Formation of Palygorskite Clay from Treated Diatomite and its Application for the Removal of Heavy Metals from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Palygorskite
2.3. Kinetics Batch Experiments
2.4. Analysis
3. Results and Discussions
3.1. Characterization of Adsorbents
3.1.1. XRF Studies
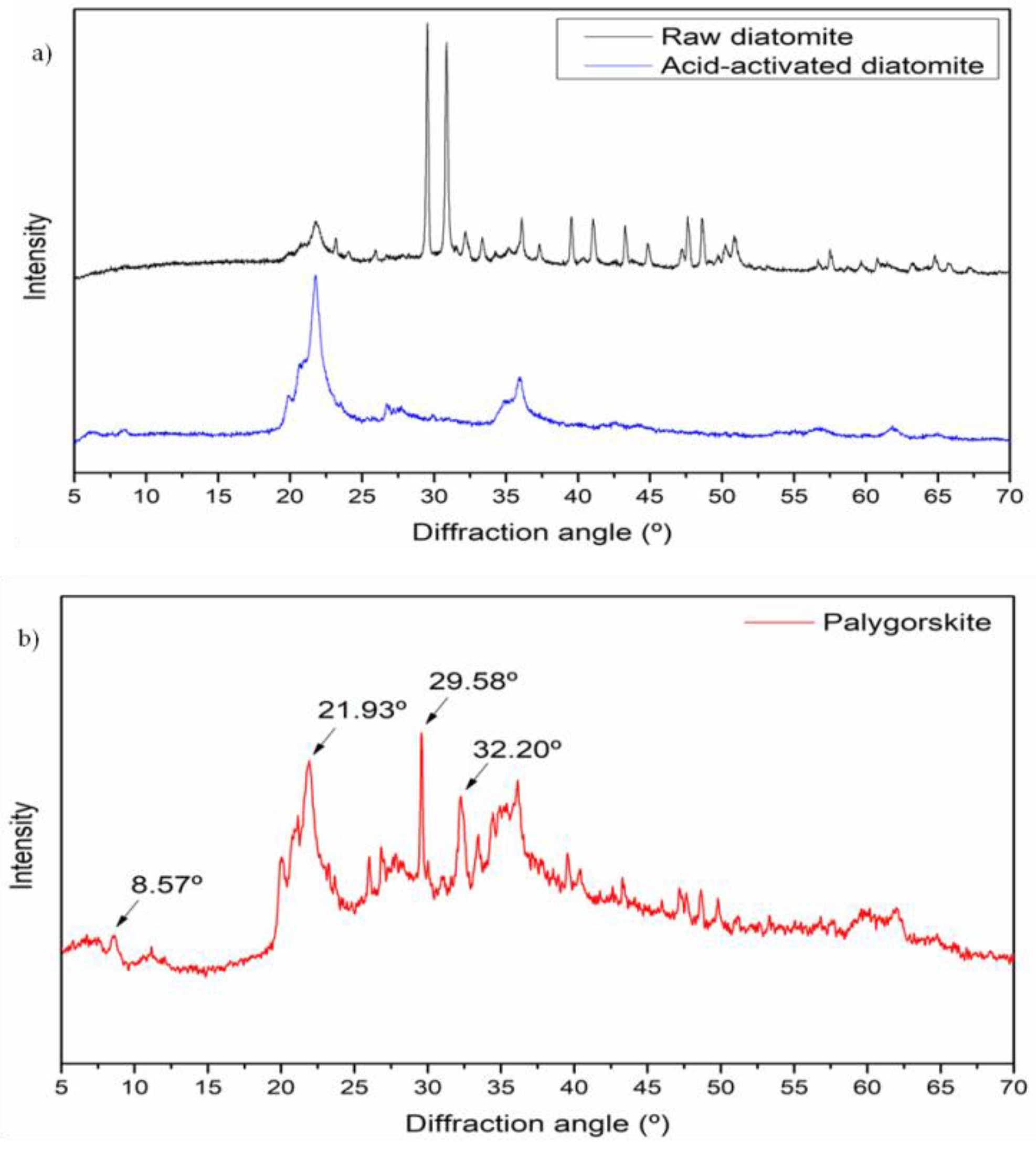
3.1.2. XRD Studies
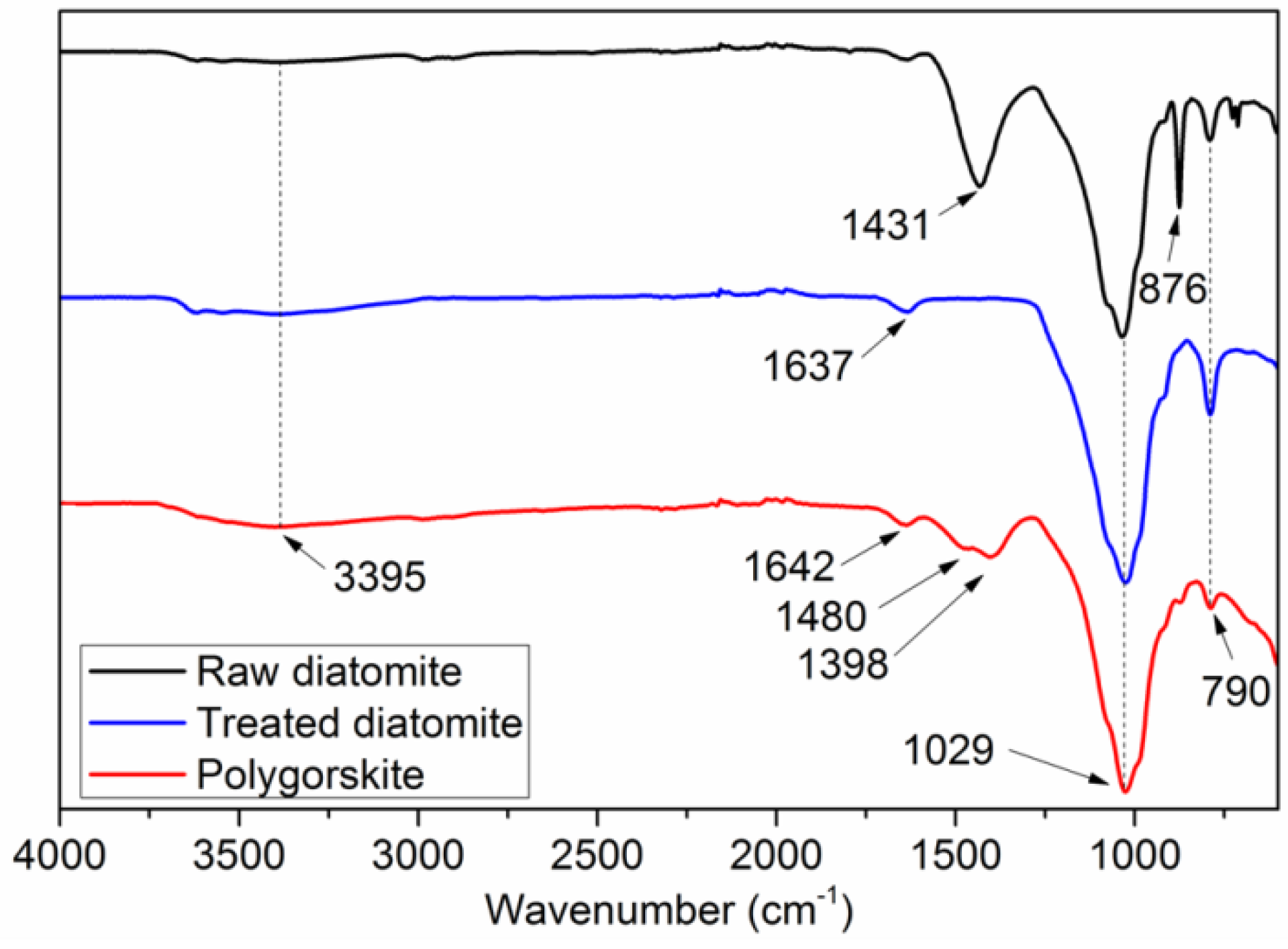
3.1.3. FTIR studies
3.2. Adsorption Kinetics
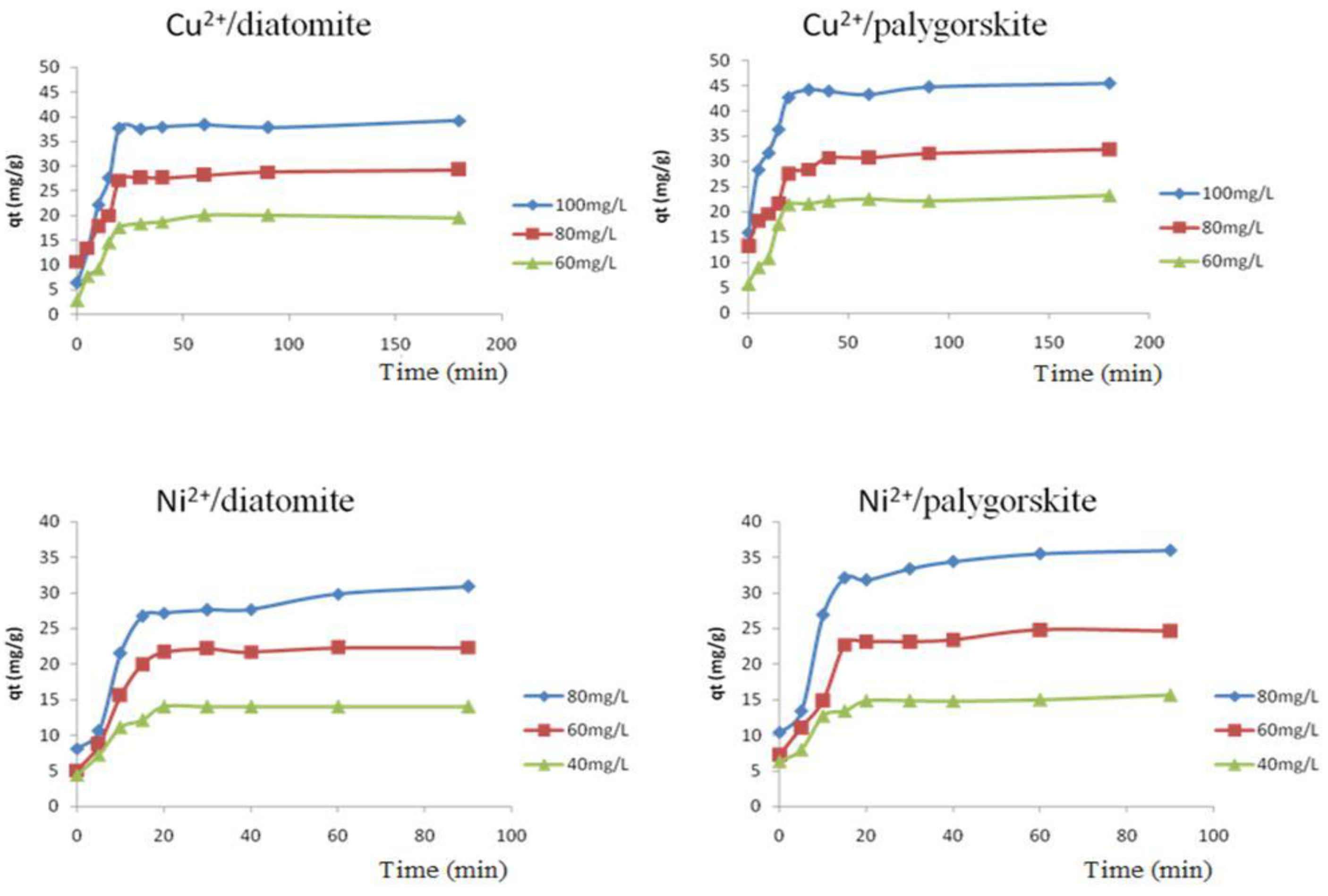
3.2.1. Effect of Contact Time and Initial Concentration of the Adsorption of Copper and Nickel by Treated Diatomite and Palygorskite
3.2.2. Comparative Experiment
3.2.3. Effect of pH
3.2.4. Effect of Temperature
3.2.5. Stimulation Modeling of Adsorption Data
3.3. Adsorption Isotherms
3.3.1. Langmuir Isotherm
3.3.2. Freundlich Isotherm
3.4. Thermodynamic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barros, F.C.; Sousa, F.W.; Cavalcante, R.M.; Carvalho, T.V.; Dias, F.S.; Queiroz, D.C.; Nascimento, R.F. Removal of Copper, Nickel and Zinc Ions from Aqueous Solution by Chitosan-8-Hydroxyquinoline Beads. CLEAN–Soil Air Water 2008, 36, 292–298. [Google Scholar] [CrossRef]
- Revathi, M.; Ahmed Basha, C.; Velan, M. Removal of copper (II) ions from synthetic electroplating rinse water using polyethyleneimine modified ion-exchange resin. Desalin. Water. Treat. 2016, 57, 20350–20367. [Google Scholar] [CrossRef]
