Understanding the Ecological Response of Planktic and Benthic Epipelic Algae to Environmental Factors in an Urban Rivers System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling of Planktic Algae, Epipelic Algae, and Microscopic Examination
2.3. Sampling and Analysis of Water Samples
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Water Quality
3.2. Characteristics of Algae Community
3.2.1. Species, Abundance, and Biomass of Planktic and Epipelic Algae
3.2.2. Characteristics of Algae Community and Distribution of Planktic and Epipelic Algae
3.2.3. Seasonal Changes of Planktic and Epipelic Algae
3.3. The Correlations between the Pollution Characteristics and Algae Distribution
3.4. Multivariate Stepwise Regression Analysis (MSRA)
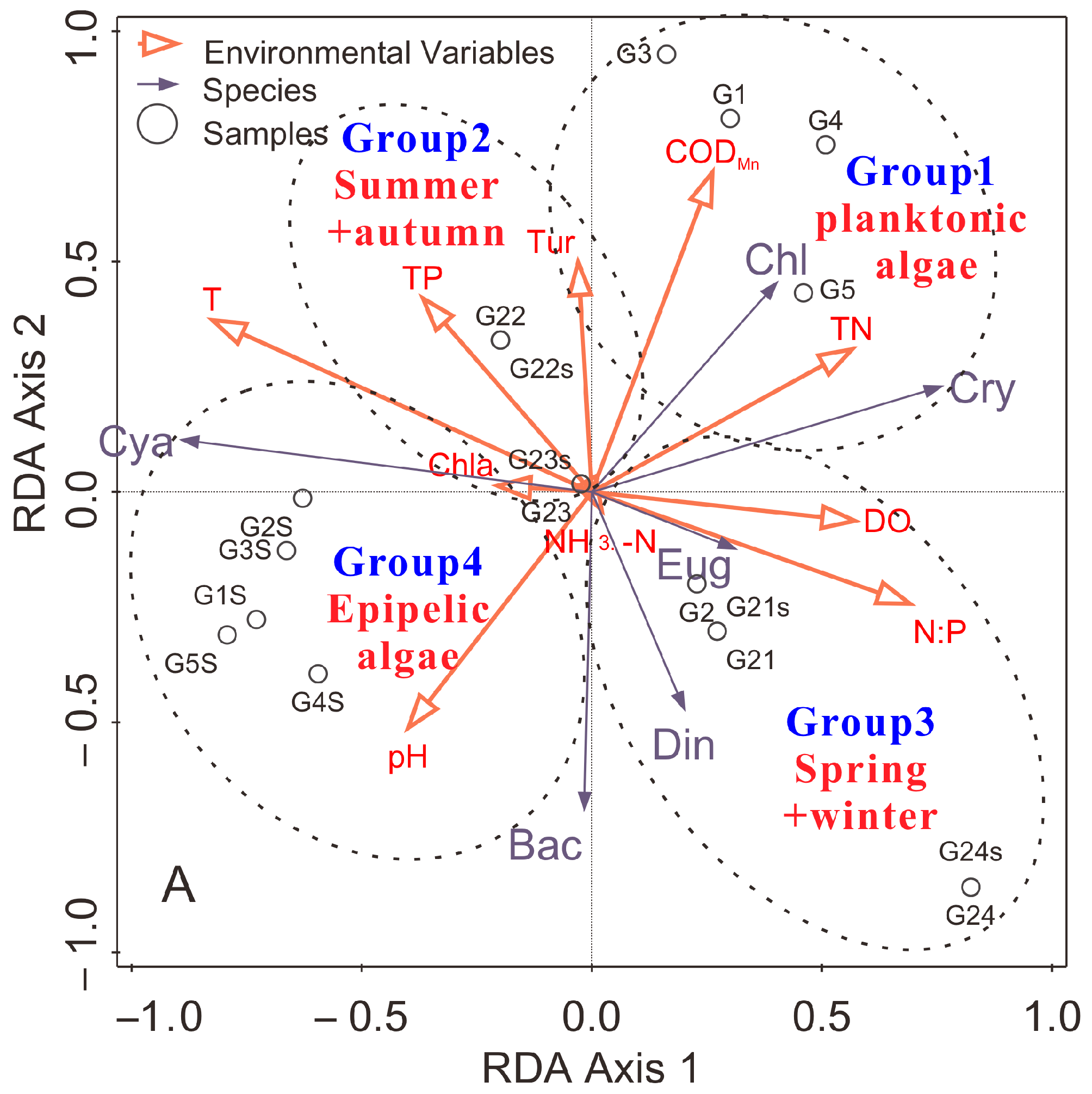
3.5. Redundancy Analysis (RDA) of Algae and Environmental Factors
3.6. Identification of Environmental Factors Affecting the Distribution of Algae
3.7. Understanding the Relation between River Pollution and Algae Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nunes, M.; Adams, J.B.; Bate, G.C.; Bornman, T.G. Abiotic characteristics and microalgal dynamics in South Africa’s largest estuarine lake during a wet to dry transitional phase. Estuar. Coast. Shelf Sci. 2017, 198, 236–248. [Google Scholar] [CrossRef]
- Nazeer, S.; Khan, M.U.; Malik, R.N. Phytoplankton Spatio-temporal dynamics and its relation to nutrients and water retention time in multi-trophic system of Soan River, Pakistan. Environ. Technol. Innov. 2018, 9, 38–50. [Google Scholar] [CrossRef]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.-H.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, L.; Wu, C.; Chen, Y.; Xu, H.; Chen, C.; Lin, G. Inter-annual and seasonal variations of phytoplankton community and its relation to water pollution in Futian Mangrove of Shenzhen, China. Cont. Shelf Res. 2018, 166, 138–147. [Google Scholar] [CrossRef]
- Çelekli, A.; Kap, E.; Soysal, Ç.; Arslanargun, H.; Bozkurt, H. Evaluating biochemical response of filamentous algae integrated with different water bodies. Ecotox. Environ. Safe. 2017, 142, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Abboud-Abi Saab, M.; Hassoun, A.E.R. Effects of organic pollution on environmental conditions and the phytoplankton community in the central Lebanese coastal waters with special attention to toxic algae. Reg. Stud. Mar. Sci. 2017, 10, 38–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Yang, Z. Investigation on Water Pollution by Algae at Locations of Water Collection in Chaohu Lake. J. Environ. Health 2002, 19, 316–318. [Google Scholar]
- Skuras, D.; Tyllianakis, E. The perception of water related risks and the state of the water environment in the European Union. Water Res. 2018, 143, 198–208. [Google Scholar] [CrossRef]
- Salem, Z.; Ghobara, M.; El Nahrawy, A.A. Spatio-temporal evaluation of the surface water quality in the middle Nile Delta using Palmer’s algal pollution index. Egypt. J. Basic Appl. Sci. 2017, 4, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Al-Saadi, H.A.; Kassim, T.I.; Al-Lami, A.A.; Salman, S.K. Spatial and seasonal variations of phytoplankton populations in the upper region of the Euphrates River, Iraq. Limnol.Ecol. Manag. Inland Waters 2000, 30, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Cerco, C.F.; Seitzinger, S.P. Measured and modeled effects of benthic algae on eutrophication in Indian River—Rehoboth Bay, Delaware. Estuaries 1997, 20, 231–248. [Google Scholar] [CrossRef]
- Kies, L. Distribution, biomass and production of planktonic and benthic algae in the Elbe estuary. Oceanogr. Lit. Rev. 1997, 11, 1328. [Google Scholar]
- Light, B.R.; Beardall, J. Distribution and spatial variation of benthic microalgal biomass in a temperate, shallow-water marine system. Aquat. Bot. 1998, 61, 39–54. [Google Scholar] [CrossRef]
