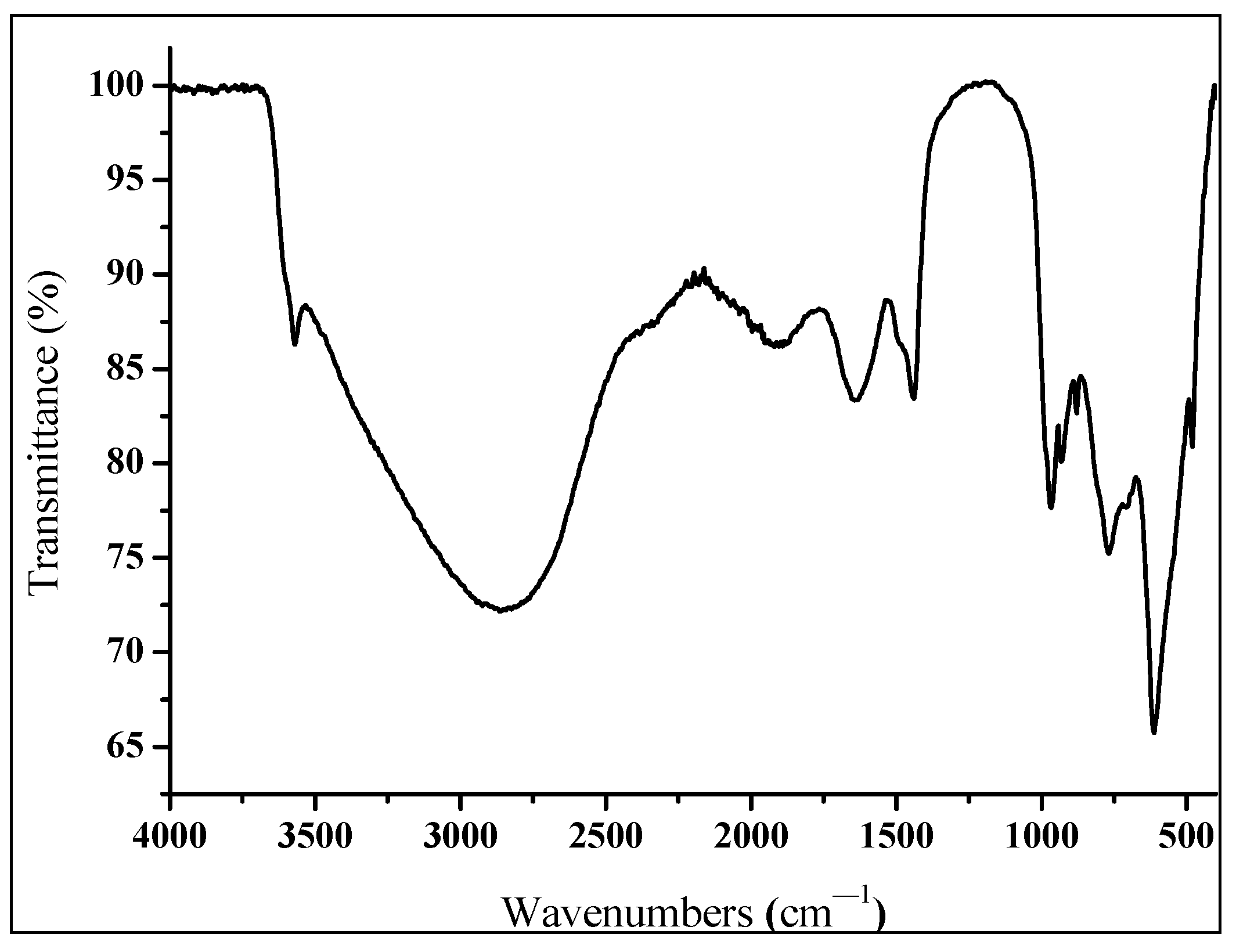
2.4.1. Characterization of Sodium Ferrate (VI) Using FT-IR
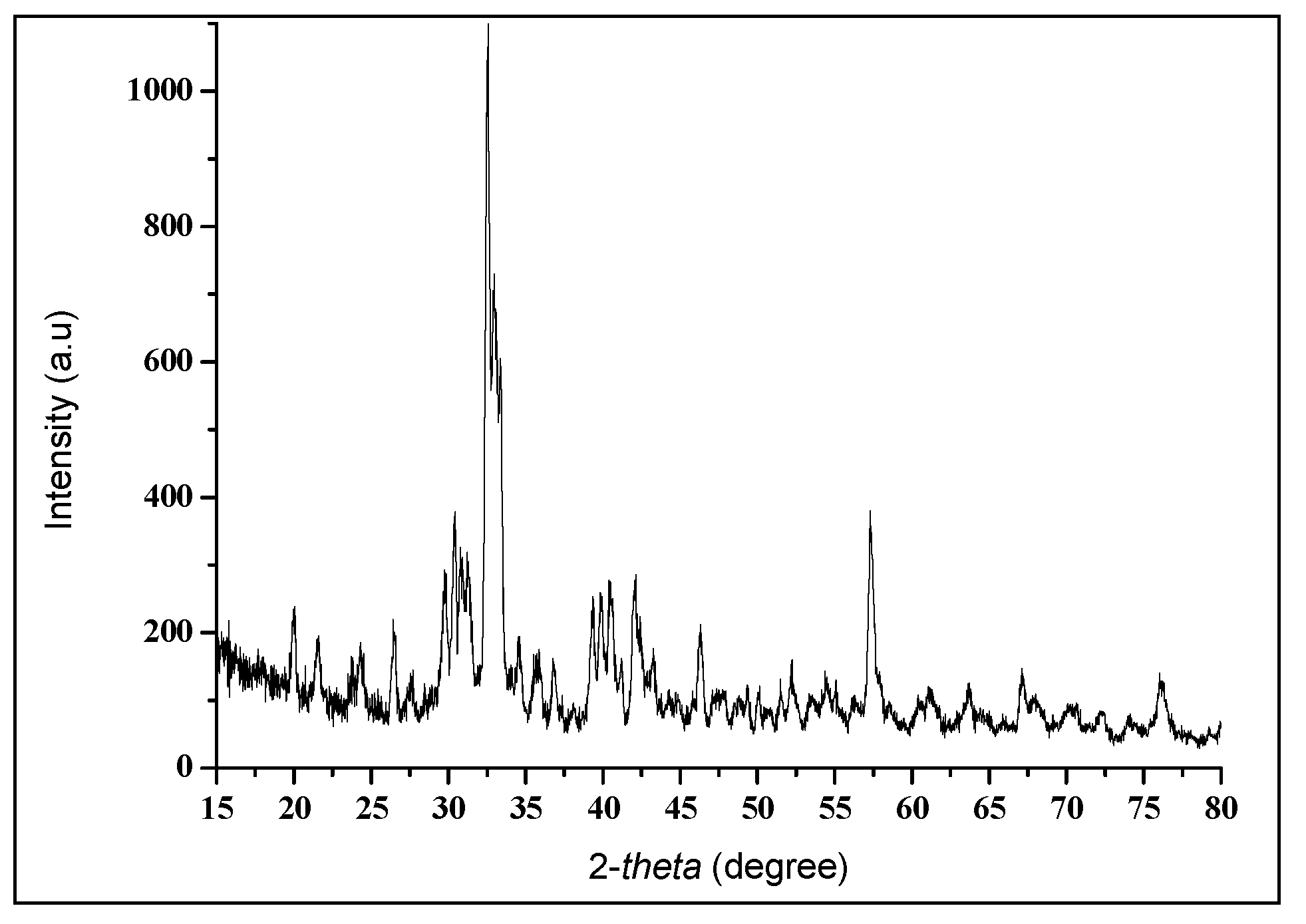
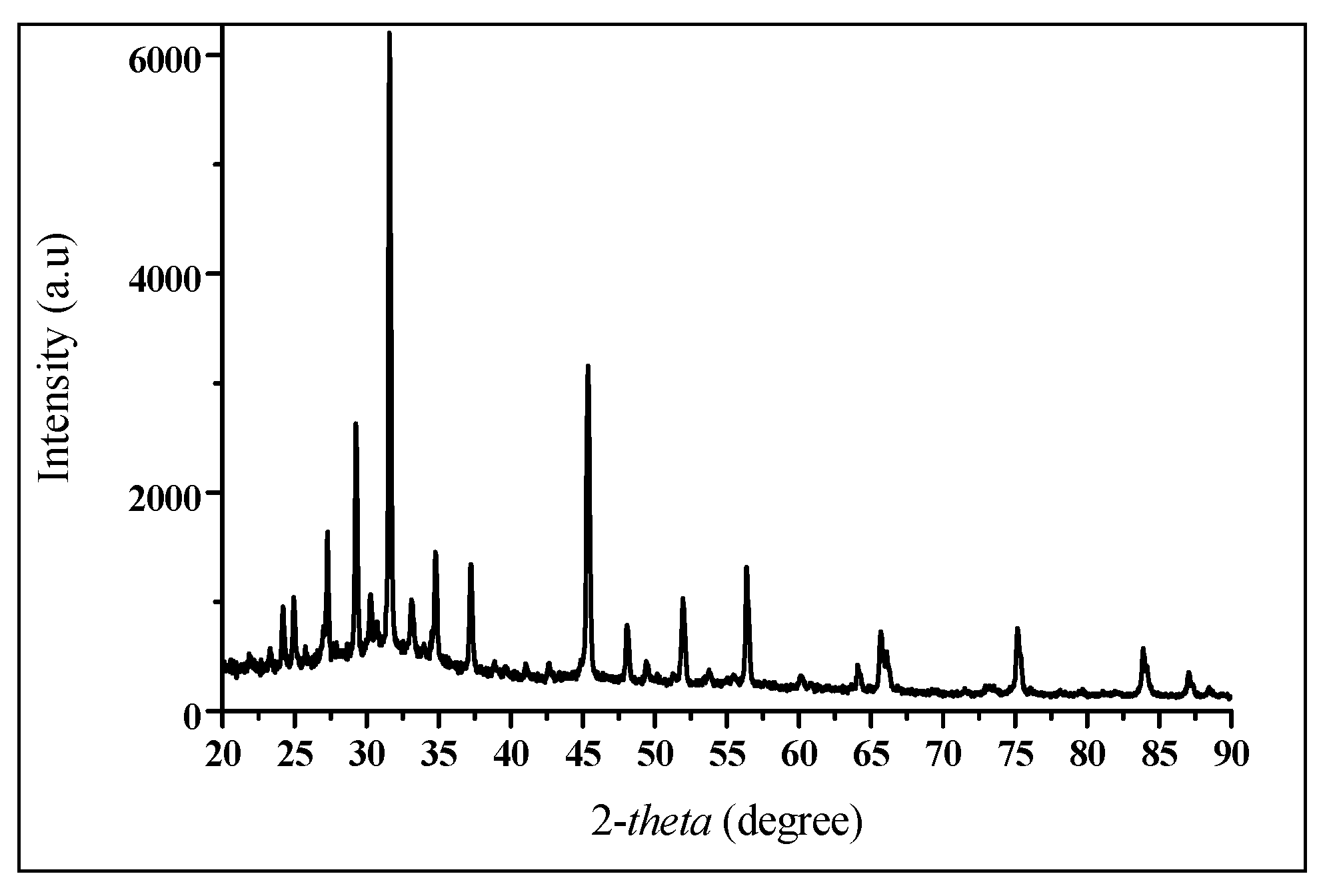
