Macroinvertebrate Biodiversity Trends and Habitat Relationships within Headwater Rivers of the Qinghai-Tibet Plateau
Abstract
1. Introduction
2. Study Areas and Methods
2.1. Study Areas
2.2. Environmental Variable Measurement
2.3. Macroinvertebrate Sampling
2.4. Data Analyses
3. Results
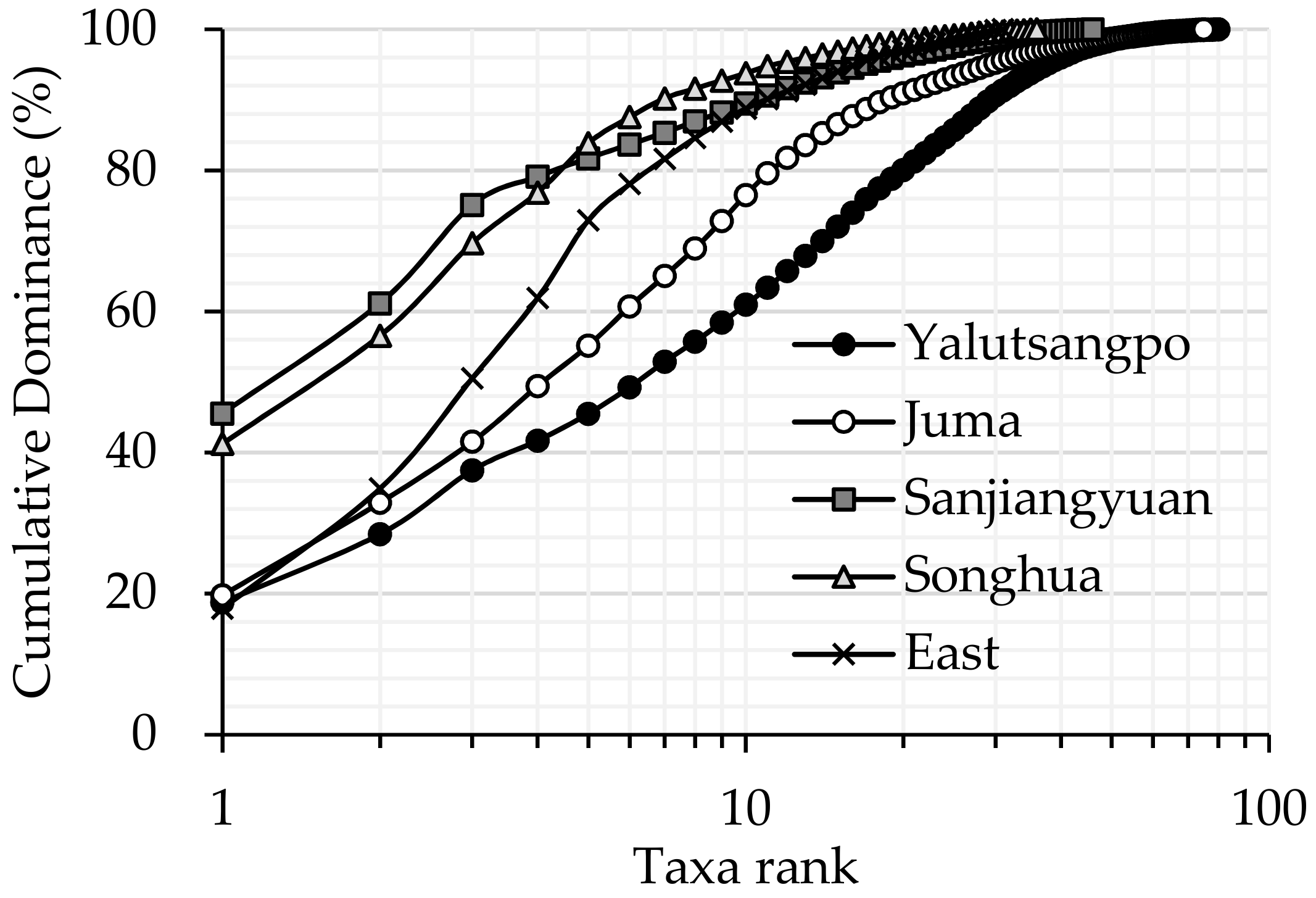
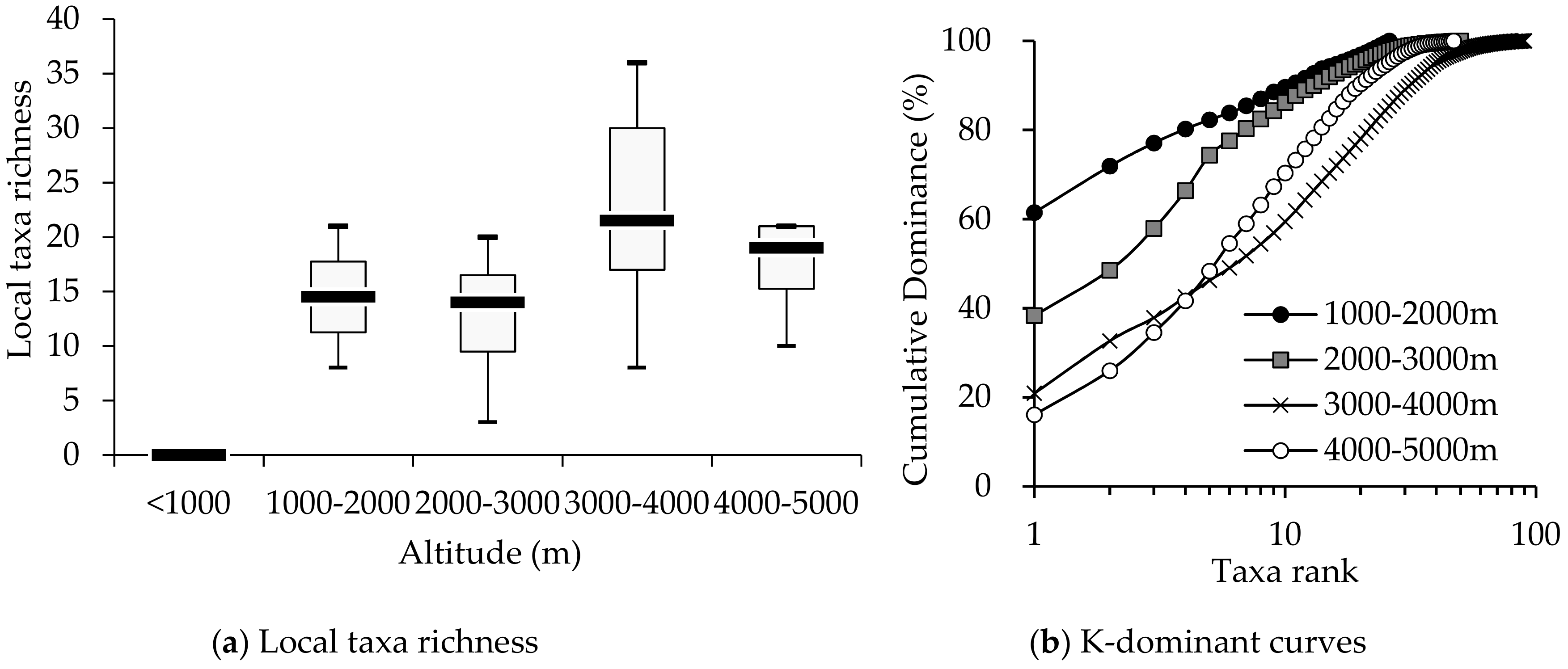
3.1. Comparison of Macroinvertebrate Biodiversity in Highland and Lowland Rivers
3.2. Relationships of Macroinvertebrate Communities with Stream Habitat Variables of the Yalutsangpo River
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Site | H (m) | D50 (mm) | h (cm) | v (m/s) | DO (mg/L) | T (°C) | Bed Structure Score | Riparian Condition Score | Stream Condition Score = Bed Structure Score × Riparian Condition Score |
|---|---|---|---|---|---|---|---|---|---|
| S0 | 4873 | 5 | 50 | 0.05 | 6.4 | 2.4 | 4 | 8 | 32 |
| S1 | 4484 | 40 | 15 | 0.4 | 8.4 | 1.3 | 9 | 10 | 90 |
| S2 | 4228 | 100 | 30 | 0.83 | 8.1 | 3.5 | 6 | 10 | 60 |
| S3 | 4014 | 50 | 20 | 0.4 | 7.9 | 4.7 | 3 | 2 | 6 |
| S4 | 3916 | 5 | 15 | 0.3 | 6.15 | 10.5 | 4.5 | 10 | 45 |
| S5 | 3901 | 5 | 30 | 0.1 | 7.9 | 3 | 2 | 1 | 2 |
| S6 | 3768 | 10 | 20 | 0.3 | 9.6 | 17.4 | 4.5 | 1 | 4.5 |
| S7 | 3752 | 300 | 25 | 0.4 | 8.5 | 2 | 3.5 | 4 | 14 |
| S8 | 3598 | 150 | 35 | 0.6 | 10.3 | 10.5 | 4.5 | 6 | 27 |
| S9 | 3566 | 200 | 35 | 0.3 | 7.7 | 12.4 | 2.5 | 1 | 2.5 |
| S10 | 3514 | 500 | 30 | 0.4 | 8.1 | 7.2 | 9 | 2 | 18 |
| S11 | 3237 | 1000 | 100 | 1 | 8 | 8 | 9 | 1 | 9 |
| S12 | 2993 | 800 | 35 | 0 | 6.7 | 13 | 2.5 | 1 | 2.5 |
| S13 | 2959 | 300 | 30 | 2 | 7.8 | 8 | 3.5 | 6 | 21 |
| S14 | 2948 | 30 | 70 | 0.9 | 7.5 | 15 | 4.5 | 7 | 31.5 |
| S15 | 2744 | 150 | 30 | 0.75 | 8.5 | 10.1 | 6 | 4 | 24 |
| S16 | 2228 | 150 | 15 | 0.3 | 9.6 | 10.3 | 3.5 | 8 | 28 |
| S17 | 2208 | 5 | 30 | 0.1 | 7.9 | 13.2 | 1.5 | 1 | 1.5 |
| S18 | 2008 | 180 | 20 | 0.4 | 9.6 | 11 | 6 | 6 | 36 |
| S19 | 1100 | 50 | 15 | 0.55 | 9.5 | 15 | 6 | 7 | 42 |
| S20 | 1001 | 120 | 15 | 0.15 | 9.3 | 14.4 | 9 | 7 | 63 |
| S21 | 718 | 300 | 25 | 0.4 | 9.2 | 13.5 | 2.5 | 1 | 2.5 |
| S22 | 705 | 200 | 20 | 0.4 | 9.5 | 14.6 | 3.5 | 1 | 3.5 |
| S23 | 688 | 200 | 20 | 0.45 | 9.4 | 14.1 | 2.5 | 1 | 2.5 |
References