- Medvedeva, L.A. Biodiversity of aquatic algal communities in the Sikhote-Alin biosphere reserve (Russia): Biodiversité des communautés algales de la réserve de la biosphère Sikhote-Alin (Russie). Cryptogam. Algol. 2001, 22, 65–100. [Google Scholar] [CrossRef]
- Dalu, T.; Wasserman, R.J. Cyanobacteria dynamics in a small tropical reservoir: Understanding spatio-temporal variability and influence of environmental variables. Sci. Total. Environ. 2018, 643, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C. What factors influence the species composition of phytoplankton in lakes of different trophic status? Hydrobiologia 1998, 369–370, 11–26. [Google Scholar] [CrossRef]
- Zhao, Z.; Mi, T.; Xia, L.; Yan, W.; Jiang, Y.; Gao, Y. Understanding the patterns and mechanisms of urban water ecosystem degradation: Phytoplankton community structure and water quality in the Qinhuai River, Nanjing City, China. Environ. Sci. Pollut. Res. 2013, 20, 5003–5012. [Google Scholar] [CrossRef]
- Winter, J.G.; Young, J.D.; Landre, A.; Stainsby, E.; Jarjanazi, H. Changes in phytoplankton community composition of Lake Simcoe from 1980 to 2007 and relationships with multiple stressors. J. Great Lakes Res. 2011, 37, 63–71. [Google Scholar] [CrossRef]
- Naselli-Flores, L. Phytoplankton assemblages in twenty-one Sicilian reservoirs: Relationships between species composition and environmental factors. Hydrobiologia 2000, 424, 1–11. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Barone, R. Phytoplankton dynamics in two reservoirs with different trophic state (Lake Rosamarina and Lake Arancio, Sicily, Italy). Hydrobiologia 1998, 369–370, 163–178. [Google Scholar] [CrossRef]
- Habib, O.A.; Tippett, R.; Murphy, K.J. Seasonal changes in phytoplankton community structure in relation to physico-chemical factors in Loch Lomond, Scotland. Hydrobiologia 1997, 350, 63–79. [Google Scholar] [CrossRef]
- Montoya, J.V.; Roelke, D.L.; Winemiller, K.O.; Cotner, J.B.; Snider, J.A. Hydrological seasonality and benthic algal biomass in a Neotropical floodplain river. J. North Am. Benthol. Soc. 2006, 25, 157–170. [Google Scholar] [CrossRef]
- Lu, N.; Yin, H.; Deng, J.; Gao, F.; Hu, W.; Gao, J. Spring community structure of phytoplankton from Lake Chaohu and its relationship to environmental factors. J. Lake Sci. 2010, 22, 950–956. [Google Scholar]
- Tian, W.; Zhang, H.; Zhao, L.; Huang, H. Responses of a phytoplankton community to seasonal and environmental changes in Lake Nansihu, China. Mar. Freshw. Res. 2017, 68, 1877–1886. [Google Scholar] [CrossRef]
- Liu, X.; Lu, X.; Chen, Y. The effects of temperature and nutrient ratios on Microcystis blooms in Lake Taihu, China: An 11-year investigation. Harmful Algae 2011, 10, 337–343. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, X.; Tang, H.; Song, J.; Zhou, J.; Liu, H.; Wang, Q. Seasonal variations in the phytoplankton community and the relationship between environmental factors of the sea around Xiaoheishan Island in China. Chin. J. Oceanol. Limnol. 2017, 35, 163–173. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Shen, H. Seasonal variations of phytoplankton community structure in relation to physico-chemical factors in Lake Baiyangdian, China. Procedia Environ. Sci. 2010, 2, 1622–1631. [Google Scholar] [CrossRef] [Green Version]
- Oukarroum, A.; Polchtchikov, S.; Perreault, F.; Popovic, R. Temperature influence on silver nanoparticles inhibitory effect on photosystem II photochemistry in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Environ. Sci. Pollut. Res. 2012, 19, 1755–1762. [Google Scholar] [CrossRef]
- Rochelle-Newall, E.J.; Chu, V.T.; Pringault, O.; Amouroux, D.; Arfi, R.; Bettarel, Y.; Bouvier, T.; Bouvier, C.; Got, P.; Nguyen, T.M.H.; et al. Phytoplankton distribution and productivity in a highly turbid, tropical coastal system (Bach Dang Estuary, Vietnam). Mar. Pollut. Bull. 2011, 62, 2317–2329. [Google Scholar] [CrossRef]