2.4.2. Characterization Using an X-ray Diffraction (XRD) Spectroscopy
2.5. Optimization of Parameters
2.6. Analytical Techniques
2.7. Flow Diagram of Real AMD Treatment Using Sodium Ferrate (FeO42−)
3. Results and Discussions
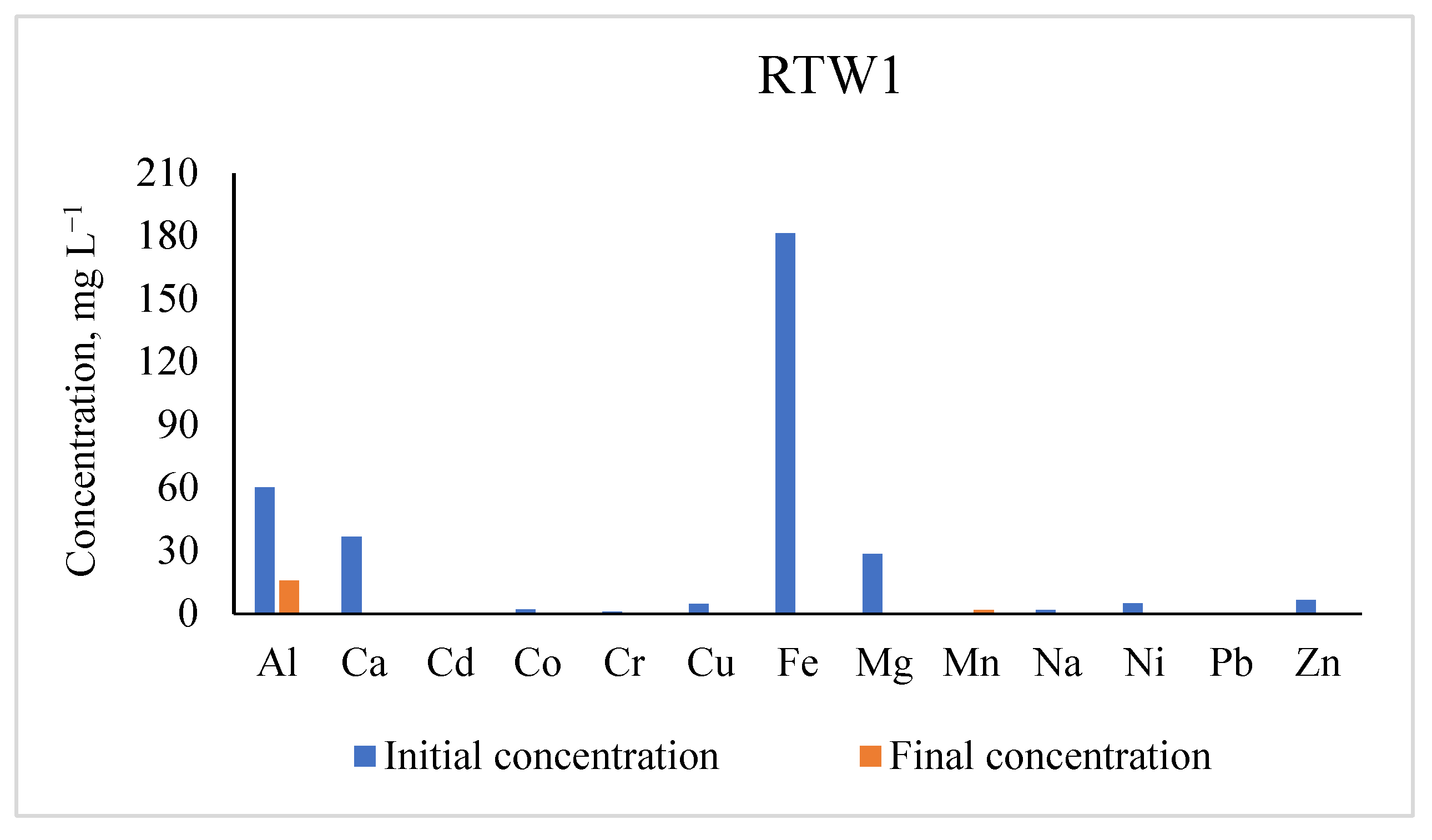
3.1. Concentrations of Metals in RTW1
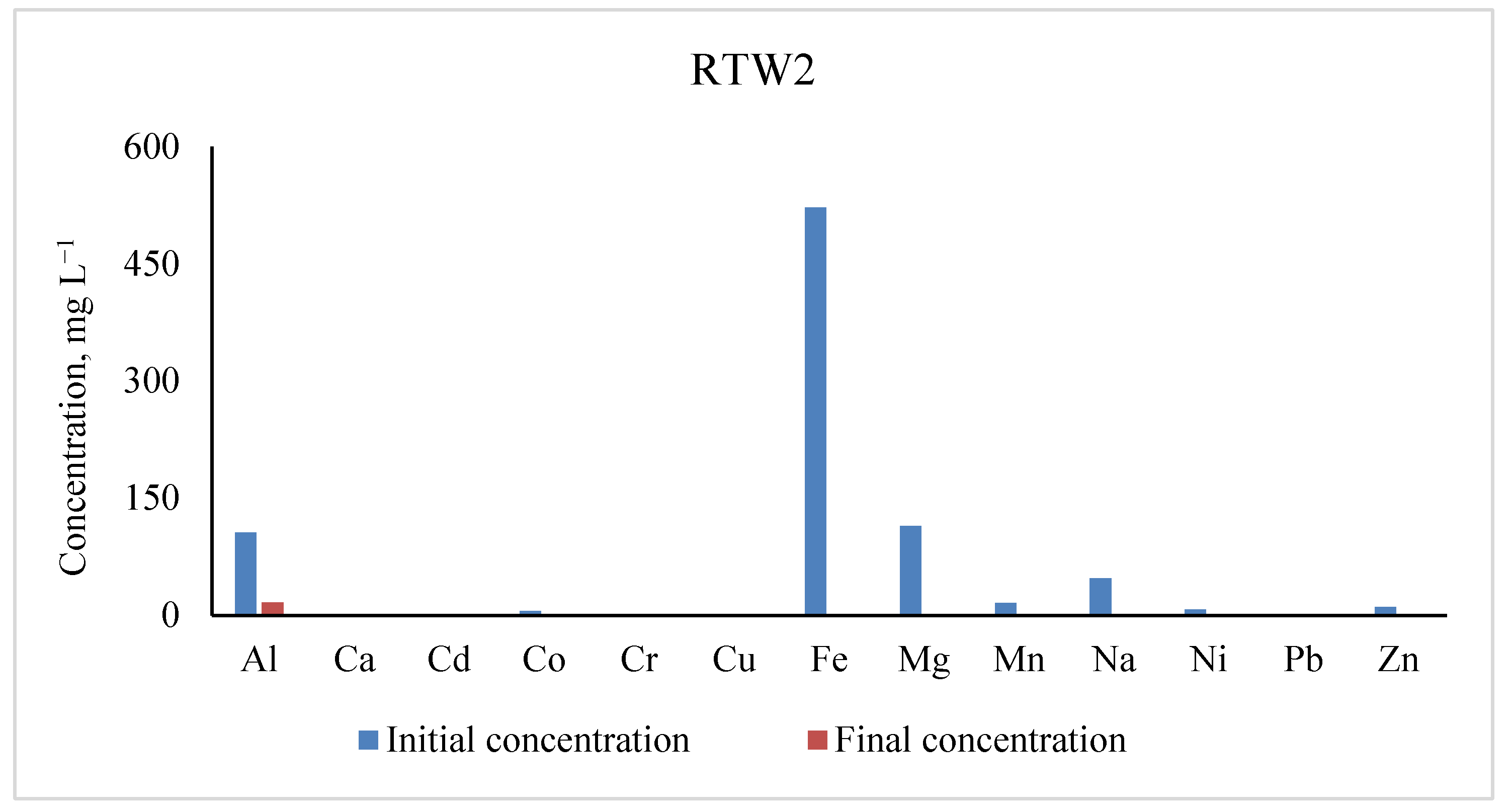
3.2. Concentrations of Metals in RTW2
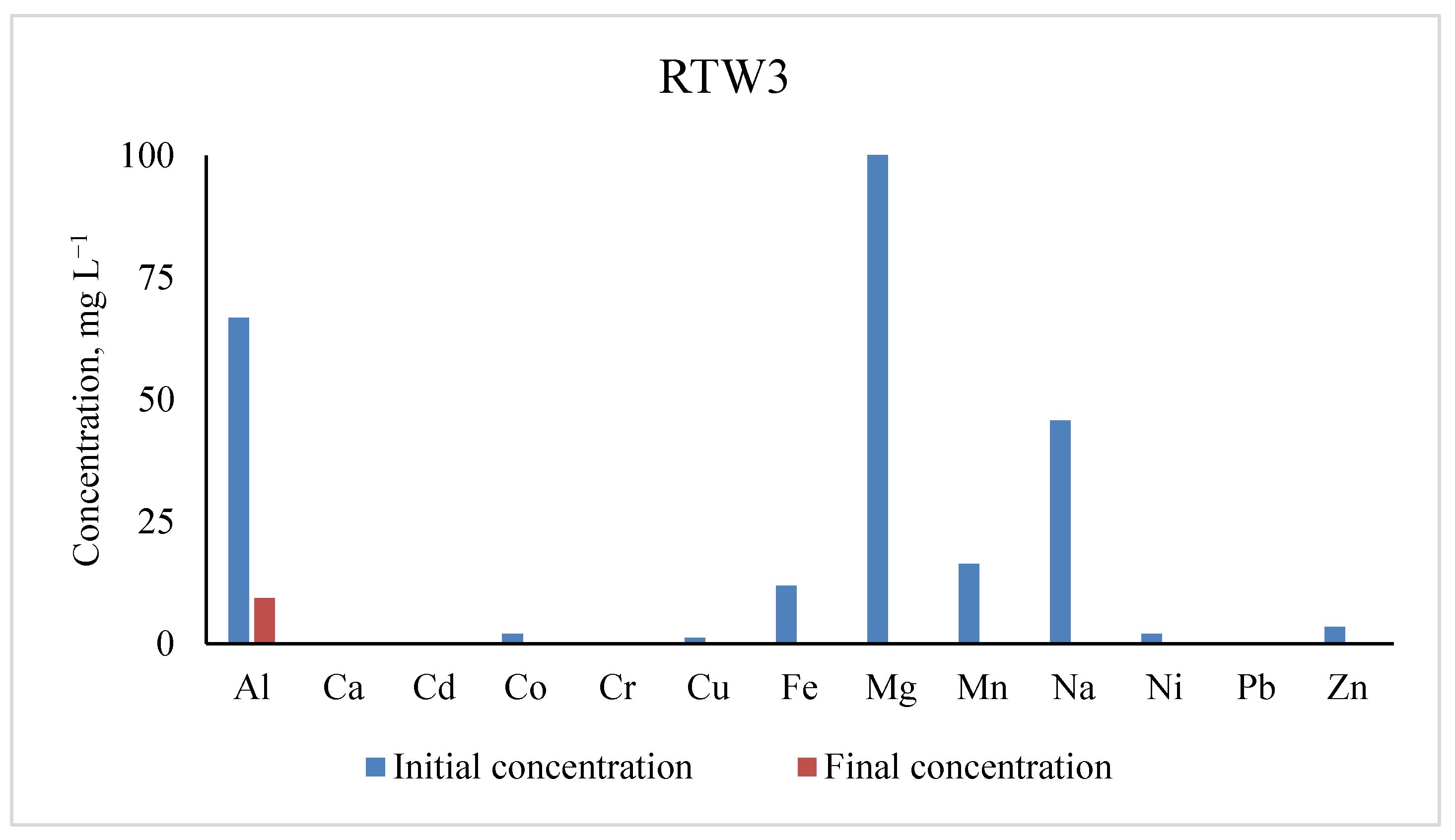
3.3. Concentrations of Metals in RTW3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younger, P.L. Mine Waste or Mine Voids: Which is the Most Important Long-Term Source of Polluted Mine Drainage? In the United Nations Environment Program 2002, Mineral Resources Forum: Current Feature Paper. Available online: www.mineralresourcesforum.org/docs/pdfs/younger1102.pdf (accessed on 8 August 2021).