- Aliabadi, M.; Irani, M.; Ismaeili, J.; Najafzadeh, S. Design and evaluation of chitosan/hydroxyapatite composite nanofiber membrane for the removal of heavy metal ions from aqueous solution. J. Taiwan Inst. Chem. 2014, 45, 518–526. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, T.; Liu, H.; Xu, B.; Xie, J. Kinetics and thermodynamics of Eu (III) and U (VI) adsorption onto palygorskite. J. Mol. Liq. 2016, 219, 272–278. [Google Scholar] [CrossRef]
- Yariv, S.; Borisover, M.; Lapides, I. Few introducing comments on the thermal analysis of organoclays. J. Therm. Anal. Calorim. 2011, 105, 897–906. [Google Scholar] [CrossRef]
- Gionis, V.; Kacandes, G.H.; Kastritis, I.D.; Chryssikos, G.D. Combined near-infrared and X-ray diffraction investigation of the octahedral sheet composition of palygorskite. Clays Clay Miner. 2007, 55, 543–553. [Google Scholar] [CrossRef]
- Fois, E.; Gamba, A.; Tilocca, A. On the unusual stability of Maya blue paint: molecular dynamics simulations. Microporous Mesoporous Mater. 2003, 57, 263–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Zhang, J.; Liu, P.; Wang, A. A comparative study about adsorption of natural palygorskite for methylene blue. Chem. Eng. J. 2015, 262, 390–398. [Google Scholar] [CrossRef]
- Khademi, H.; Arocena, J.M. Kaolinite formation from palygorskite and sepiolite in rhizosphere soils. Clays Clay Miner. 2008, 56, 429–436. [Google Scholar] [CrossRef]