- Nayar, S.; Goh, B.P.L.; Chou, L.M. Environmental impact of heavy metals from dredged and resuspended sediments on phytoplankton and bacteria assessed in in situ mesocosms. Ecotox. Environ. Safe. 2004, 59, 349–369. [Google Scholar] [CrossRef]
- Tien, C.-J. Some aspects of water quality in a polluted lowland river in relation to the intracellular chemical levels in planktonic and epilithic diatoms. Water Res. 2004, 38, 1779–1790. [Google Scholar] [CrossRef]
- Shou, W.; Zong, H.; Ding, P.; Hou, L. A modelling approach to assess the effects of atmospheric nitrogen deposition on the marine ecosystem in the Bohai Sea, China. Estuar. Coast. Shelf Sci. 2018, 208, 36–48. [Google Scholar] [CrossRef]
- Rolland, A.; Bertrand, F.; Maumy, M.; Jacquet, S. Assessing phytoplankton structure and spatio-temporal dynamics in a freshwater ecosystem using a powerful multiway statistical analysis. Water Res. 2009, 43, 3155–3168. [Google Scholar] [CrossRef] [PubMed]
- Manual, H.C. Guidelines for monitoring of phytoplankton species composition, abundance and biomass. In Manuals and Guidelines; STATECON group of HELCOM, Ed.; HELCOM Combine Manual: Helsinki, Finland, 2006 with update to 24/01/2020; p. 22. Available online: https://helcom.fi/wp-content/uploads/2020/01/HELCOM-Guidelines-for-monitoring-of-phytoplankton-species-composition-abundance-and-biomass.pdf (accessed on 24 April 2020).
- UNESCO. Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; Karlson, B., Cusack, C., Bresna, E., Eds.; IOC Manuals and Guides No. 55; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2010. [Google Scholar]
- Brierley, B.; Carvalho, L.; Davies, S.; Krokowski, J. Guidance on the Quantitative Analysis of Phytoplankton in Freshwater Samples; report to sniffer (project wfd80); Water Framework Directive – United Kingdom Technical Advisory Group (WFD-UKTAG): Edinburgh, UK, 2007. [Google Scholar]
- Jin, Q.; Lyu, H.; Shi, L.; Miao, S.; Wu, Z.; Li, Y.; Wang, Q. Developing a two-step method for retrieving cyanobacteria abundance from inland eutrophic lakes using MERIS data. Ecol. Indic. 2017, 81, 543–554. [Google Scholar] [CrossRef]
- Yicheng, X.; Qijun, K. Study on the phytoplankton · in a large reservoir. Chin. J. Oceanol. Limnol. 1992, 10, 359–370. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef] [Green Version]
- Fetahi, T.; Schagerl, M.; Mengistou, S. Key drivers for phytoplankton composition and biomass in an Ethiopian highland lake. Limnologica 2014, 46, 77–83. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.-D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- LeGresley, M.; McDermott, G. Counting Chamber Methods for Quantitative Phytoplankton Analysis: Haemocytometer, Palmer-Maloney Cell and Sedgewick-Rafter Cell. In Microscopic and Molecular Methods for Quantitative Phytoplankton Analysis; Karlson, B., Godhe, A., Cusack, C., Bresnan, E., Eds.; Manuals and Guides 55; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2010; Volume 55, pp. 25–30. [Google Scholar]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. In Baltic Sea Environment Proceedings No. 106; Baltic Marine Environment Protection Commission–Helsinki Commission: Helsinki, Finland, 2006; Volume 106, p. 144. [Google Scholar]
- Napiórkowska-Krzebietke, A.; Kobos, J. Assessment of the cell biovolume of phytoplankton widespread in coastal and inland water bodies. Water Res. 2016, 104, 532–546. [Google Scholar] [CrossRef]
- Baker, P.D.; Fabbro, L.D. A Guide to the Identification of Common Blue-Green Algae (Cyanoprokaryotes) in Australian Freshwaters; Albury, N.S.W., Ed.; Cooperative Research Centre for Freshwater Ecology: Thurgoona, NSW, Australia, 2002. [Google Scholar]