- Maiolini, B.; Lencioni, V. Longitudinal distribution of macroinvertebrate assemblages in a glacially influenced stream system in the Italian Alps. Freshw. Biol. 2001, 46, 1625–1639. [Google Scholar] [CrossRef]
- Jiang, X.M.; Xie, Z.C.; Chen, Y.F. Longitudinal patterns of macroinvertebrate communities in relation to environmental factors in a Tibetan-Plateau river system. Quat. Int. 2013, 304, 107–114. [Google Scholar] [CrossRef]
- Brierley, G.J.; Li, X.; Cullum, C.; Gao, J. Introduction: Landscape and ecosystem diversity in the Yellow River Source Zone. In Landscape and Ecosystem Diversity, Dynamics and Management in the Yellow River Source Zone; Brierley, G.J., Li, X., Cullum, C., Gao, J., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 1–34. [Google Scholar]
- Chiang, S.C.; Shen, Y.F.; Gong, X.J. Aquatic Invertebrate in Tibet, 1st ed.; Science Press: Beijing, China, 1983; (In Chinese, Latin Names). [Google Scholar]
- Xu, M.Z.; Wang, Z.Y.; Pan, B.Z.; Yu, G.A. The assemblage characteristics of benthic macroinvertebrates in the Yalutsangpo Basin, the highest-altitude major river in the world. Front. Earth Sci. 2014, 8, 351–361. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Xie, C. Eco-environmental degradation in the source region of the Yellow River, Northeast Qinghai-Xizang Plateau. Environ. Monit. Assess. 2006, 122, 125–143. [Google Scholar] [CrossRef] [PubMed]
- McGregor, G.R. Climate variability and change in the Sanjiangyuan region. In Landscape and Ecosystem Diversity, Dynamics and Management in the Yellow River Source Zone; Brierley, G.J., Li, X., Cullum, C., Gao, J., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 35–57. [Google Scholar]
- Chang, G.; Li, L.; Zhu, X.; Wang, Z.; Xiao, J.; Li, F. Influencing factors of water resources in the source region of the Yellow River. J. Geogr. Sci. 2007, 17, 131–140. [Google Scholar] [CrossRef]
- Smith, M.J.; Kay, W.R.; Edward, D.H.D.; Papas, P.J.; St. J. Richardson, K.; Simpson, J.C.; Pinder, A.M.; Cale, D.J.; Horwitz, P.H.J.; Davis, J.A.; et al. Using macroinvertebrates to assess ecological condition of rivers in Western Australia. Freshw. Biol. 1999, 41, 269–282. [Google Scholar] [CrossRef]