- Chaisena, A.; Rangsriwatananon, K. Synthesis of sodium zeolites from natural and modified diatomite. Mater. Lett. 2005, 59, 1474–1479. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lai, C.W.; Hsien, K.J. Characterization and adsorption properties of diatomaceous earth modified by hydrofluoric acid etching. J. Colloid Interface Sci. 2006, 297, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, M.L.; Jones, H.; Garelick, H.; Mohamedbakr, H.G.; Burkitbayev, M. The removal of arsenate from water using iron-modified diatomite (D-Fe): isotherm and column experiments. Environ. Sci. Pollut. Res. 2014, 21, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, X.; Wang, X.; Zhang, J.; Wang, H.; Song, J.; Zhao, J. Synthesis and Characterization of MgO Modified Diatomite for Phosphorus Recovery in Eutrophic Water. J. Chem. Eng. Data. 2016, 62, 226–235. [Google Scholar] [CrossRef]
- He, X.; Yang, Q.; Fu, L.; Yang, H. Synthesis and magnetic property of SiO2 coated Fe3O4/palygorskite. Funct. Mater. Lett. 2015, 8, 56–155. [Google Scholar] [CrossRef]
- Lai, S.; Yue, L.; Zhao, X.; Gao, L. Preparation of silica powder with high whiteness from palygorskite. Appl. Clay Sci. 2010, 50, 432–437. [Google Scholar] [CrossRef]
- Korus, I.; Rumińska, M. UV spectrophotometric studies of Cu (II) ions separation by ultrafiltration enhanced with poly (sodium acrylate). Desalin. Water 2016, 57, 1436–1442. [Google Scholar] [CrossRef]