- Strosnider WH, J.; López, F.L.; LaBar, J.A.; Palmer, K.J.; Nairn, R.W. Unabated acid mine drainage from Cerro Rico de Potosí, Bolivia: Uncommon constituents of concern impact the Río Pilcomayo headwaters. Environ. Earth Sci. 2014, 71, 3223–3234. [Google Scholar] [CrossRef]
- Sun, X.H.; Wang, L.; Li, W.C.; Tuo, W.Q. Preparation of liquid ferrate and the optimization of process parameters. In Advanced Materials Research; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2013; Volume 772, pp. 884–887. [Google Scholar] [CrossRef]
- Goodwill, J.E.; Mai, X.; Jiang, Y.; Reckhow, D.A.; Tobiason, J.E. Oxidation of manganese (II) with ferrate: Stoichiometry, kinetics, products and impact of organic carbon. Chemosphere 2016, 159, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of passive systems for acid mine drainage treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Munyengabe, A.; Zvinowanda, C. Production, characterization and application of ferrate (vi) in water and wastewater treatments. Braz. J. Anal. Chem. 2019, 6, 40–57. [Google Scholar] [CrossRef]
- Lv, D.; Zheng, L.; Zhang, H.; Deng, Y. Coagulation of colloidal particles with ferrate (VI). Environ. Sci. Water Res. Technol. 2018, 4, 701–710. [Google Scholar] [CrossRef]
- Zheng, L.; Deng, Y. Settleability and characteristics of ferrate (VI)-induced particles in advanced wastewater treatment. Water Res. 2016, 93, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Deng, Y.; Liu, S.; Song, Y.; Li, N.; Zhou, J. Formation of bromate during ferrate (VI) oxidation of bromide in water. Chemosphere 2016, 155, 528–533. [Google Scholar] [CrossRef] [Green Version]
- De Luca, S.J.; Cantelli, M.; De Luca, M.A. Ferrate vs traditional coagulants in the treatment of combined industrial wastes. Water Sci. Technol. 1992, 26, 2077–2080. [Google Scholar] [CrossRef]
- Alsheyab, M.; Jiang, J.Q.; Stanford, C. Online production of ferrate with an electrochemical method and its potential application for wastewater treatment: A review. J. Environ. Manag. 2009, 90, 1350–1356. [Google Scholar] [CrossRef]
- Dubrawski, K.L.; Cataldo, M.; Dubrawski, Z.; Mazumder, A.; Wilkinson, D.P.; Mohseni, M. In-situ electrochemical Fe (VI) for removal of microcystin-LR from drinking water: Comparing dosing of the ferrate ion by electrochemical and chemical means. J. Water Health 2018, 16, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Stanford, C.; Jiang, J.Q.; Alsheyab, M. Electrochemical production of ferrate (iron VI): Application to the wastewater treatment on a laboratory scale and comparison with iron (III) coagulant. Water Air Soil Pollut. 2010, 209, 483–488. [Google Scholar] [CrossRef]
- Ockerman, L.T.; Schreyer, J.M. Preparation of sodium ferrate (VI). J. Am. Chem. Soc. 1951, 73, 5478. [Google Scholar] [CrossRef]
- Munyengabe, A.; Zvinowanda, C.; Zvimba, J.N.; Ramontja, J. Innovative oxidation and kinetic studies of ferrous ion by sodium ferrate (VI) and simultaneous removal of metals from a synthetic acid mine drainage. Phys. Chem. Earth Parts A/B/C 2020, 102932, In Press, Corrected Proof. [Google Scholar] [CrossRef]
- Lei, B.; Zhou, G.; Cheng, T.; Du, J. Synthesis of potassium ferrate by chemical dry oxidation and its properties in the degradation of methyl orange. Asian J. Chem. 2013, 25, 27. [Google Scholar] [CrossRef]
- Kooti, M.; Jorfi, M.; Javadi, H. Rapid chemical synthesis of four ferrate (VI) compounds. J. Iran. Chem. Soc. 2010, 7, 814–819. [Google Scholar] [CrossRef]
- Yates, B.J.; Zboril, R.; Sharma, V.K. Engineering aspects of ferrate in water and wastewater treatment: A review. J. Environ. Sci. Health 2014, 49, 1603–1614. [Google Scholar] [CrossRef]
- Lee, Y.; Zimmermann, S.G.; Kieu, A.T.; von Gunten, U. Ferrate (Fe (VI)) application for municipal wastewater treatment: A novel process for simultaneous micropollutant oxidation and phosphate removal. Environ. Sci. Technol. 2009, 43, 3831–3838. [Google Scholar] [CrossRef]
- Waite, T.D.; Gray, K.A. Oxidation and coagulation of wastewater effluent utilising ferrate (VI) ion. In Studies in Environmental Science 23; Elsevier: Amsterdam, The Netherlands, 1984; pp. 407–420. [Google Scholar] [CrossRef]
- Sharma, V.K. Ferrate (V) oxidation of pollutants: A premix pulse radiolysis study. Radiat. Phys. Chem. 2002, 65, 349–355. [Google Scholar] [CrossRef]
- Nguema, P.F.; Jun, M. Application of ferrate (VI) as a disinfectant in drinking water treatment processes: A review. Int. J. 2016, 7, 53–62. [Google Scholar] [CrossRef]
- Song, Y.R.; Ma, J.W. Development of ferrate (VI) salt as an oxidant and coagulant for water and wastewater treatment. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2013; Volume 361, pp. 658–661. [Google Scholar] [CrossRef]
- Fernandes, H.M.; Reinhart, D.; Franklin, M.R. Use of Na-Ferrate (VI) to prevent acid drainage from uranium mill tailings. In Uranium 2008, Mining and Hydrogeology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 261–270. [Google Scholar] [CrossRef]
- Goodwill, J.E.; LaBar, J.; Slovikosky, D.; Strosnider, W.H. Preliminary assessment of ferrate treatment of metals in acid mine drainage. J. Environ. Qual. 2019, 48, 1549–1556. [Google Scholar] [CrossRef] [Green Version]
- Momtazpour, H.; Jorfi, S.; Tabatabaie, T.; Pazira, A.A. Application of sodium ferrate produced from industrial wastes for TOC removal of surface water. Water Sci. Technol. 2019, 79, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Weissenstein, K.; Sinkala, T. Soil pollution with heavy metals in mine environments, impact areas of mine dumps particularly of gold-and copper mining industries in Southern Africa. Arid Ecosyst. 2011, 1, 53–58. [Google Scholar] [CrossRef]
- SANS (South African National Standards) 241:2015. Drinking-Water. Available online: https://selectech.co.za/whatyoushouldknowaboutthenew2015bluedroplimitssans2412015drinkingwater/#:~:text=The%20new%20SANS%2041%3A2015 (accessed on 8 August 2020).
