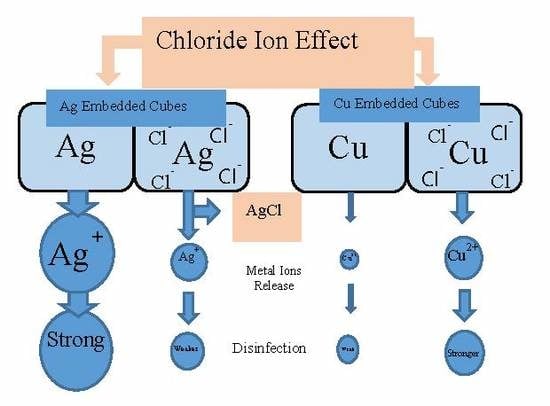
Effect of Chloride Ions on the Point-of-Use Drinking Water Disinfection Performance of Porous Ceramic Media Embedded with Metallic Silver and Copper
Abstract
:1. Introduction
2. Materials and Methods
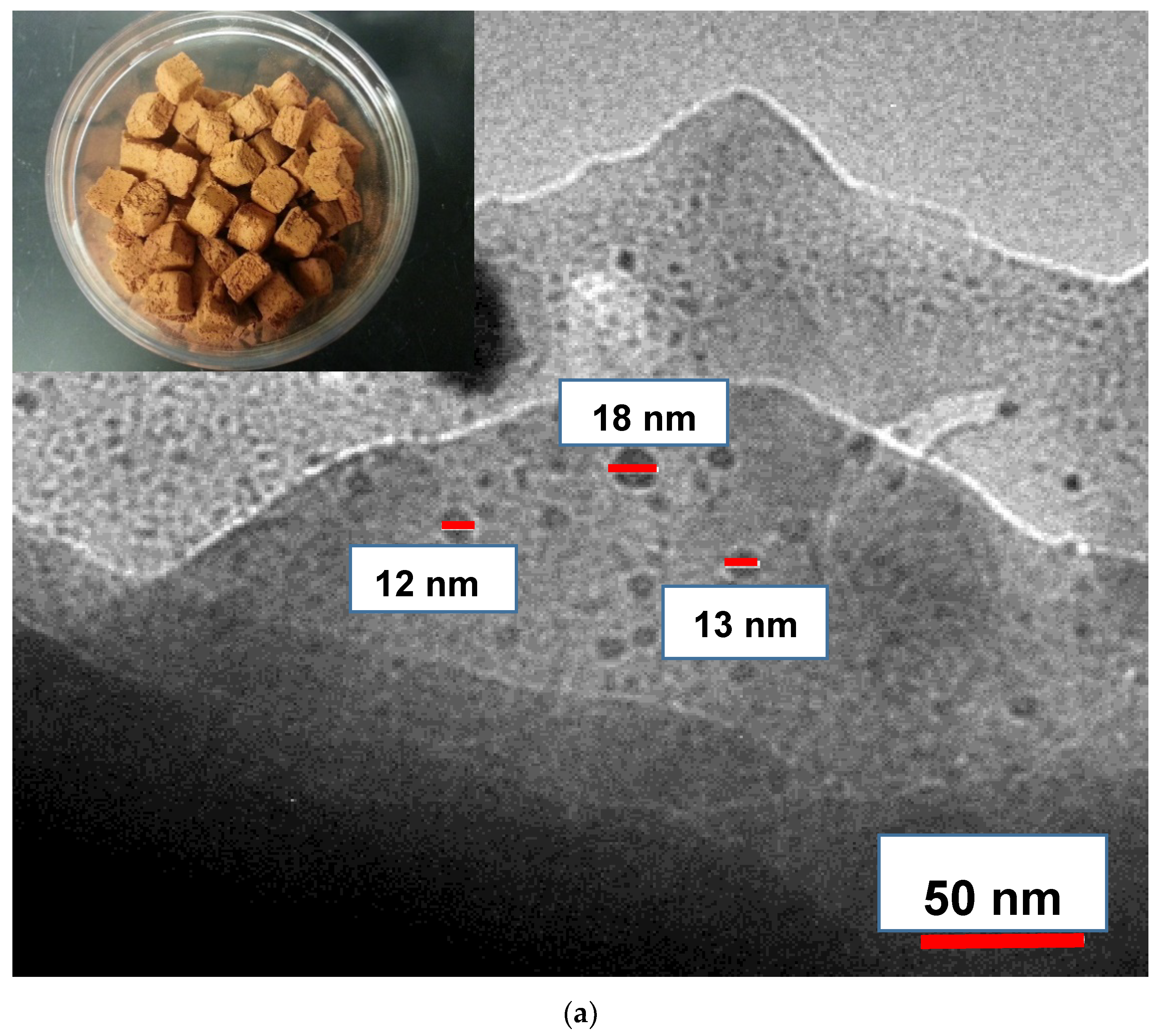
2.1. Silver and Copper Ceramic Cubes
2.2. Experimental Methods
2.2.1. Batch Experiment—E. coli Disinfection by Silver and Copper Ions
2.2.2. Metals Release and E. coli Disinfection Experiments
2.3. Analytical Methods
2.3.1. Characterization of Silver
2.3.2. Silver and Copper Analysis
2.3.3. E. coli Measurement
3. Results and Discussion
3.1. Characterization Study
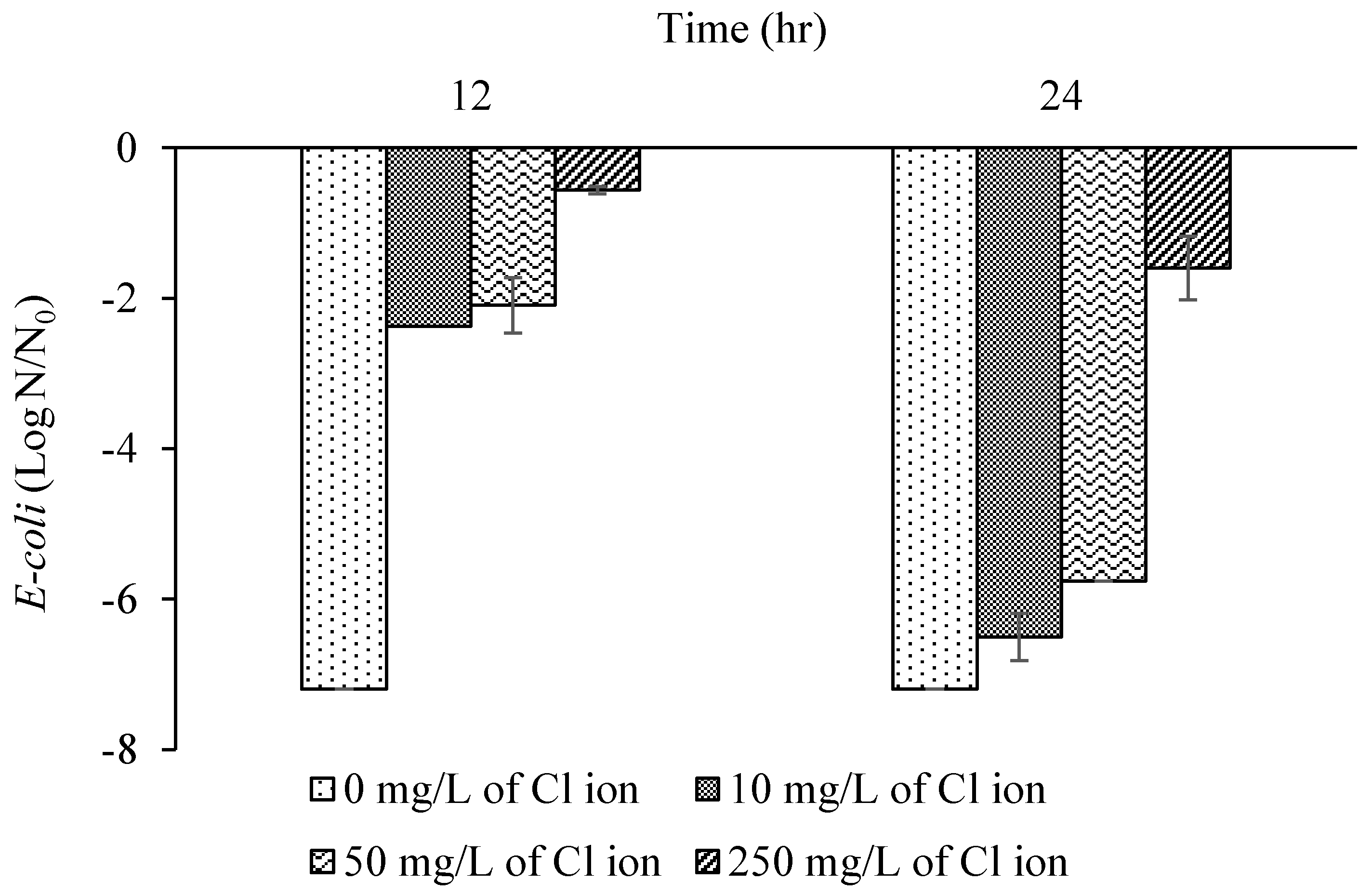
3.2. Batch Experiments
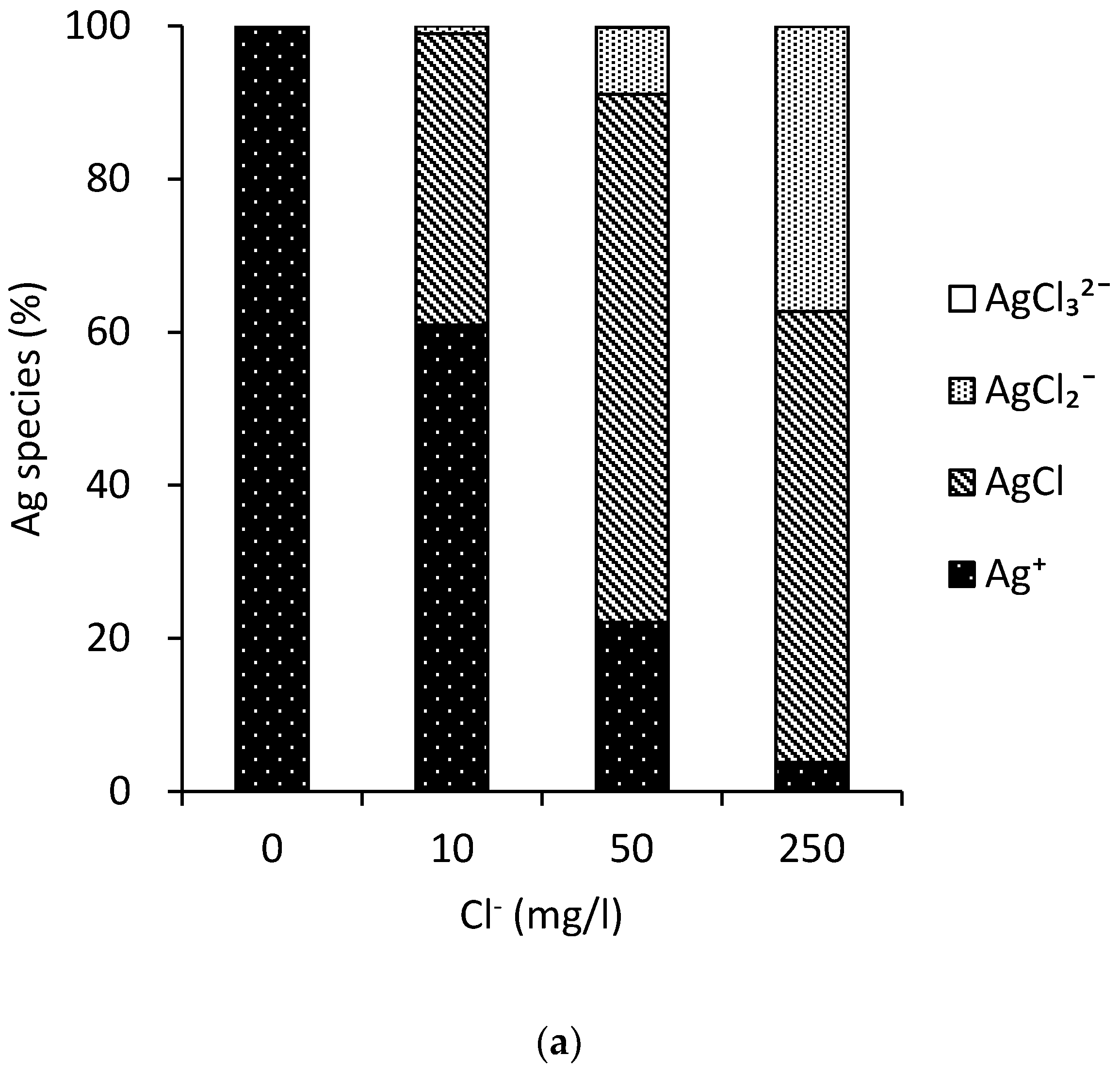
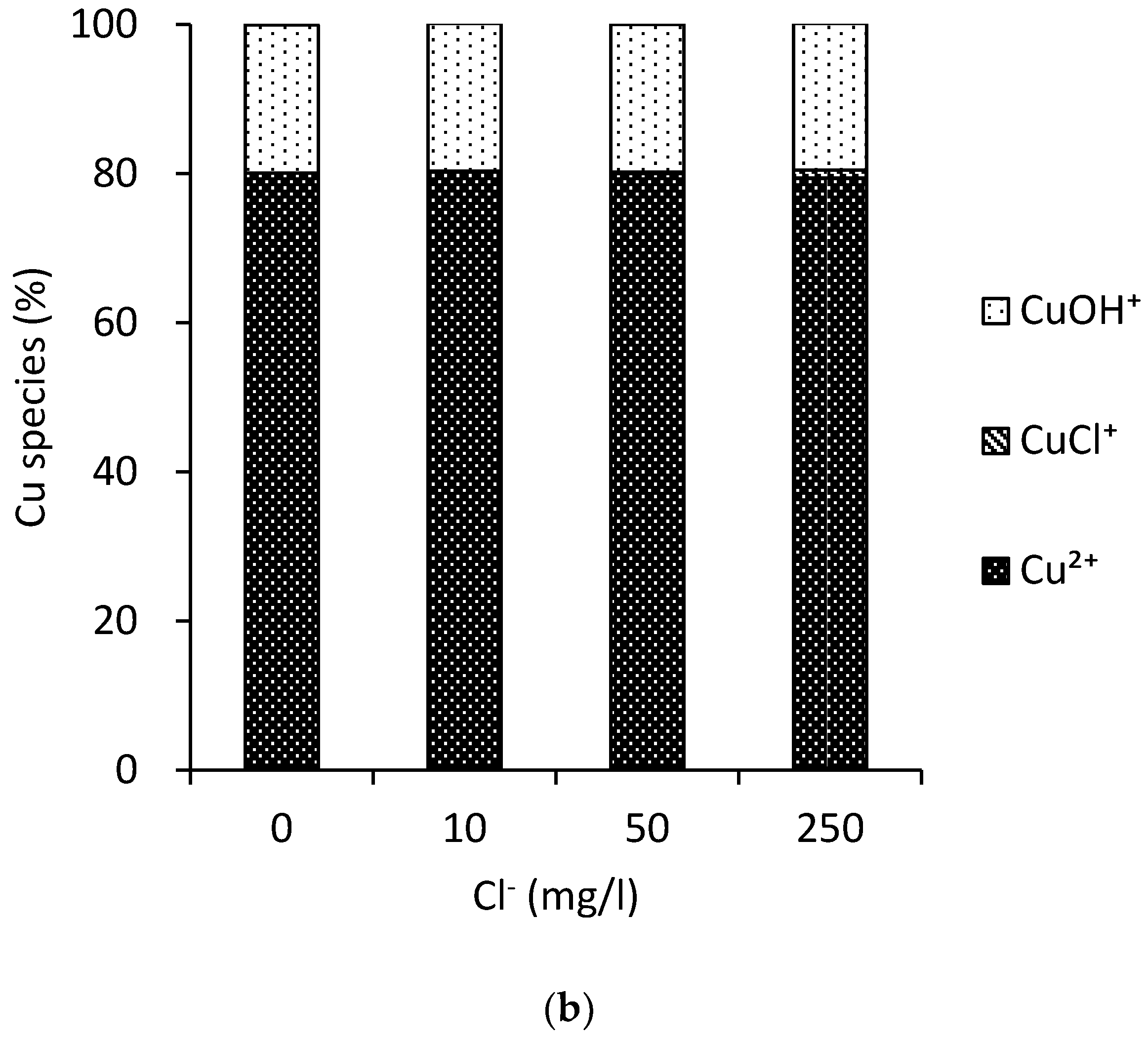
3.3. MINTEQ Model
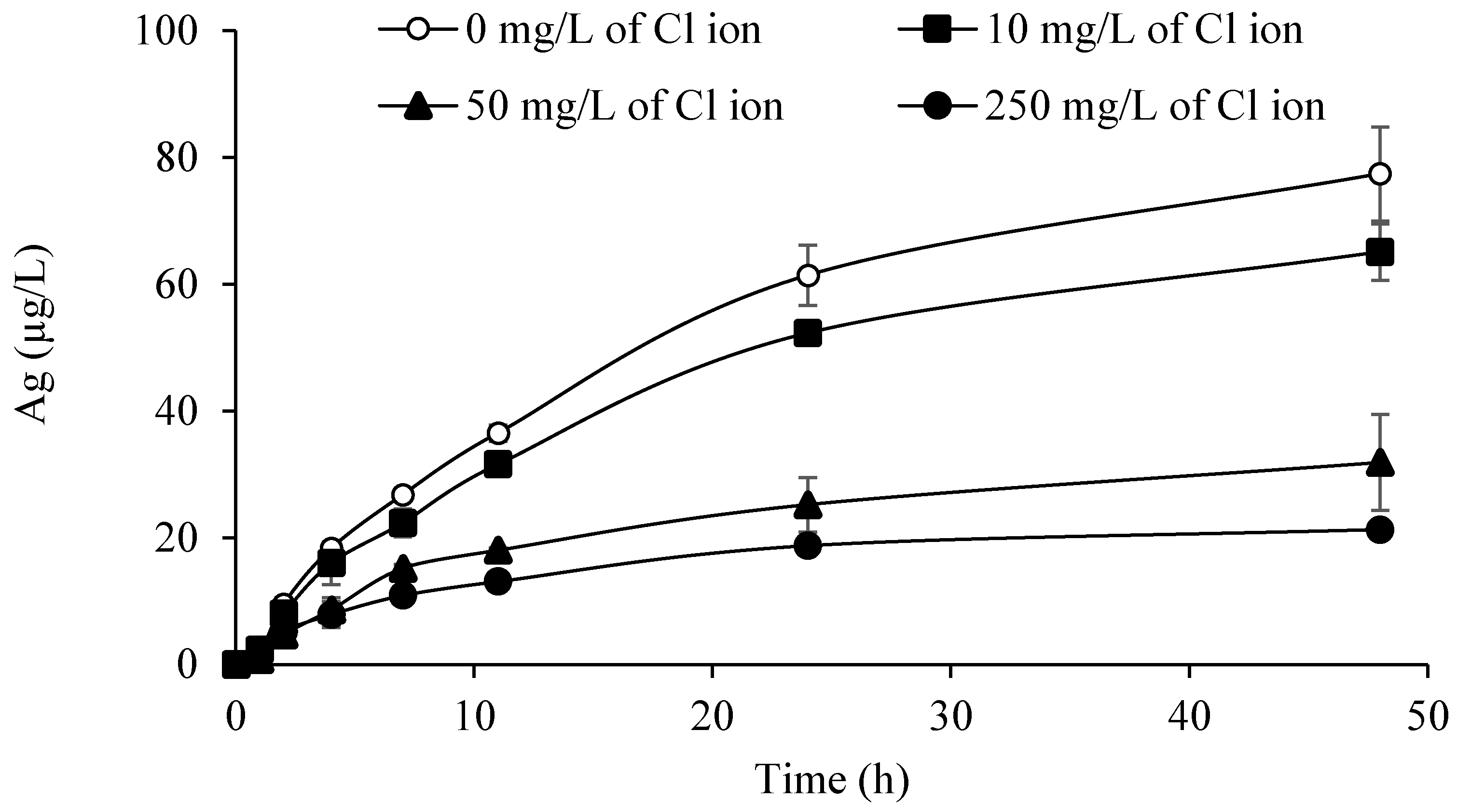
3.4. Silver Trend Analytical Results
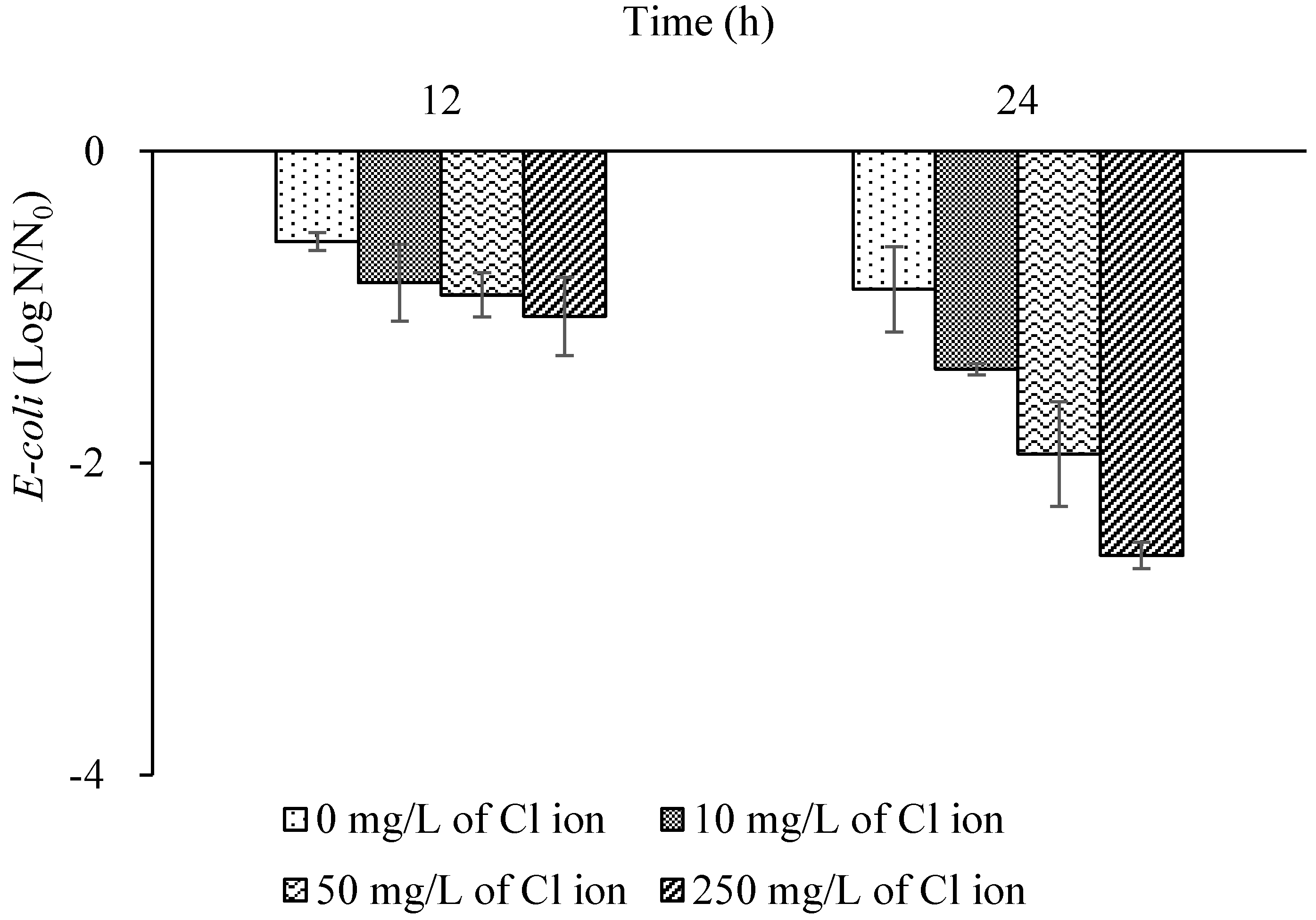
3.5. Copper Disinfection Trend
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamichhane, S.; Kansakar, B.R. Comparison of the Performance of Ceramic Filters in Drinking Water Treatment. Int. J. Eng. Innov. Technol. 2013, 3, 481–484. [Google Scholar]