- Botes, L. Phytoplankton Identification Catalogue: Saldanha Bay, April 2001. Programme Coordination Unit, Global Ballast Water Management Programme; International Maritime Organization, London: London, UK, 2003. [Google Scholar]
- Taylor, J.C.; Harding, W.R.; Archibald, C. An Illustrated Guide to Some Common Diatom Species from South Africa; Water Research Commission: Pretoria, South Africa, 2007. [Google Scholar]
- Dantas, Ê.W.; Bittencourt-Oliveira, M.d.C.; Moura, A.d.N. Dynamics of phytoplankton associations in three reservoirs in northeastern Brazil assessed using Reynolds’ theory. Limnologica 2012, 42, 72–80. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K.; Ettl, H.; Gärtner, G.; Heynig, H.D.M. Cyanoprokaryota: 1. Teil: Chroococcales. In Sußwasserflora Von Mitteleuropa 19/1; Ettl, H., Gärtner, G., Heynig, H., Eds.; Gustav Fischer: Berlin, Germany, 1999; Volume 1, p. 548. [Google Scholar]
- Komárek, J.; Anagnostidis, K.; Büdel, B.L.K.; Gärtner, G.M.S. Cyanoprokaryota: 2. Teil: Oscillatoriales. In Sußwasserflora Von Mitteleuropa 19/2; Büdel, B., Gärtner, G., Eds.; Elsevier Spektrum: Heidelberg, Germany, 2005; Volume 2, p. 759. [Google Scholar]
- Komárek, J. Cyanoprocaryota 3. Teil: Heterocytous Genera. In Sußwasserflora Von Mitteleuropa 19/3; Gustav Fischer: Berlin, Germany, 2013; Volume 3, p. 1130. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Fusheng, W. Determination Methods for Examination of Water and Wastewater, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Pápista, É.; Ács, É.; Böddi, B. Chlorophyll-a determination with ethanol—A critical test. Hydrobiologia 2002, 485, 191–198. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, Y.; Xia, L.; Mi, T.; Yan, W.; Gao, Y.; Jiang, X.; Fawundu, E.; Hussain, J. Application of canonical correspondence analysis to determine the ecological contribution of phytoplankton to PCBs bioaccumulation in Qinhuai River, Nanjing, China. Environ. Sci. Pollut. Res. 2014, 21, 3091–3103. [Google Scholar] [CrossRef] [PubMed]
- Ter Braak, C.; Smilauer, P. CANOCO Reference Manual and Cano Draw for Windows User s Guide:Software for Canonical Community Ordination, (version 4.5); Microcomputer Power: New York, NY, USA, 2002. [Google Scholar]
- Angers, B.; Magnan, P.; Plante, M.; Bernatchez, L. Canonical correspondence analysis for estimating spatial and environmental effects on microsatellite gene diversity in brook charr (Salvelinus fontinalis). Mol. Ecol. 1999, 8, 1043–1053. [Google Scholar] [CrossRef]
- Sierra, M.; Gomez, N. Structural Characteristics and Oxygen Consumption of the Epipelic Biofilm in Three Lowland Streams Exposed to Different Land Uses. WaterAir Soil Pollut. 2007, 186, 115–127. [Google Scholar] [CrossRef]
- Casamatta, D.A.; Hašler, P. Blue-Green Algae (Cyanobacteria) in Rivers. In River Algae; Necchi, O., Jr., Ed.; Springer: Cham, Switzerland, 2016; pp. 5–34. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Jin, X.; Xu, J.; Zhang, H.; Yu, J.; Sun, Q.; Gao, C.; Wang, L. Analysis of algae growth mechanism and water bloom prediction under the effect of multi-affecting factor. Saudi J. Biol. Sci. 2017, 24, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Liess, A.; Piggott, J.J.; Townsend, C.R.; Matthaei, C.D. Light, nutrients and grazing interact to determine stream diatom community composition and functional group structure. Freshwat. Biol. 2011, 56, 264–278. [Google Scholar] [CrossRef]
- Hou, W.; Dong, H.; Wang, S.; Jiang, H.; Wu, G.; Yang, J.; Li, G. Distribution and diversity of cyanobacteria and eukaryotic algae in Qinghai–Tibetan lakes. Geomicrobiol. J. 2016, 33, 860–869. [Google Scholar] [CrossRef]