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: The Fourth Edition Incorporating the First Addendum. License: CC BY-NC-SA 3.0 IGO. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/254637/9789241549950-eng.pdf;jsessionid=F099F3FEEEBB3C1A1A81964E06B241FC?sequence=1 (accessed on 8 August 2020).
- Thompson, G.W.; Ockerman, L.; Schreyer, J.M. Preparation and purification of potassium ferrate (VI). J. Am. Chem. Soc. 1951, 73, 1379–1381. [Google Scholar] [CrossRef]
- El Maghraoui, A.; Zerouale, A.; Ijjaali, M. Process for the synthesis of ferrate (VI) Alkali metal dry. Adv. Mater. Phys. Chem. 2015, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- El Maghraoui, A.; Zerouale, A.; Ijjaali, M.; Sajieddine, M. Synthesis and characterization of ferrate (VI) alkali metal by electrochemical method. Adv. Mater. Phys. Chem. 2013, 3, 83–87. Available online: http://www.scirp.org/journal/PaperInformation.aspx?PaperID=29310 (accessed on 15 July 2021). [CrossRef] [Green Version]
- Bouzek, K.; Schmidt, M.J.; Wragg, A.A. Influence of electrolyte composition on current yield during ferrate (VI) production by anodic iron dissolution. Electrochem. Commun. 1999, 1, 370–374. [Google Scholar] [CrossRef]
- Sharma, V.K.; Zboril, R.; Varma, R.S. Ferrates: Greener oxidants with multimodal action in water treatment technologies. Acc. Chem.Res. 2015, 48, 182–191. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kazama, F.; Jiangyong, H.; Ray, A.K. Ferrates (iron (VI) and iron (V)): Environmentally friendly oxidants and disinfectants. J. Water Health 2005, 3, 45–58. [Google Scholar] [CrossRef]
- Batarseh, E.S.; Reinhart, D.R.; Daly, L. Liquid sodium ferrate and Fenton’s reagent for treatment of mature landfill leachate. J. Environ. Eng. 2007, 133, 1042–1050. [Google Scholar] [CrossRef]
- Munyengabe, A.; Zvinowanda, C. Synthesis and chemical stability studies of sodium ferrate (VI) solution. Asian J. Chem. 2019, 31, 3029–3034. [Google Scholar] [CrossRef]
- Koltypin, M.; Licht, S.; Nowik, I.; Vered, R.T.; Levi, E.; Gofer, Y.; Aurbach, D. Study of various (“super iron”) MFeO4 compounds in Li salt solutions as potential cathode materials for Li batteries. J. Electrochem. Soc. 2006, 153, A32–A41. Available online: https://www.cheric.org/research/tech/periodicals/doi.php?art_seq=527715 (accessed on 15 August 2020). [CrossRef]
- Cui, P.; Chen, S.S.; Li, D.S.; Zhao, P. Preparation of Sodium Ferrate by Microwave Method; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2013; Volume 781, pp. 2225–2228. [Google Scholar] [CrossRef]
- Laksono, F.B.; Kim, I. Removal of 2-Bromophenol by advanced oxidation process with in-situ liquid ferrate (VI). Int. Proc. Chem. Biol. Environ. Eng. 2016, 94, 128–135. [Google Scholar] [CrossRef]
- Martinez-Tamayo, E.; Beltran-Porter, A.; Beltran-Porter, D. Iron compounds in high oxidation states: II. Reaction between Na2O2 and FeSO4. Thermochim. Acta 1986, 97, 243–255. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Umukoro, E.H.; Tshwenya, L.; Moutloali, R.; Babalola, J.O.; Arotiba, O.A. Photo-electrocatalytic water treatment systems: Degradation, kinetics and intermediate products studies of sulfamethoxazole on a TiO2-exfoliated graphite electrode. RSC Adv. 2017, 7, 40571–40580. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.M.; Jun, M.; Liu, W. Enhanced removal of lead (II) and cadmium (II) from water in alum coagulation by ferrate (VI) pretreatment. Water Environ. Res. 2007, 79, 2420–2426. [Google Scholar] [CrossRef]