- Mellor, J.E.; Kallman, E.; Oyanedel-Craver, V.; Smith, J.A. Comparison of Three Household Water Treatment Technologies in San Mateo Ixtatán, Guatemala. J. Environ. Eng. ASCE 2015, 14, 04014085. [Google Scholar] [CrossRef]
- Jackson, K.N.; Smith, J.A. A New Method for the Deposition of Metallic Silver on Porous Ceramic Water Filters. J. Nanotechnol. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Kallman, E.N.; Oyanedel-Craver, V.A.; Smith, J.A. Ceramic Filters Impregnated with Silver Nanoparticles for Point-of-Use Water Treatment in Rural Guatemala. J. Environ. Eng. ASCE 2011, 137, 407–415. [Google Scholar] [CrossRef]
- Bielefeldt, A.R.; Stewart, M.W.; Mansfield, E.; Summers, R.S.; Ryan, J.N. Effects of chlorine and other water quality parameters on the release of silver nanoparticles from a ceramic surface. Water Res. 2013, 47, 4032–4039. [Google Scholar] [CrossRef]
- Ehdaie, B.; Krause, C.; Smith, J.A. Porous Ceramic Tablet Embedded with Silver Nanopatches for Low-Cost Point-of-Use Water Purification. Environ. Sci. Technol. 2014, 48, 13901–13908. [Google Scholar] [CrossRef]
- Kahler, D.M.; Koermer, N.T.; Reichl, A.R.; Samie, A.; Smith, J.A. Performance and Acceptance of Novel Silver-Impregnated Ceramic Cubes for Drinking Water Treatment in Two Field Sites: Limpopo Province, South Africa and Dodoma Region, Tanzania. Water 2016, 8, 95. [Google Scholar] [CrossRef]
- Ehdaie, B.; Rento, C.T.; Son, V.; Turner, S.S.; Samie, A.; Dillingham, R.A.; Smith, J.A. Evaluation of a Silver-Embedded Ceramic Tablet as a Primary and Secondary Point-of-Use Water Purification Technology in Limpopo Province, S. Africa. PLoS ONE 2017, 12, e0169502. [Google Scholar] [CrossRef]
- Jackson, K.N.; Smith, J.A.; Edokpayi, J.N. New Method for the Deposition of Metallic Silver and Metallic Copper on Full-Size Porous Ceramic Water Filters. Environ. Eng. Sci. 2019, 36, 2–11. [Google Scholar] [CrossRef]