- Bermejo-Barrera, A.; Bermejo-Barrera, P.; Martinez, F.B. Simultaneous determination of copper and cobalt with EDTA using derivative spectrophotometry. Analyst 1985, 110, 1313–1315. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X. Adsorption and desorption of nickel (II) ions from aqueous solution by a lignocellulose/montmorillonite nanocomposite. PLoS ONE 2015, 10, e0117077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, M.; Zhang, Y.; Yu, Z.; Meng, C. Synthesis of zeolite Y from diatomite and its modification by dimethylglyoxime for the removal of Ni (II) from aqueous solution. J. Sol-Gel Sci. Technol. 2016, 80, 215–225. [Google Scholar] [CrossRef]
- Li, X.Y.; Jiang, Y.; Liu, X.Q.; Shi, L.Y.; Zhang, D.Y.; Sun, L.B. Direct Synthesis of Zeolites from a Natural Clay, Attapulgite. ACS Sustain. Chem. Eng. 2017, 5, 6124–6130. [Google Scholar] [CrossRef]
- Simões, K.M.; Novo, B.L.; Felix, A.A.; Afonso, J.C.; Bertolino, L.C.; Silva, F.A. Ore Dressing and Technological Characterization of Palygorskite from Piauí/Brazil for Application as Adsorbent of Heavy Metals:In Characterization of Minerals, Metals and Materials. In Characterization of Minerals, Metals and Materials; Springer: Cham, Switzerland, 2017; pp. 261–267. [Google Scholar]
- Belaroui, L.S.; Dali Youcef, L.; Ouali, A.F.F.A.F.; Bengueddach, A.B.D.E.L.K.A.D.E.R.; Lopez Galindo, A. Mineralogical and chemical characterization of palygorskite from East-Algeria. Rev. Soc. Esp. Miner. Macla 2014, 19. Available online: http://www.ehu.eus/sem/macla_pdf/macla19/Belaroui.et.al_SEM2014.WEB.pdf (accessed on 14 September 2018).