- Pan, B.Z.; Wang, Z.Y.; Li, Z.W.; Yu, G.A.; Xu, M.Z.; Zhao, N.; Brierley, G. An exploratory analysis of benthic macroinvertebrates as indicators of the ecological status of the Upper Yellow and Yangtze Rivers. J. Geogr. Sci. 2013, 23, 871–882. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Xu, M.Z.; Wang, Z.Y.; Pan, B.Z.; Zhao, N. Distribution and species composition of macroinvertebrates in the hyporheic zone of bed sediment. Int. J. Sediment Res. 2012, 27, 129–140. [Google Scholar] [CrossRef]
- Subcommittee on Sediment Terminology. Report on the Subcommittee on Sediment Terminology, American Geophysical Union. Transit. Am. Geophys. Union 1947, 28, 936–938. [Google Scholar] [CrossRef]
- Duan, X.H.; Wang, Z.Y.; Xu, M.Z.; Zhang, K. Effect of streambed sediment on benthic ecology. Int. J. Sediment Res. 2009, 24, 325–338. [Google Scholar] [CrossRef]
- Wiggins, G.B. Larvae of the North American Caddisfly Genera (Trichoptera), 2nd ed.; University of Toronto Press: Toronto, ON, Canada, 2015. [Google Scholar]
- Epler, J.H. Identification Manual for the Larval Chironomidae (Diptera) of North and South Carolina: A Guide to the Taxonomy of the Midges of the Southeastern United States, Including Florida; Special Publication SH2001-SP13; North Carolina Department of Environment and Natural Resources: Raleigh, NC, USA, 2001. [Google Scholar]
- Morse, J.C.; Yang, L.F.; Tian, L.X. Aquatic Insects of China Useful for Monitoring Water Quality, 1st ed.; Hohai University Press: Nanjing, China, 1994. [Google Scholar]
- Liang, Y.L.; Wang, H.Z. Zoobenthos. In Advanced Hydrobiology; Liu, J.K., Ed.; Science Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Jacobsen, D. Contrasting patterns in local and zonal family richness of stream invertebrates along an Andean altitudinal gradient. Freshw. Biol. 2004, 49, 1293–1305. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance, 2nd ed.; Harper and Row Press: New York, NY, USA, 1978. [Google Scholar]
- Wang, Z.Y.; Lee, J.H.W.; Melching, C.S. River Dynamics and Integrated River Management, 1st ed.; Tsinghua University Press: Beijing, China, 2014. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: New York, NY, USA, 2003. [Google Scholar]
- Scheibler, E.E.; Claps, M.C.; Roig-Juñent, S.A. Temporal and Altitudinal Variations in Benthic Macroinvertebrate Assemblages in an Andean River Basin of Argentina. J. Limnol. 2014, 73, 92–108. [Google Scholar] [CrossRef]