- Solari, L.C.; Claps, M.C. Planktonic and benthic algae of a pampean river (Argentina): Comparative analysis. Ann. Limnol. Int. J. Lim. 1996, 32, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Geider, R.J.; Maclntyre, H.L.; Kana, T.M. A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol. Oceanogr. 1998, 43, 679–694. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H.; Lohman, K. Nitrogen and phosphorus relationships to benthic algal biomass in temperate streams. Can. J. Fish. Aquat. Sci. 2002, 59, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Jiang, H.; Liu, W.; Wang, B. Benthic Algal Community Structures and Their Response to Geographic Distance and Environmental Variables in the Qinghai-Tibetan Lakes With Different Salinity. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potapova, M.G.; Charles, D.F. Benthic diatoms in USA rivers: Distributions along spatial and environmental gradients. J. Biogeogr. 2002, 29, 167–187. [Google Scholar] [CrossRef]
- Sommer, U. Nutrient status and nutrient competition of phytoplankton in a shallow, hypertrophic lake. Limnol. Oceanogr. 1989, 34, 1162–1173. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Kilham, S.S.; Kilham, P. Phytoplankton Community Ecology: The Role of Limiting Nutrients. Annu. Rev. Ecol. Syst. 1982, 13, 349–372. [Google Scholar] [CrossRef]
- Riegman, R. Nutrient-related selection mechanisms in marine phytoplankton communities and the impact of eutrophication on the planktonic food web. Water Sci. Technol. 1995, 32, 63–75. [Google Scholar] [CrossRef]
- Hernández Fariñas, T.; Ribeiro, L.; Soudant, D.; Belin, C.; Bacher, C.; Lampert, L.; Barillé, L. Contribution of benthic microalgae to the temporal variation in phytoplankton assemblages in a macrotidal system. J. Phycol. 2017, 53, 1020–1034. [Google Scholar] [CrossRef]
- Cantonati, M.; Lowe, R.L. Lake benthic algae: Toward an understanding of their ecology. Freshw. Sci. 2014, 33, 475–486. [Google Scholar] [CrossRef]
- Cathy, H.L.; Clare, B.; Patrick, M.H. Benthic-pelagic exchange of microalgae at a tidal flat. 2. Taxonomic analysis. Mar. Ecol. Prog. Ser. 2001, 212, 39–52. [Google Scholar]
- Abrantes, N.; Antunes, S.C.; Pereira, M.J.; Goncalves, F. Seasonal succession of cladocerans and phytoplankton and their interactions in a shallow eutrophic lake (Lake Vela, Portugal). Acta Oecologica 2006, 29, 54–64. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, B.; Teubner, K.; Dokulil, M.T. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J. Plankton Res. 2003, 25, 445–453. [Google Scholar] [CrossRef]
- Poulíčková, A.; Hašler, P.; Lysáková, M.; Spears, B. The ecology of freshwater epipelic algae: An update. Phycologia 2008, 47, 437–450. [Google Scholar] [CrossRef] [Green Version]
- McGregor, G.; Marshall, J.; Thoms, M. Spatial and temporal variation in algal-assemblage structure in isolated dryland river waterholes, Cooper Creek and Warrego River, Australia. Mar. Freshw. Res 2006, 57, 453–466. [Google Scholar] [CrossRef]
- Moore, J.W. Attached and planktonic algal communities in some inshore areas of Great Bear Lake. Can. J. Bot. 1980, 58, 2294–2308. [Google Scholar] [CrossRef]
- Feng, H.; Li, W.; Yang, Z.; Ruan, X.; Xing, Y. Characteristics of adsorption and desorption of phosphate by sediments in the Suzhou channels, East China. Earth Sci. Front. 2006, 31, 113–118. [Google Scholar]
- Jiang, X.; Ruan, X.; Xing, Y.; Zhao, Z. Effects of nutrient concentration and DO status of heavily polluted urban stream water on nitrogen release from sediment. Environ. Sci. 2007, 28, 87–91. [Google Scholar]


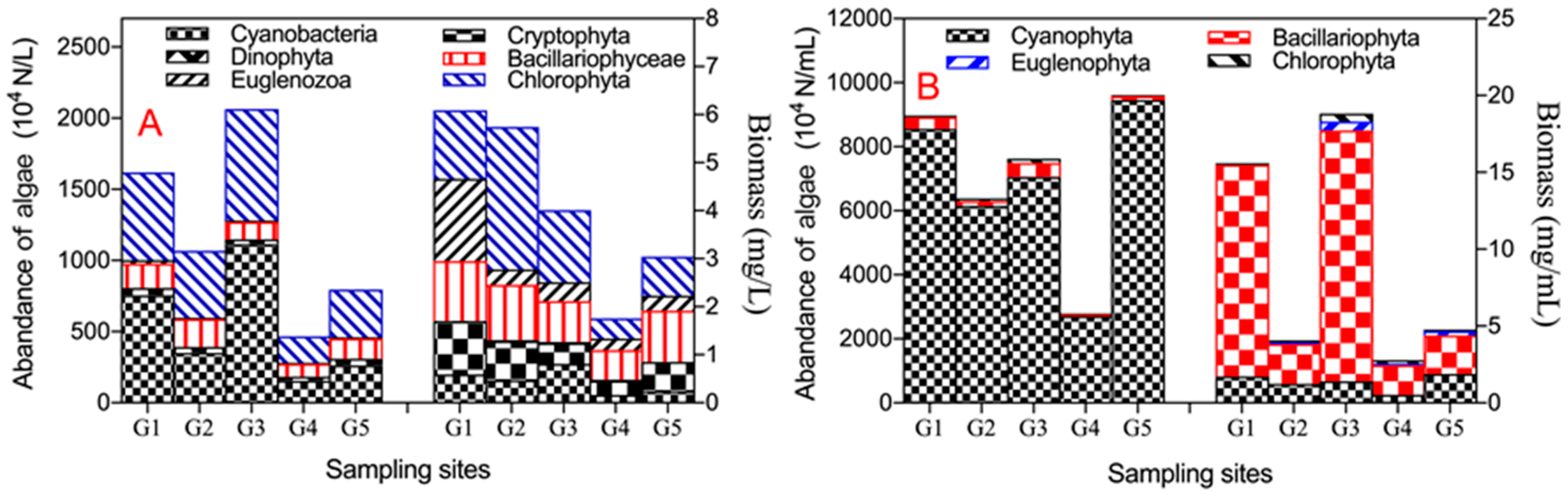


| Algae | Spring | Summer | Autumn | Winter | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A 1) | B 2) | A 1) | B 2) | A 1) | B 2) | A 1) | B 2) | A 1) | B 2) | ||
| Cyanobacteria | Anagnostidinema amphibium | 167 | 0.012 | 6580 | 0.461 | 567 | 0.004 | 0 | 0 | 1829 | 0.128 |
| Spirulina platensis | 0 | 0 | 767 | 0.153 | 417 | 0.083 | 0 | 0 | 296 | 0.059 | |