- Swathy, J.R.; Sankar, M.U.; Chaudhary, A.; Anshup, S.A.; Pradeep, T. Antimicrobial silver: An unprecedented anion effect. Sci. Rep. Nat. 2014, 4, 7161. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.P.; Gopal, K. Evaluation of bactericidal efficacy of silver ions on Escherichia coli for drinking water disinfection. Environ. Sci. Pollut. Res. 2012, 19, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Halem, D.; Laan, H.; Heijman, S.G.J.; Dijk, J.C.; Amy, G.L. Assessing the sustainability of the silver-impregnated ceramic pot filter for low-cost household drinking water treatment. Phys. Chem. Earth 2009, 34, 36–42. [Google Scholar] [CrossRef]
- Singh, R.; Rento, C.; Son, V.; Turner, S.; Smith, J.A. Optimization of Silver Ion Release from Silver-Ceramic Porous Media for Household Level Water Purification. Water 2019, 11, 816. [Google Scholar] [CrossRef] [Green Version]
- Olivares, M.; Uauy, R. Copper as an essential nutrient. Am. J. Clin. Nutr. 1996, 63, 791–796. [Google Scholar] [CrossRef]
- Chen, N.H.; Chung, C.J.; Chiang, C.C.; Chen, K.C.; He, J.L. Antimicrobial and decorative ion-plated copper-containing ceramic coatings. Surf. Coat. Technol. 