- Füreder, L.; Schütz, C.; Wallinger, M. Physico-chemistry and aquatic insects of a glacier-fed and a spring-fed alpine stream. Freshw. Biol. 2001, 46, 1673–1690. [Google Scholar] [CrossRef]
- Milner, A.M.; Petts, G.E. Glacial rivers: Physical habitat and ecology. Freshw. Biol. 1994, 32, 295–307. [Google Scholar] [CrossRef]
- Liu, W.; Xu, M.Z.; Zhao, N.; Zhou, X.D.; Pan, B.Z.; Lei, F.K.; Tian, S.M. Aquatic Ecology and Water Quality Assessment of the Yellow River Headwater Region Based on the Multiple Traits of Macroinvertebrate Community; Inner Report of River Research Institute [2018.1.30]; Tsinghua University: Beijing, China, 2018. [Google Scholar]
- Beisel, J.N.; Usseglio-Polatera, P.; Moreteau, J.C. The spatial heterogeneity of a river bottom, a key factor determining macroinvertebrate communities. Hydrobiologia 2000, 422/423, 163–171. [Google Scholar] [CrossRef]
- Skjelkvåle, B.L.; Wright, R.F. Mountain lakes; sensitivity to acid deposition and global climate change. AMBIO 1998, 27, 280–286. [Google Scholar]
- Schwendel, A.C.; Death, R.G.; Fuller, I.C.; Tonkin, J.D. A new approach to assess bed stability relevant for invertebrate Communities in upland streams. River Res. Appl. 2012, 28, 1726–1739. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, Z.Y.; Pan, B.Z.; Xu, M.Z.; Li, Z.W. Macroinvertebrate assemblages in mountain streams with different streambed stability. River Res. Appl. 2014, 31, 825–833. [Google Scholar] [CrossRef]
- Zhou, X.D.; Wang, Z.Y.; Xu, M.Z.; Yu, B.F.; Liu, W.; Pan, B.Z.; Zhao, N.; Shao, X.J. The stream power parameter as a predictive indicator of aquatic macroinvertebrate assemblages in the Yarlung Tsangpo River Basin (Tibetan Plateau). Hydrobiologia 2017, 797, 215–230. [Google Scholar] [CrossRef]


| SR | H | Substrate Composition | D50 (mm) | W (m) | H (m) | V (m/s) | Alpha-Diversity Indices | ||
|---|---|---|---|---|---|---|---|---|---|
| S | H′ | B | |||||||
| YA | 3500–4500 | five sites with boulder, pebble, cobble, sand, and aquatic macrophytes; two with sand, gravel | 20–200 | 5–40 | 0–0.5 | 0.3–0.8 | 17–33 | 1.7–2.7 | 9.8–19.2 |
| SA | 3500–4900 | five sites with gravel, sand, and aquatic macrophytes; two with silt and gravel | 0.5–5.0 | 5–60 | 0–0.6 | 0–0.5 | 6–21 | 0.5–2.1 | 3.0–10.0 |
| SO | <500 | five sites with gravel, sand, and aquatic macrophytes; two with silt, fluid mud | 0.01–5.0 | 5–30 | 0.3–1.5 | 0–0.8 | 6–13 | 1.2–1.8 | 3.7–8.9 |