| Samples and Guidelines | Temperature °C | pH at 25 °C | EC µS cm−1 | TDS (mg L−1) |
|---|---|---|---|---|
| RTW1 | 26.2 | 2.58 | 262 | >1000 |
| RTW2 | 26.7 | 2.50 | 263 | 960 |
| RTW3 | 25.0 | 3.13 | 226 | >1000 |
| SANS guidelines | <30 | ≥5 to ≤9.7 | ≤170 | ≤1200 |
| WHO guidelines | 6.5–9.5 | 600 |
| Elements | SANS 241-2015 (Limits for Drinkability) (µg L−1) | WHO Guidelines [29] (mg L−1) |
|---|---|---|
| Al | ≤300 | 0.2 |
| Ca | 100–300 | |
| Cd | ≤3 | 0.003 |
| Co | ||
| Cr | ≤50 | 0.05 |
| Cu | ≤2000 | |
| Fe | ≤2000 | 0.5–50 |
| Mg | ||
| Mn | ≤400 | 0.4 |
| Na | ≤200 | 200 |
| Ni | ≤70 | 0.07 |
| Pb | ≤10 | 0.01 |
| Zn | ≤5 | <3 |
| Time (min) | pH | Volume of Fe2+ (mL) | Volume of Ferrate (mL) | Concentration of Ferrate (mmol L−1) |
|---|---|---|---|---|
| 30 | 3.0 | 15 | 5 | 5 × 10−2 |
| Elements | Initial Concentrations (mg L−1) | Residual Concentrations (mg L−1) | % Removal |
|---|---|---|---|
| Al | 60.35 ± 0.50 | 15.77 | 73.90 |
| Ca | 36.66 ± 0.00 | 0.103 | 99.72 |
| Cd | 0.005 ± 0.00 | 0.004 | 26.00 |
| Co | 2.10 ± 0.00 | 0.0006 | 99.97 |
| Cr | 0.97 ± 0.00 | 0.370 | 61.72 |
| Cu | 4.74 ± 0.00 | 0.030 | 99.40 |
| Fe | 181.30 ± 0.00 | 0.540 | 99.70 |
| Mg | 28.53 ± 0.00 | 0.00 | 100.00 |
| Mn | 0.590 ± 0.01 | bdl | |
| Na | 1.77 ± 0.00 | 0.00 | 100.00 |
| Ni | 4.87 ± 0.01 | 0.00 | 100.00 |
| Pb | 0.002 ± 0.00 | bdl | |
| Zn | 6.490 ± 0.00 | 0.280 | 95.70 |
| Elements | Initial Concentrations (mg L−1) | Residual Concentrations (mg L−1) | % Removal |
|---|---|---|---|
| Al | 105.00 ± 0.40 | 16.560 | 84.23 |
| Ca | bdl | bdl | |
| Cd | bdl | bdl | |
| Co | 5.16 ± 0.00 | 0.011 | 99.78 |
| Cr | 1.02 ± 0.00 | 0.117 | 88.40 |
| Cu | 1.30 ± 0.00 | 0.007 | 99.45 |
| Fe | 521.40 ± 0.05 | 0.088 | 99.98 |
| Mg | 113.70 ± 0.04 | 0 | 100.00 |
| Mn | 15.48 ± 0.01 | 0.002 | 100.00 |
| Na | 47.07 ± 0.04 | 0.00 | 100.00 |
| Ni | 7.34 ± 0.01 | 0.00 | 100.00 |
| Pb | 0.12 ± 0.00 | 0.001 | 98.85 |
| Zn | 10.75 ± 0.00 | 0.024 | 99.77 |
| Elements | Initial Concentrations (mg L−1) | Residual Concentrations (mg L−1) | % Removal |
|---|---|---|---|
| Al | 66.67 ± 0.21 | 9.321 | 86.02 |
| Ca | |||
| Cd | 0.003 ± 0.00 | 0.001 | 70.00 |
| Co | 1.929 ± 0.00 | 0.010 | 99.48 |
| Cr | 0.092 ± 0.00 | bdl | |
| Cu | 1.159 ± 0.00 | 0.008 | 99.32 |
| Fe | 11.79 ± 0.00 | 0.017 | 99.85 |
| Mg | 104.30 ± 0.03 | 0 | 100.00 |
| Mn | 16.32 ± 0.00 | 0.002 | 99.98 |
| Na | 45.65 ± 0.05 | 0 | 100 |
| Ni | 1.949 ± 0.00 | bdl | |
| Pb | 0.014 ± 0.00 | 0.002 | |
| Zn | 3.374 ± 0.00 | 0.016 | 99.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munyengabe, A.; Zvinowanda, C.; Ramontja, J.; Zvimba, J.N. Effective Desalination of Acid Mine Drainage Using an Advanced Oxidation Process: Sodium Ferrate (VI) Salt. Water 2021, 13, 2619. https://doi.org/10.3390/w13192619
Munyengabe A, Zvinowanda C, Ramontja J, Zvimba JN. Effective Desalination of Acid Mine Drainage Using an Advanced Oxidation Process: Sodium Ferrate (VI) Salt. Water. 2021; 13(19):2619. https://doi.org/10.3390/w13192619
Chicago/Turabian StyleMunyengabe, Alexis, Caliphs Zvinowanda, James Ramontja, and John Ngoni Zvimba. 2021. "Effective Desalination of Acid Mine Drainage Using an Advanced Oxidation Process: Sodium Ferrate (VI) Salt" Water 13, no. 19: 2619. https://doi.org/10.3390/w13192619