| Sum | 1530 | 0.060 | 10166 | 0.743 | 2966 | 0.227 | 117 | 0.004 | 3695 | 0.259 | |
| Cryptophyta | Cryptomonas ovata | 517 | 2.067 | 183 | 0.733 | 350 | 1.400 | 67 | 0.267 | 279 | 1.072 |
| Chroomonas acuta | 727 | 0.073 | 200 | 0.020 | 150 | 0.015 | 100 | 0.010 | 294 | 0.029 | |
| Sum | 1243 | 1.959 | 383 | 0.753 | 500 | 1.415 | 167 | 0.277 | 573 | 1.101 | |
| Dinophyta | Gymnodinium aeruginosum | 0 | 0 | 17 | 0.050 | 16.7 | 0.050 | 0 | 0 | 8.3 | 0.005 |
| Sum | 0 | 0 | 17 | 0.050 | 16.7 | 0.050 | 0 | 0 | 8.3 | 0.025 | |
| Bacillario-phyceae | Cyclotella meneghiniana | 1193 | 0.931 | 500 | 0.393 | 917 | 0.715 | 697 | 0.546 | 828 | 0.646 |
| Stephanodiscus hantzschii | 680 | 1.363 | 1330 | 0.267 | 350 | 0.700 | 33 | 0.067 | 299 | 0.599 | |
| Aulacoseira granulata | 67 | 0.022 | 2617 | 0.864 | 850 | 0.281 | 167 | 0.055 | 925 | 0.305 | |
| Sum | 2110 | 2.688 | 3283 | 1.590 | 2183 | 1.836 | 983 | 0.851 | 2140 | 1.741 | |
| Euglenozoa | Euglena viridis | 67 | 0.467 | 50 | 0.350 | 83 | 0.817 | 0 | 0 | 58 | 0.408 |
| Sum | 573 | 1.520 | 67 | 0.417 | 166.7 | 0.950 | 33 | 0.067 | 210 | 0.738 | |
| Chlorophyta | Pandorina morum | 0 | 0 | 2667 | 4.000 | 200 | 0.300 | 0 | 0 | 717 | 1.075 |
| Oocystis lacustris | 633 | 0.317 | 467 | 0.233 | 283 | 0.142 | 217 | 0.108 | 400 | 0.200 | |
| Scenedesmus sp. 3) | 1133 | 0.227 | 1700 | 0.340 | 667 | 0.133 | 333 | 0.067 | 960 | 0.192 | |
| Sum | 4637 | 1.686 | 9267 | 5.880 | 3400 | 0.770 | 1116 | 0.253 | 4605 | 2.147 | |
| Algae | Spring | Summer | Autumn | Winter | Average | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A 1) | B 2) | A 1) | B 2) | A 1) | B 2) | A 1) | B 2) | A 1) | B 2) | ||
| Cyano- bacteria | Anagnostidinema amphibium | 250 | 0.050 | 4209 | 0.838 | 1625 | 0.325 | 0 | 0 | 1659 | 0.331 |
| Sum | 406 | 0.096 | 5258 | 1.273 | 2700 | 0.541 | 1.4 | 0.0001 | 2091 | 0.478 | |
| Bacillario-phyceae | Cyclotella meneghiniana | 1.1 | 0.043 | 7.6 | 0.304 | 6.1 | 0.245 | 1.1 | 0.044 | 4.3 | 0.173 |
| Stephanodiscus hantzschii | 7.4 | 0.296 | 4.3 | 0.171 | 11.5 | 0.460 | 8.1 | 0.325 | 8.5 | 0.341 | |
| Aulacoseira granulata | 7.0 | 0.228 | 28.5 | 0.707 | 19.2 | 0.470 | 6.4 | 0.045 | 16.7 | 0.396 | |
| Navicula aitchelbee | 10.2 | 0.371 | 1.4 | 0.023 | 11.7 | 0.229 | 22.1 | 0.441 | 12.3 | 0.290 | |
| Fragilaria amphicephaloides | 4.6 | 0.457 | 0.7 | 0.066 | 0.5 | 0.050 | 1.8 | 0.177 | 2.1 | 0.205 | |
| Nitzschia palea | 7.6 | 0.303 | 1.5 | 0.044 | 1.0 | 0.030 | 10.2 | 0.803 | 5.5 | 0.322 | |
| Gomphonema parvulum | 27.0 | 0.540 | 0.6 | 0.011 | 0.6 | 0.011 | 46.6 | 0.931 | 20.4 | 0.407 | |
| Pinnularia microstauron | 1.7 | 0.167 | 1.6 | 0.245 | 0.5 | 0.050 | 2.8 | 0.283 | 1.8 | 0.203 | |
| Tabellaria fenestrata | 2.8 | 0.225 | 0.8 | 0.083 | 0.8 | 0.083 | 4.3 | 0.344 | 2.4 | 0.201 | |
| Sum | 84.5 | 2.887 | 52.5 | 1.791 | 85.9 | 2.664 | 140.5 | 4.152 | 90.8 | 2.874 | |
| Planktic Algae | DO | CODMn | NH3-N | TN | TP | Chl-a | T | Tur | pH | N:P | |
| Cya2) | P 1) | −0.06 | 0.42 | −0.11 | 0.45 | 0.58 | 0.33 | 0.61 *3) | 0.03 | −0.23 | −0.38 |
| K | 0.00 | 0.28 | −0.06 | 0.28 | 0.46 * | 0.28 | 0.44 * | 0.17 | −0.06 | −0.39 | |
| S | −0.08 | 0.33 | 0.02 | 0.40 | 0.61 * | 0.37 | 0.50 | 0.15 | −0.03 | −0.50 | |
| Cry | P | 0.55 | −0.01 | −0.01 | −0.38 | −0.36 | 0.59 * | 0.05 | −0.04 | −0.03 | 0.22 |
| K | 0.06 | −0.11 | −0.11 | −0.44 * | −0.29 | 0.22 | 0.06 | 0.00 | 0.11 | −0.11 | |
| S | 0.17 | −0.18 | −0.30 | −0.67 * | −0.39 | 0.33 | 0.13 | 0.03 | 0.15 | −0.10 | |
| Din | P | −0.01 | −0.43 | −0.76 ** | −0.33 | −0.22 | 0.15 | 0.74 * | −0.40 | 0.52 | 0.02 |
| K | −0.09 | −0.09 | −0.34 | 0.15 | 0.10 | 0.09 | 0.65 * | −0.28 | 0.46 * | −0.09 | |
| S | −0.13 | −0.20 | −0.45 | 0.17 | 0.10 | 0.14 | 0.78 ** | −0.30 | 0.57 | −0.13 | |
| Bac | P | 0.56 | −0.44 | −0.83 ** | −0.19 | −0.45 | 0.73 * | 0.77 ** | −0.46 | 0.29 | 0.40 |