- Cai, Y.; Xue, J.; Polya, D.A. A Fourier transform infrared spectroscopic study of Mg-rich, Mg-poor and acid leached palygorskites. Spectrochim. Acta Part A 2007, 66, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Wang, S.; Hu, J.; Lu, Y.; Li, J.; Dong, Y.; Wang, X. Adsorption of Pb (II) on diatomite as affected via aqueous solution chemistry and temperature. Coll. Surf. A 2009, 339, 159–166. [Google Scholar] [CrossRef]
- da Silva, M.L.D.G.; Fortes, A.C.; Oliveira, M.E.R.; de Freitas, R.M.; da Silva Filho, E.C.; Soares, M.F.D.L.R.; da Silva Leite, C.M. Palygorskite organophilic for dermopharmaceutical application. J. Therm. Anal. Calorim. 2014, 115, 2287–2294. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Nikaeen, M.; Khiadani, H.H. Removal of benzene, toluene, ethylbenzene and xylene (BTEX) from aqueous solutions by montmorillonite modified with nonionic surfactant: Equilibrium, kinetic and thermodynamic study. Chem. Eng. J. 2012, 191, 341–348. [Google Scholar] [CrossRef]
- Mohammed, J.; Nasri, N.S.; Ahmad Zaini, M.A.; Hamza, U.D.; Ani, F.N. Adsorption of benzene and toluene onto KOH activated coconut shell based carbon treated with NH3. Int. Biodeterior. Biodegrad. 2015, 102, 245–255. [Google Scholar] [CrossRef]
- Sheshdeh, R.K.; Abbasizadeh, S.; Nikou, M.R.K.; Badii, K.; Sharafi, M.S. Liquid Phase adsorption kinetics and equilibrium of toluene by novel modified-diatomite. J. Environ. Heal. Sci. Eng. 2014, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Rusmin, R.; Sarkar, B.; Biswas, B.; Churchman, J.; Liu, Y.; Naidu, R. Structural, electrokinetic and surface properties of activated palygorskite for environmental application. Appl. Clay Sci. 2016, 134, 95–102. [Google Scholar] [CrossRef]
- Wan, M.W.; Kan, C.C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Labidi, A.; Salaberria, A.M.; Fernandes, S.C.; Labidi, J.; Abderrabba, M. Adsorption of copper on chitin-based materials: Kinetic and thermodynamic studies. J. Taiwan Inst. Chem. 2016, 65, 10–148. [Google Scholar] [CrossRef]
- Moreno, J.C.; Gómez, R.; Giraldo, L. Removal of Mn, Fe, Ni and Cu ions from wastewater using cow bone charcoal. Materials 2010, 3, 452–466. [Google Scholar] [CrossRef]
- Šljivić, M.; Smičiklas, I.; Pejanović, S.; Plećaš, I. Comparative study of Cu2+ adsorption on a zeolite, a clay and a diatomite from Serbia. Appl. Clay Sci. 2009, 43, 33–40. [Google Scholar] [CrossRef]
- Bourliva, A.; Sikalidis, A.K.; Papadopoulou, L.; Betsiou, M. Removal of Cu2+ and Ni2+ ions from aqueous solutions by adsorption onto natural palygorskite and vermiculite. Clay Miner. 2018, 53, 1–15. [Google Scholar] [CrossRef]
- Fouladgar, M.; Beheshti, M.; Sabzyan, H. Single and binary adsorption of nickel and copper from aqueous solutions by γ-alumina nanoparticles: equilibrium and kinetic modeling. J. Mol. Liq. 2015, 211, 1060–1073. [Google Scholar] [CrossRef]
