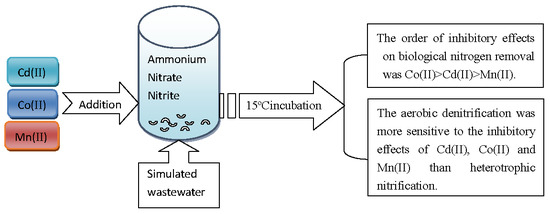
Effect of Cobalt, Cadmium and Manganese on Nitrogen Removal Capacity of Arthrobacter arilaitensis Y-10
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Medium
2.2. Assessment of the Influence of Cd(II), Co(II), and Mn(II) on the Nitrogen Bioremediation Performance of Strain Y-10
2.3. Analytical Methods
2.4. Statistical Analysis
2.5. Kinetic Model
3. Results and Discussion
3.1. Impacts of Cadmium on the Nitrogen Bioremediation Characteristics of Strain Y-10
3.2. Impacts of Cobalt on the Nitrogen Bioremediation Characteristics of Strain Y-10
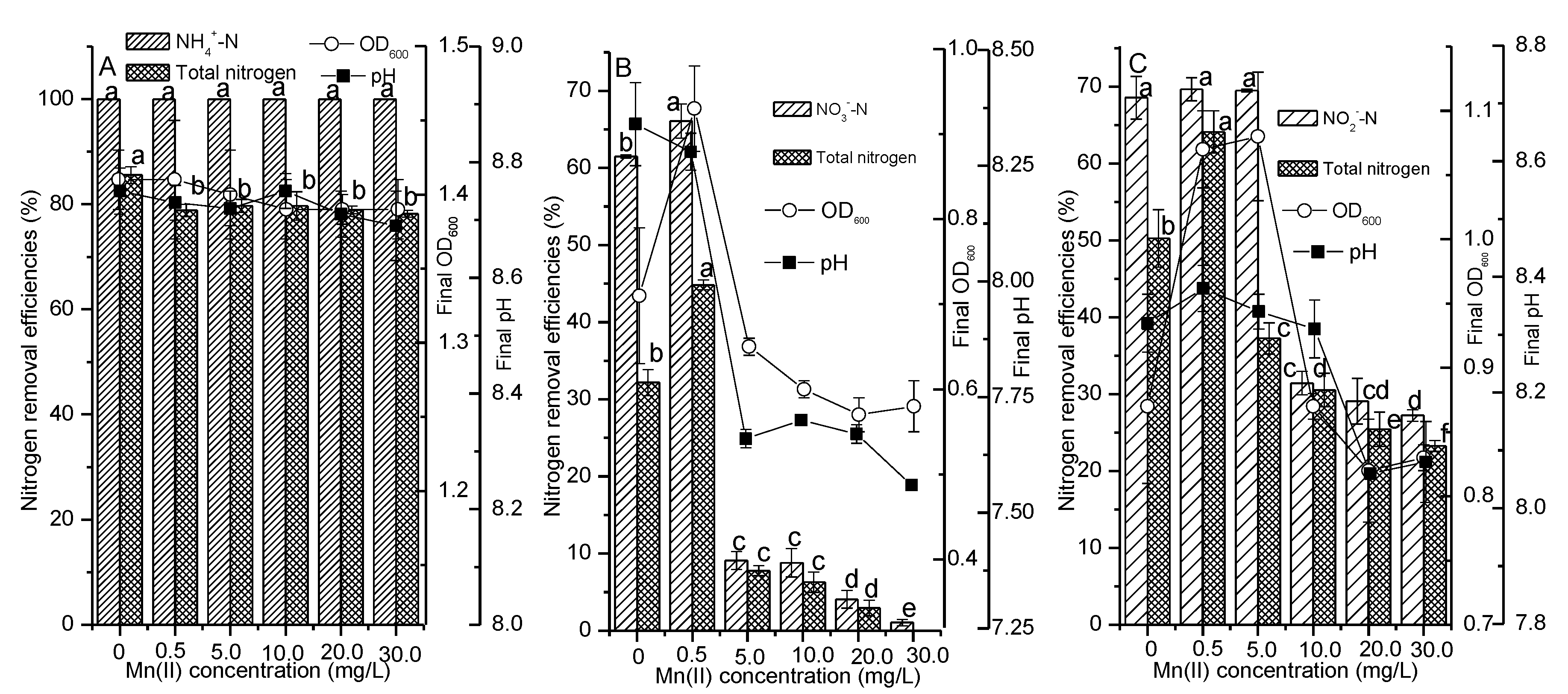
3.3. Impacts of Manganese on the Nitrogen Bioremediation Characteristics of Strain Y-10
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- He, T.; Xie, D.; Li, Z.; Ni, J.; Sun, Q. Ammonium stimulates nitrate reduction during simultaneous nitrification and denitrification process by Arthrobacter arilaitensis Y-10. Bioresour. Technol. 2017, 239, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Capodici, M.; Corsino, S.F.; Di Trapani, D.; Viviani, G. Achievement of partial nitrification under different carbon-to-nitrogen ratio and ammonia loading rate for the co-treatment of landfill leachate with municipal wastewater. Biochem. Eng. J. 2019, 149, 107229. [Google Scholar] [CrossRef]
- Jin, R.; Liu, T.; Liu, G.; Zhou, J.; Huang, J.; Wang, A. Simultaneous heterotrophic nitrification and aerobic denitrification by the marine origin bacterium Pseudomonas sp. ADN-42. Appl. Biochem. Biotechnol. 2015, 175, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, Z.; Sun, Q.; Xu, Y.; Ye, Q. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion. Bioresour. Technol. 2016, 200, 493–499. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Liu, Y.; Ai, G.-M.; Miao, L.-L.; Zheng, H.-Y.; Liu, Z.-P. The characteristics of a novel heterotrophic nitrification–aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.-S.; Jiang, C.-K.; Tang, X.; Feng, F.; Tang, J.; Tang, C.-J.; Chai, L.-Y. Insights into Anammox activity inhibition under trivalent and hexavalent chromium stresses. Biochem. Eng. J. 2019, 147, 118–125. [Google Scholar] [CrossRef]
- Redeker, E.S.; Blust, R. Accumulation and toxicity of cadmium in the aquatic oligochaete tubifex tubifex: A kinetic modeling approach. Environ. Sci. Technol. 2004, 38, 537–543. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, M.; Zhao, Y.; Zhai, H. Recovery of nitrification in cadmium-inhibited activated sludge system by bio-accelerators. Bioresour. Technol. 2016, 200, 812–819. [Google Scholar] [CrossRef]