| K | 0.33 | −0.39 | −0.61 * | −0.39 | −0.34 | 0.50 * | 0.44 * | −0.39 | 0.50 * | 0.28 | |
| S | 0.47 | −0.50 | −0.75 ** | −0.32 | −0.47 | 0.57 | 0.57 | −0.43 | 0.67 * | 0.30 | |
| Eug | P | 0.43 | 0.02 | 0.16 | −0.30 | −0.27 | 0.45 | −0.09 | −0.15 | −0.23 | 0.16 |
| K | 0.11 | 0.06 | −0.06 | −0.28 | −0.11 | 0.28 | 0.00 | −0.06 | 0.06 | −0.17 | |
| S | 0.15 | 0.08 | 0.02 | −0.33 | −0.13 | 0.35 | −0.02 | −0.02 | −0.02 | −0.30 | |
| Chl | P | 0.27 | 0.27 | −0.30 | 0.35 | 0.32 | 0.63 * | 0.708 * | −0.08 | −0.12 | −0.13 |
| K | 0.28 | 0.22 | 0.00 | 0.33 | 0.29 | 0.56 * | 0.28 | 0.11 | 0.00 | −0.11 | |
| S | 0.35 | 0.33 | −0.05 | 0.37 | 0.38 | 0.72 * | 0.33 | 0.13 | −0.03 | −0.22 | |
| Epipelic algae | DO | CODMn | NH3-N | TN | TP | Chl-a | T | Tur | pH | N:P | |
| Cya | P | −0.62 * | −.33 | −0.20 | −0.63 * | 0.25 | 0.27 | 0.70 * | 0.27 | 0.16 | −0.66 * |
| K | −0.47 * | −0.20 | −0.14 | −0.42 | 0.34 | 0.03 | 0.46 * | 0.42 | 0.15 | −0.42 | |
| S | −0.57 | −0.21 | −0.17 | −0.59 * | 0.49 | 0.05 | 0.55 | 0.47 | 0.20 | −0.64 * | |
| Bac | P | −0.47 | −0.50 | 0.03 | −0.60 * | 0.16 | −0.38 | 0.12 | 0.37 | 0.22 | −0.55 |
| K | −0.32 | −0.39 | 0.22 | −0.61 * | 0.20 | −0.50 * | −0.08 | 0.11 | 0.21 | −0.44 * | |
| S | −0.36 | −0.55 | 0.27 | −0.70 * | 0.28 | −0.63 * | −0.14 | 0.17 | 0.22 | −0.60 * | |
| Eug | P | −0.52 | −0.27 | −0.07 | −0.54 | 0.44 | −0.17 | 0.45 | 0.41 | 0.32 | −0.69 * |
| K | −0.24 | −0.15 | −0.09 | −0.32 | 0.29 | 0.03 | 0.29 | 0.32 | 0.13 | −0.49 * | |
| S | −0.33 | −0.19 | −0.17 | −0.46 | 0.39 | 0.07 | 0.41 | 0.35 | 0.16 | −0.63 * | |
| Chl | P | −0.49 | −0.39 | 0.00 | −0.62 * | 0.46 | −0.41 | 0.36 | 0.42 | 0.36 | −0.75 * |
| K | −0.32 | −0.28 | 0.00 | −0.28 | 0.48 * | −0.28 | 0.31 | 0.11 | 0.45 | −0.56 * | |
| S | −0.39 | −0.43 | −0.03 | −0.55 | 0.60 * | −0.43 | 0.44 | 0.17 | 0.57 | −0.75 ** | |
| DO | CODMn | NH3 | TN | TP | Chl-a | T | Tur | pH | N:P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Planktic algae | lgCya | −0.116 | 0.444 | −0.16 | 0.158 | 0.479 | 0.395 | 0.797 ** | 0.219 | −0.249 | −0.501 |
| lgCry | 0.409 | 0.052 | −0.101 | −0.411 | −0.254 | 0.596 * | 0.290 | 0.047 | −0.088 | 0.039 | |
| lgDin | −0.101 | −0.282 | −0.643 * | −0.244 | −0.056 | 0.096 | 0.731 * | −0.308 | 0.52 | −0.111 | |
| lgBac | 0.534 | −0.413 | −0.763 ** | −0.296 | −0.451 | 0.705 * | 0.723 * | −0.44 | 0.232 | 0.338 | |
| lgEug | 0.240 | 0.038 | 0.105 | −0.381 | −0.166 | 0.370 | 0.096 | −0.178 | −0.312 | −0.036 | |
| lgChl | 0.224 | 0.306 | −0.256 | 0.162 | 0.288 | 0.638 * | 0.730 * | 0.011 | −0.162 | −0.224 | |
| Epipelic algae | Cya | −0.621 * | −0.334 | −0.197 | −0.626 * | 0.249 | 0.272 | 0.703 * | 0.270 | 0.156 | −0.656 * |
| Bac | −0.470 | −0.503 | 0.035 | −0.603 * | 0.160 | −0.376 | 0.119 | 0.368 | 0.222 | −0.546 | |
| Eug | −0.522 | −0.272 | −0.075 | −0.540 | 0.438 | −0.167 | 0.451 | 0.408 | 0.321 | −0.685 * | |
| Chl | −0.487 | −0.388 | −0.002 | −0.620 * | 0.461 | −0.411 | 0.363 | 0.423 | 0.36 | −0.745 * | |
| Environ- mental factors | DO | 1 | |||||||||
| CODMn | −0.386 | 1 | |||||||||
| NH3 | −0.497 | 0.690 * | 1 | ||||||||
| TN | 0.021 | 0.572 | 0.277 | 1 | |||||||
| TP | −0.614 * | 0.906 ** | 0.629 * | 0.582 * | 1 | ||||||
| Chl−a | 0.840 ** | −0.023 | −0.486 | 0.180 | −0.262 | 1 | |||||
| T | 0.110 | 0.04 | −0.637 * | 0.055 | 0.079 | 0.536 | 1 | ||||
| Tur | −0.37 | 0.780 ** | 0.448 | 0.345 | 0.615 * | −0.089 | 0.047 | 1 | |||
| pH | 0.383 | −0.594 * | −0.650 * | −0.471 | −0.554 | 0.163 | 0.141 | −0.320 | 1 | ||
| N:P | 0.825 ** | −0.718 * | −0.553 | −0.045 | −0.827 ** | 0.470 | −0.116 | −0.570 | 0.414 | 1 |
| Dependent Variable | R | R2 | Adjusted R2 | Std. Error of Estimate | F | Sig. F | Durbin-Watson | |
|---|---|---|---|---|---|---|---|---|
| Planktic algae | lgCya a,1) | 0.915 a | 0.837 | 0.783 | 0.279 | 15.434 | 0.004 | 1.913 |