- Boujelben, N.; Bouzid, J.; Elouear, Z. Adsorption of nickel and copper onto natural iron oxide-coated sand from aqueous solutions: study in single and binary systems. J. Hazard. Mater. 2009, 163, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Labidi, A.; Saad, A.; Abderrabba, M. Copper adsorption onto starch as biopolymer: Isothermal equilibrium and kinetic studies. J. Chem. Pharm. Res. 2015, 7, 1274–1282. [Google Scholar]
- Lagergren, S. Zurtheorie der sogenannten adsorption gelosterstoffe, KungligaSvenskaVetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Shabani, K.S.; Ardejani, F.D.; Badii, K.; Olya, M.E. Preparation and characterization of novel nano-mineral for the removal of several heavy metals from aqueous solution: Batch and continuous systems. Arab. J. Chem. 2017, 10, S3108–S3127. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Wang, A. Removal of Cu (II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard. Mater. 2007, 149, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Petra, L.; Billik, P.; Melichová, Z.; Komadel, P. Mechanochemically activated saponite as materials for Cu2+ and Ni2+ removal from aqueous solutions. Appl. Clay Sci. 2017, 143, 22–28. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P.; Ofomaja, A. The adsorption of copper (II) ions by polyaniline graft chitosan beads from aqueous solution: equilibrium, kinetic and desorption studies. J. Eniron. Chem. Eng. 2014, 2, 362–369. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Kirupha, S.D.; Murugesan, A.; Vidhyadevi, T.; Sivanesan, S. Adsorption behavior of nickel (II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design. Chem. Eng. J. 2011, 167, 122–131. [Google Scholar] [CrossRef]
- Thevannan, A.; Mungroo, R.; Niu, C.H. Biosorption of nickel with barley straw. Bioresour. Technol. 2010, 101, 1776–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuhoglu, Y.; Malkoc, E. Thermodynamic and kinetic studies for environmentaly friendly Ni (II) biosorption using waste pomace of olive oil factory. Bioresour. Technol. 2009, 100, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; MahdaviShahri, M.; Mohamad, R. Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Eriobotrya japonica seed biocomposite efficiency for copper adsorption: isotherms, kinetics, thermodynamic and desorption studies. J. Environ. Manag. 2016, 176, 21–33. [Google Scholar] [CrossRef] [PubMed]


| Chemical Compound | Diatomite before Treatment (%) | Diatomite after Treatment (%) | Palygorskite(%) |
|---|---|---|---|
| SiO2 | 29.60 | 78.83 | 33.65 |
| Al2O3 | 2.57 | 6.53 | 3.16 |
| Fe2O3 | 1.08 | 2.52 | 9.71 |
| MnO | 0.00 | 0.00 | 0.01 |
| MgO | 5.78 | 2.01 | 9.92 |
| CaO | 0.20 | 0.59 | 5.35 |
| Na2O | 26.89 | 0.00 | 4.12 |
| K2O | 0.39 | 0.96 | 0.47 |
| TiO2 | 0.16 | 0.38 | 0.19 |
| P2O5 | 4.12 | 0.05 | 1.90 |
| LOI | 28.11 | 8.10 | 29.53 |
| LOI: Loss of ignition | |||
| Ni2+ | Cu2+ | |||||||
|---|---|---|---|---|---|---|---|---|
| Adsorbents | Kinetic Models | Parameters | 80 mg/L | 60 mg/L | 40 mg/L | 100 mg/L | 80 mg/L | 60 mg/L |
| Natural diatomite | Pseudo-first-order | qecal(mg/g) | 15.35 | 13.93 | 4.27 | 11.18 | 21.23 | 18.75 |