| JU | <300 | five sites with boulder, pebble, gravel, sand, and aquatic macrophytes; two with sand, silt | 20–100 | 5–30 | 0.2–0.7 | 0.1–0.6 | 16–38 | 1.2–2.6 | 7.5–17.3 |
| EA | <100 | three sites with gravel, sand, aquatic macrophytes; two with gravel and sand; two with fine sand, silt and clay | 0.1–2.0 | 5–40 | 0.3–1.3 | 0.3–1.0 | 6–15 | 1.1–1.9 | 4.1–10.1 |
| Phylum | Class | Family | YA | SA | SO | JU | EA |
|---|---|---|---|---|---|---|---|
| Platyhelminthes | Turbellaria | n.d. | + | + | |||
| Nematoda | Nematoda | n.d. | + | + | |||
| Annelida | Hirudinea | Glossiphonidae | + | ||||
| Glossiphoniidae | + | + | |||||
| Piscicolidae | + | ||||||
| Branchiobdellidae | + | ||||||
| Erpobdellidae | + | ||||||
| Hirudinidae | + | + | + | ||||
| Oligochaeta | Tubificidae | + | + | + | + | + | |
| Lumbriculidae | + | + | |||||
| Naididae | + | + | |||||
| Mollusca | Gastropoda | Lymnaeidae | + | + | + | + | |
| Planorbidae | + | + | + | + | |||
| Physidae | + | ||||||
| Hydrobiidae | + | + | |||||
| Pleuroceridae | + | + | |||||
| Stenothyridae | + | + | |||||
| Turbinidae | + | ||||||
| Viviparidae | + | ||||||
| Bivalvia | Corbiculidae | + | + | + | |||
| Unionidae | + | ||||||
| Arthropoda | Crustacea | Gammaridae | + | + | + | ||
| Palaemonidae | + | + | |||||
| Atyidae | + | ||||||
| Arachnida | Hydrachnidae | + | + | + | + | ||
| Insecta | Baetidae | + | + | + | + | + | |
| Heptageniidae | + | + | + | + | |||
| Ephemerellidae | + | + | + | + | |||
| Ephemeridae | + | ||||||
| Leptophlebiidae | + | + | + | + | |||
| Siphlonuridae | + | + | |||||
| Caenidae | + | + | + | ||||
| Potamanthidae | + | ||||||
| Isonychiidae | + | ||||||
| Neoephemeridae | + | ||||||
| Coenagrionidae | + | + | |||||
| Gomphidae | + | + | |||||
| Corduliidae | + | + | |||||
| Macromiidae | + | ||||||
| Platycnemididae | + | + | |||||
| Agriidae | + | ||||||
| Perlidae | + | + | + | ||||
| Chloroperlidae | + | ||||||
| Nemouridae | + | + | |||||
| Capniidae | + | ||||||
| Pteronarcidae | + | ||||||
| Perlodidae | + | ||||||
| Naucoridae | + | + | + | + | |||
| Corixidae | + | + | |||||
| Sisyridae | + | ||||||
| Corydalidae | + | + | |||||
| Hydropsychidae | + | + | + | + | |||
| Hydroptilidae | + | ||||||