| lgDin b | 0.731 b | 0.534 | 0.468 | 0.374 | 8.034 | 0.025 | 1.302 | |
| lgBac c | 0.763 c | 0.582 | 0.522 | 0.123 | 9.737 | 0.017 | 1.429 | |
| lgChl d | 0.730 d | 0.533 | 0.466 | 0.213 | 7.986 | 0.026 | 1.932 | |
| Epipelic algae | Cyae | 0.861 e | 0.742 | 0.656 | 2009.850 | 8.615 | 0.017 | 1.986 |
| Bacf | 0.603 f | 0.363 | 0.273 | 121.639 | 3.997 | 0.046 | 2.099 | |
| Eugg | 0.685 g | 0.469 | 0.393 | 1.889 | 6.171 | 0.042 | 2.081 | |
| Chlh | 0.745 h | 0.555 | 0.492 | 31.962 | 8.741 | 0.021 | 2.102 |
| Dependent Variable | Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | Collinearity Statistics | |||
|---|---|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Tolerance | VIF | |||||
| Planktic algae | lgCya 1) | (Constant) | −1.639 | 1.209 | −1.356 | 0.224 | |||
| T | 0.133 | 0.024 | 1.168 | 5.470 | 0.002 | 0.595 | 1.681 | ||
| NH3-N | 0.345 | 0.126 | 0.583 | 2.732 | 0.034 | 0.595 | 1.681 | ||
| lgDin | (Constant) | −1.063 | 0.559 | −1.903 | 0.099 | ||||
| T | 0.071 | 0.025 | 0.731 | 2.834 | 0.025 | 1.000 | 1.000 | ||
| lgBac | (Constant) | 4.064 | 0.275 | 14.771 | 0.000 | ||||
| NH3-N | −0.134 | 0.043 | −0.763 | −3.120 | 0.017 | 1.000 | 1.000 | ||
| lgChl | (Constant) | 2.723 | 0.318 | 8.557 | 0.000 | ||||
| T | 0.041 | 0.014 | 0.730 | 2.826 | 0.026 | 1.000 | 1.000 | ||
| Epipelic algae | Cya | (Constant) | 4965.604 | 5195.977 | 0.956 | 0.376 | |||
| T | 343.227 | 120.476 | 0.603 | 2.849 | 0.029 | 0.961 | 1.040 | ||
| TN | −1137.081 | 473.557 | −0.508 | −2.401 | 0.053 | 0.961 | 1.040 | ||
| Bac | (Constant) | 600.277 | 220.626 | 2.721 | 0.030 | ||||
| TN | −56.186 | 28.103 | −0.603 | −1.999 | 0.086 | 1.000 | 1.000 | ||
| Eug | (Constant) | 8.040 | 2.236 | 3.595 | 0.009 | ||||
| N:P 2) | −0.173 | 0.070 | −0.685 | −2.484 | 0.042 | 1.000 | 1.000 | ||
| Chl | (Constant) | 149.144 | 37.844 | 3.941 | 0.006 | ||||
| N:P | −3.482 | 1.178 | −0.745 | −2.957 | 0.021 | 1.000 | 1.000 | ||
| Condition Index | Variance Proportions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Dimension | Eigenvalue | (Constant) | T | NH3-N | TN | N:P | ||
| planktic algae | lgCya | 1 | 2.938 | 1.000 | 0.00 | 0.00 | 0.00 | / | / |
| 2 | 0.059 | 7.079 | 0.00 | 0.29 | 0.09 | / | / | ||
| 3 | 0.004 | 27.667 | 1.00 | 0.70 | 0.91 | / | / | ||
| lgDin | 1 | 1.975 | 1.000 | 0.01 | 0.01 | / | / | / | |
| 2 | 0.025 | 8.837 | 0.99 | 0.99 | / | / | / | ||
| lgBac | 1 | 1.989 | 1.000 | 0.01 | / | 0.01 | / | / | |
| 2 | 0.011 | 13.334 | 0.99 | / | 0.99 | / | / | ||
| lgChl | 1 | 1.975 | 1.000 | 0.01 | 0.01 | / | / | / | |
| 2 | 0.025 | 8.837 | 0.99 | 0.99 | / | / | / | ||
| Epipelic algae | Cya | 1 | 2.939 | 1.000 | 0.00 | 0.01 | / | 0.00 | / |
| 2 | 0.050 | 7.635 | 0.00 | 0.54 | / | 0.27 | / | ||
| 3 | 0.011 | 16.413 | 0.99 | 0.46 | / | 0.72 | / | ||
| Bac | 1 | 1.983 | 1.000 | 0.01 | / | / | 0.01 | / | |
| 2 | 0.017 | 10.790 | 0.99 | / | / | 0.99 | / | ||
| Eug | 1 | 1.960 | 1.000 | 0.02 | / | / | / | 0.02 | |
| 2 | 0.040 | 6.961 | 0.98 | / | / | / | 0.98 | ||
| Chl | 1 | 1.960 | 1.000 | 0.02 | / | / | / | 0.02 | |
| 2 | 0.040 | 6.961 | 0.98 | / | / | / | 0.98 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, L.; Zhu, Y.; Zhao, Z. Understanding the Ecological Response of Planktic and Benthic Epipelic Algae to Environmental Factors in an Urban Rivers System. Water 2020, 12, 1311. https://doi.org/10.3390/w12051311
Xia L, Zhu Y, Zhao Z. Understanding the Ecological Response of Planktic and Benthic Epipelic Algae to Environmental Factors in an Urban Rivers System. Water. 2020; 12(5):1311. https://doi.org/10.3390/w12051311
Chicago/Turabian StyleXia, Liling, Yuelong Zhu, and Zhenhua Zhao. 2020. "Understanding the Ecological Response of Planktic and Benthic Epipelic Algae to Environmental Factors in an Urban Rivers System" Water 12, no. 5: 1311. https://doi.org/10.3390/w12051311