2013, 236, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Palza, H.; Quijada, R.; Delgado, K. Antimicrobial polymer composites with copper micro- and nanoparticles: Effect of particle size and polymer matrix. J. Bioact. Compat. Polym. 2015, 30, 366–380. [Google Scholar] [CrossRef]
- Drelich, A.J.; Miller, J.; Donofrio, R.W.; Drelich, J.W. Novel Durable Antimicrobial Ceramic with Embedded Copper Sub-Microparticles for a Steady-State Release of Copper Ions. Materials 2017, 10, 775. [Google Scholar] [CrossRef] [Green Version]
- Dankovich, T.A.; Smith, J.A. Incorporation of copper nanoparticles into paper for point-of-use water purification. Water Res. 2014, 63, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Singh, R.; Edokpayi, J.N.; Odiyo, J.O.; Smith, J.A. E. coli Inactivation by Metals and Effects of Changes in Water Chemistry. J. Environ. Eng. 2019, 145, 040181. [Google Scholar] [CrossRef]
- Levard, C.; Mitra, S.; Yang, T.; Jew, A.D.; Badireddy, A.R.; Lowry, G.V.; Brown, G.E., Jr. Effect of Chloride on the Dissolution Rate of Silver Nanoparticles and Toxicity to E. coli. Environ. Sci. Technol. 