- Olabarrieta, I.; L’Azou, B.; Yuric, S.; Cambar, J.; Cajaraville, M. In vitro effects of cadmium on two different animal cell models. Toxicol. Vitr. 2001, 15, 511–517. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, A.; Tang, C. Ammonium-based fertilizers enhance Cd accumulation in Carpobrotus rossii grown in two soils differing in pH. Chemosphere 2017, 188, 689–696. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Li, C.; Yang, W.; Song, T.; Tang, C.; Meng, Y.; Dai, S.; Wang, H.; Chai, L.; et al. Synthesis of core–shell magnetic Fe3O4@poly(m-Phenylenediamine) particles for chromium reduction and adsorption. Environ. Sci. Technol. 2015, 49, 5654–5662. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef] [PubMed]
- Khaokaew, S.; Chaney, R.L.; Landrot, G.; Ginder-Vogel, M.; Sparks, N.L. Speciation and release kinetics of cadmium in an alkaline paddy soil under various flooding periods and draining conditions. Environ. Sci. Technol. 2011, 45, 4249–4255. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.P.; Barber, J.B.; Chandran, K.; Constable, S.; Darnell, C.; DiMenna, R.; Gaines, B.; Goodman, A.; Hlavek, R.; Johns, F.J.; et al. Industrial wastewater management, treatment, and disposal. In WEF Manual of Practice No. FD-3rd; Mcgraw-Hill: New York, NY, USA, 2008; pp. 474–489. [Google Scholar]
- Hu, Z.; Chandran, K.; Grasso, D.; Smets, B.F. Impact of metal sorption and internalization on nitrification inhibition. Environ. Sci. Technol. 2003, 37, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Gikas, P. Single and combined effects of nickel (Ni(II)) and cobalt (Co(II)) ions on activated sludge and on other aerobic microorganisms: A review. J. Hazard. Mater. 2008, 159, 187–203. [Google Scholar] [CrossRef]
- Li, H.; Yao, H.; Zhang, D.; Zuo, L.; Ren, J.; Ma, J.; Pei, J.; Xu, Y.; Yang, C. Short- and long-term effects of manganese, zinc and copper ions on nitrogen removal in nitritation-anammox process. Chemosphere 2017, 193, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Tekerlekopoulou, A.; Vayenas, D. Ammonia, iron and manganese removal from potable water using trickling filters. Desalination 2007, 210, 225–235. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kappelhof, J.; Groenendijk, M.; Schippers, J.C. Comparison of physicochemical iron removal mechanisms in filters. J. Water Supply: Res. Technol. 2001, 50, 187–198. [Google Scholar] [CrossRef]
- Huang, X.; Gao, D.-W.; Peng, S.; Tao, Y. Effects of ferrous and manganese ions on anammox process in sequencing batch biofilm reactors. J. Environ. Sci. 2014, 26, 1034–1039. [Google Scholar] [CrossRef]
- Hernandez-Martinez, G.R.; Ortiz-Alvarez, D.; Perez-Roa, M.; Urbina-Suárez, N.; Thalasso, F. Multiparameter analysis of activated sludge inhibition by nickel, cadmium, and cobalt. J. Hazard. Mater. 2018, 351, 63–70. [Google Scholar] [CrossRef]