| qeexp(mg/g) | 28.65 | 22.02 | 14.02 | 38.09 | 28.08 | 19.06 | ||
| K1(min−1) | 0.036 | 0.047 | 0.049 | 0.025 | 0.046 | 0.047 | ||
| R2 | 0.815 | 0.796 | 0.765 | 0.553 | 0.869 | 0.965 | ||
| Pseudo-second-order | qecal(mg/g) | 32.25 | 23.81 | 14.49 | 41.67 | 30.3 | 20.41 | |
| qeexp(mg/g) | 30.91 | 22.02 | 14.02 | 38.09 | 28.08 | 19.06 | ||
| K2(g/mg.min) | 0.0067 | 0.013 | 0.034 | 0.0051 | 0.0081 | 0.0108 | ||
| R2 | 0.991 | 0.993 | 0.997 | 0.996 | 0.996 | 0.995 | ||
| Palygorskite | Pseudo-first-order | qecal(mg/g) | 24,77 | 13,39 | 6.92 | 17.46 | 19.055 | 9.42 |
| qeexp(mg/g) | 34.231 | 23,83 | 15.04 | 44.07 | 30.17 | 22.2 | ||
| K1(min−1) | 0.052 | 0.0743 | 0.047 | 0.032 | 0.034 | 0.0343 | ||
| R2 | 0.925 | 0.811 | 0.803 | 0.68 | 0.907 | 0.753 | ||
| Pseudo-second-order | qecal(mg/g) | 38.46 | 26.32 | 16.13 | 47.62 | 33.33 | 24.39 | |
| qeexp(mg/g) | 34.231 | 23.83 | 15.04 | 44.07 | 30.17 | 22.2 | ||
| K2(g/mg.min) | 0.0068 | 0.0103 | 0.027 | 0.081 | 0.00671 | 0.00804 | ||
| R2 | 0.993 | 0.993 | 0.997 | 0.999 | 0.998 | 0.996 | ||
| Adsorbents | Metals ions | Parameters of Freundlich | Parameters of Langmuir | ||||
|---|---|---|---|---|---|---|---|
| Kf | 1/n | R2 | qm(mg/g) | Kl (L/mg) | R2 | ||
| Palygorskite | Cu2+ | 2.028 | 0.914 | 0.995 | 333.33 | 0.005 | 0.994 |
| Ni2+ | 2.87 | 0.848 | 0.994 | 166.67 | 0.014 | 0.992 | |
| Cu2+ | 3.062 | 0.694 | 0.999 | 111.10 | 0.014 | 0.999 | |
| Natural Diatomite | Ni2+ | 2.42 | 0.757 | 0.995 | 90.91 | 0.0176 | 0.997 |
| Materials | Metals Ions | T (°C) | 𝛥°H(KJ·mol−1) | 𝛥°S(KJ·mol−1) | 𝛥°G(KJ·mol−1) |
|---|---|---|---|---|---|
| 25 | −23.15 | −0.069 | −2.46 | ||
| Cu2+ | 35 | −1.93 | |||
| 45 | −1.07 | ||||
| Palygorskite | 25 | −17.70 | −0.46 | −4.10 | |
| Ni2+ | 35 | −3.71 | |||
| 45 | −9.19 | ||||
| 25 | −22.34 | −0.07 | −0.56 | ||
| Cu2+ | 35 | 0.51 | |||
| Diatomite | 45 | 0.85 | |||
| 25 | −13.64 | −0.069 | −2.72 | ||
| Ni2+ | 35 | −2.17 | |||
| 45 | −1.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nefzi, H.; Abderrabba, M.; Ayadi, S.; Labidi, J. Formation of Palygorskite Clay from Treated Diatomite and its Application for the Removal of Heavy Metals from Aqueous Solution. Water 2018, 10, 1257. https://doi.org/10.3390/w10091257
Nefzi H, Abderrabba M, Ayadi S, Labidi J. Formation of Palygorskite Clay from Treated Diatomite and its Application for the Removal of Heavy Metals from Aqueous Solution. Water. 2018; 10(9):1257. https://doi.org/10.3390/w10091257
Chicago/Turabian StyleNefzi, Houwaida, Manef Abderrabba, Sameh Ayadi, and Jalel Labidi. 2018. "Formation of Palygorskite Clay from Treated Diatomite and its Application for the Removal of Heavy Metals from Aqueous Solution" Water 10, no. 9: 1257. https://doi.org/10.3390/w10091257
APA StyleNefzi, H., Abderrabba, M., Ayadi, S., & Labidi, J. (2018). Formation of Palygorskite Clay from Treated Diatomite and its Application for the Removal of Heavy Metals from Aqueous Solution. Water, 10(9), 1257. https://doi.org/10.3390/w10091257