2013, 47, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Mittelman, A.M.; Lantagne, D.S.; Rayner, J.; Pennell, K.D. Silver Dissolution and Release from Ceramic Water Filters. Environ. Sci. Technol. 2015, 49, 8515–8522. [Google Scholar] [CrossRef] [PubMed]
- Hanim, S.; Malek, N.; Ibrahim, Z. Analyses of surface area, porosity, silver release and antibacterial activity of amine-functionalized, silver-exchanged zeolite NaY. Vacuum 2017, 143, 344–347. [Google Scholar] [CrossRef]
- González, D.M.; Santana, C.J.M.; González, A.G.; Pérez, N.; Millero, F.J. Oxidation of copper(I) in seawater at nanomolar levels. Mar. Chem. 2009, 115, 118–124. [Google Scholar] [CrossRef]
- Arjmand, F.; Adriaens, A. Influence of pH and Chloride Concentration on the Corrosion Behavior of Unalloyed Copper in NaCl Solution: A Comparative Study between the Micro and Macro Scales. Materials 2012, 5, 2439–2464. [Google Scholar] [CrossRef] [Green Version]
- El Warraky, A.; El Shayeb, H.; Sherif, E. Pitting corrosion of copper in chloride solutions. Anti Corros. Methods Mater. 2004, 51, 52–61. [Google Scholar] [CrossRef]