- He, T.; Li, Z.-L. Identification and denitrification characterization of a novel hypothermia and aerobic nitrite-denitrifying bacterium, Arthrobacter arilaitensis strain Y-10. Desalin. Water Treat. 2015, 57, 1–9. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Chen, H.; Chen, Q.-Q.; Jiang, X.-Y.; Hu, H.-Y.; Shi, M.-L.; Jin, R.-C. Insight into the short- and long-term effects of Cu(II) on denitrifying biogranules. J. Hazard. Mater. 2015, 304, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Semerci, N.; Cecen, F. Importance of cadmium speciation in nitrification inhibition. J. Hazard. Mater. 2007, 147, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, D.; Li, X.; Yang, Q.; Luo, K.; Zeng, G.-M.; Tang, M.-L. Effects of Cd(II) on wastewater biological nitrogen and phosphorus removal. Chemosphere 2014, 117, 27–32. [Google Scholar] [CrossRef]
- He, T.; Xie, D.; Ni, J.; Li, Z.; Li, Z. Nitrous oxide produced directly from ammonium, nitrate and nitrite during nitrification and denitrification. J. Hazard. Mater. 2020, 388, 122114. [Google Scholar] [CrossRef]
- Kapoor, V.; Li, X.; Elk, M.; Chandran, K.; Impellitteri, C.A.; Domingo, J.W.S. Impact of heavy metals on transcriptional and physiological activity of nitrifying bacteria. Environ. Sci. Technol. 2015, 49, 13454–13462. [Google Scholar] [CrossRef]
- Gui, M.; Chen, Q.; Ma, T.; Zheng, M.; Ni, J. Effects of heavy metals on aerobic denitrification by strain Pseudomonas stutzeri PCN-1. Appl. Microbiol. Biotechnol. 2017, 101, 1717–1727. [Google Scholar] [CrossRef]
- Rao, S.S.; Srinath, E.G. Influence of cobalt on the synthesis of vitamin B12 in sewage during aerobic & anaerobic treatment. J. Sci. Ind. Res. C Biol. Sci. 1961, 20, 261–265. [Google Scholar]
- Fang, Y.F.; Lin, F.K.; Zhu, L.U. Nutrient supplements to optimize treatment of industrial wastewater by activated sludge system. Technol. Water. Treat. 2006, 32, 15–18. [Google Scholar]
- Kim, J.K.; Park, K.J.; Cho, K.S.; Nam, S.-W.; Park, T.-J.; Bajpai, R. Aerobic nitrification–denitrification by heterotrophic Bacillus strains. Bioresour. Technol. 2005, 96, 1897–1906. [Google Scholar] [CrossRef]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chang, Y.; Liang, J.; Chen, C.; Qu, J. Treatment of groundwater containing Mn(II), Fe(II), As(III) and Sb(III) by bioaugmented quartz-sand filters. Water Res. 2016, 106, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Su, J.; Shao, P.; Liu, H.; Luo, X. Efficient autotrophic denitrification performance through integrating the bio-oxidation of Fe(II) and Mn(II). Chem. Eng. J. 2018, 348, 669–677. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, W.; Huang, X.; Qin, W. Effect of trace elements and optimization of their composition for the nitrification of a heterotrophic nitrifying bacterium, Acinetobacter harbinensis HITLi7T, at low temperature. Ann. Microbiol. 2017, 67, 715–725. [Google Scholar] [CrossRef]
- Kimura, Y.; Isaka, K. Evaluation of inhibitory effects of heavy metals on anaerobic ammonium oxidation (anammox) by continuous feeding tests. Appl. Microbiol. Biotechnol. 2014, 98, 6965–6972. [Google Scholar] [CrossRef]
- Jin, R.-C.; Yang, G.; Yu, J.-J.; Zheng, P. The inhibition of the Anammox process: A review. Chem. Eng. J. 2012, 197, 67–79. [Google Scholar] [CrossRef]
- Lotti, T.; Cordola, M.; Kleerebezem, R.; Caffaz, S.; Lubello, C.; Van Loosdrecht, M.C.M. Inhibition effect of swine wastewater heavy metals and antibiotics on anammox activity. Water Sci. Technol. 2012, 66, 1519–1526. [Google Scholar] [CrossRef]
- Mertoglu, B.; Semerci, N.; Guler, N.; Calli, B.; Çeçen, F.; Saatçı, A.M.; Saatci, A.M. Monitoring of population shifts in an enriched nitrifying system under gradually increased cadmium loading. J. Hazard. Mater. 2008, 160, 495–501. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Ma, Y.; Zhao, J.; Chen, T.; Fu, H.; Zhai, H. Acute and persistent toxicity of Cd(II) to the microbial community of Anammox process. Bioresour. Technol. 2018, 261, 453–457. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.-X.; Chai, L.; Duan, C.-S.; Tang, C.-J.; Ali, M.; Peng, C. Comparative evaluation of short-term stress of Cd(II), Hg(II), Pb(II), As(III) and Cr(VI) on anammox granules by batch test. J. Biosci. Bioeng. 2016, 122, 722–729. [Google Scholar] [CrossRef]
- Çeçen, F.; Semerci, N.; Geyik, A.G.; Çeçen, F.; Geyik, A.G. Inhibitory effects of Cu, Zn, Ni and Co on nitrification and relevance of speciation. J. Chem. Technol. Biotechnol. 2010, 85, 520–528. [Google Scholar]
- Xu, J.-J.; Zhang, Z.-Z.; Chen, Q.-Q.; Ji, Z.-Q.; Zhu, Y.-H.; Jin, R.-C. The short- and long-term effects of Mn2+ on biogranule-based anaerobic ammonium oxidation (anammox). Bioresour. Technol. 2017, 241, 750–759. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, T.; Xie, D.; Ni, J.; Li, Z.; Li, Z. Effect of Cobalt, Cadmium and Manganese on Nitrogen Removal Capacity of Arthrobacter arilaitensis Y-10. Water 2020, 12, 1701. https://doi.org/10.3390/w12061701
He T, Xie D, Ni J, Li Z, Li Z. Effect of Cobalt, Cadmium and Manganese on Nitrogen Removal Capacity of Arthrobacter arilaitensis Y-10. Water. 2020; 12(6):1701. https://doi.org/10.3390/w12061701
Chicago/Turabian StyleHe, Tengxia, Deti Xie, Jiupai Ni, Zhu Li, and Zhenlun Li. 2020. "Effect of Cobalt, Cadmium and Manganese on Nitrogen Removal Capacity of Arthrobacter arilaitensis Y-10" Water 12, no. 6: 1701. https://doi.org/10.3390/w12061701