- World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illness with Limited Resources; World Health Organization: Geneva, Switzerland, 2005; Available online: http://www.who.int/child adolescent_health/documents_9241546700/en/index.html (accessed on 26 March 2020).
- Sobsey, M.D.; Stauber, C.E.; Casanova, L.M.; Brown, J.M.; Elliott, M.A. Point of use household drinking water filtration: A practical effective solution for providing sustained access to safe drinking water in the developing world. Environ. Sci. Technol. 2008, 42, 4261–4267. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Lisa, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterial for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial Food Animal Production, Antimicrobial Resistance, and Human Health. Ann. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in Gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Erickson, R.J.; Brooke, L.T.; Kahl, M.D.; Venter, F.V.; Harting, S.L.; Markee, T.P.; Spehar, R.L. Effects of laboratory test conditions on the toxicity of silver to aquatic organisms. Environ. Toxicol. Chem. 1998, 17, 572–578. [Google Scholar] [CrossRef]
- Fabrega, J.; Fawcett, S.R.C.; Renshaw, J.C.; Lead, J.R. Silver Nanoparticle Impact on Bacterial Growth: Effect of pH, Concentration, and Organic Matter. Environ. Sci. Technol. 2009, 43, 7285–7290. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Kim, W.; Smith, J.A. Effect of Chloride Ions on the Point-of-Use Drinking Water Disinfection Performance of Porous Ceramic Media Embedded with Metallic Silver and Copper. Water 2020, 12, 1625. https://doi.org/10.3390/w12061625
Singh R, Kim W, Smith JA. Effect of Chloride Ions on the Point-of-Use Drinking Water Disinfection Performance of Porous Ceramic Media Embedded with Metallic Silver and Copper. Water. 2020; 12(6):1625. https://doi.org/10.3390/w12061625
Chicago/Turabian StyleSingh, Rekha, Woohang Kim, and James A. Smith. 2020. "Effect of Chloride Ions on the Point-of-Use Drinking Water Disinfection Performance of Porous Ceramic Media Embedded with Metallic Silver and Copper" Water 12, no. 6: 1625. https://doi.org/10.3390/w12061625