| Rhyacophilidae | + | + | |||||
| Limnephilidae | + | + | |||||
| Polycentropodidae | + | ||||||
| Stenopsychidae | + | ||||||
| Leptoceridae | + | + | |||||
| Psychomyiidae | + | ||||||
| Molannidae | + | ||||||
| Brachycentridae | + | + | |||||
| Hydrobiosidae | + | + | |||||
| Arctopsychidae | + | ||||||
| Glossosomatidae | + | ||||||
| Philopotamidae | + | ||||||
| Pyralididae | + | ||||||
| Dytiscidae | + | + | + | ||||
| Elmidae | + | + | + | + | + | ||
| Hydrophilidae | + | ||||||
| Chrysomelidae | + | ||||||
| Entomobryomorpha | + | ||||||
| Tipulidae | + | + | + | + | + | ||
| Simuliidae | + | + | + | + | |||
| Ephydridae | + | ||||||
| Culicidae | + | + | + | ||||
| Ceratopogonidae | + | + | + | ||||
| Psychodidae | + | + | |||||
| Dolichopodidae | + | ||||||
| Empididae | + | + | |||||
| Stratiomyiidae | + | ||||||
| Tabanidae | + | + | |||||
| Blephariceridae | + | ||||||
| Muscidae | + | ||||||
| Chironomidae | + | + | + | + | + |
| Variable | HVT | Method | Statistical Value | Sig. |
|---|---|---|---|---|
| Taxa richness | 0.140 | ANOVA | 14.410 | 0.000 * |
| Density (ind./m2) | 0.000 | KW | 15.230 | 0.004 * |
| Shannon-Wiener index | 0.165 | ANOVA | 4.377 | 0.005 * |
| Improved Shannon-Wiener index | 0.905 | ANOVA | 11.766 | 0.000 * |
| Pairwise Comparison | Taxa Richness. p-Value | Density. p-Value | Shannon-Wiener Index. p-Value | Improved Shannon-Wiener Index. p-Value |
|---|---|---|---|---|
| SO-EA | 0.655 | 0.559 | 0.942 | 0.496 |
| SO-SA | 0.999 | 1.000 | 0.938 | 0.996 |
| SO-JU | 0.000 *** | 1.000 | 0.100 | 0.007 ** |
| SO-YA | 0.261 | 0.588 | 0.662 | 0.024 * |
| EA-SA | 0.825 | 0.750 | 1.000 | 0.774 |
| EA-JU | 0.000 *** | 0.012 * | 0.009 ** | 0.000 *** |
| EA-YA | 0.006 ** | 0.002 ** | 0.177 | 0.000 *** |
| SA-JU | 0.000 *** | 1.000 | 0.020 * | 0.003 ** |
| SA-YA | 0.197 | 0.602 | 0.244 | 0.012 * |
| JU-YA | 0.016 * | 1.000 | 0.708 | 0.976 |
| MRPP Statistics | SO | EA | SA | JM | YA |
|---|---|---|---|---|---|
| delta | 0.8978 | 0.9200 | 0.8557 | 0.8005 | 0.8989 |
| n | 8 | 12 | 7 | 8 | 9 |
| Chance corrected within-group agreement A: 0.0721; Based on observed delta 0.8797 and expected delta 0.948. | |||||
| Pairs | F Model | R2 | p-Value | Adjusted p |
|---|---|---|---|---|
| SO-EA | 1.640 | 0.084 | 0.037 | 0.37 |
| SO-SA | 2.144 | 0.142 | 0.002 | 0.02 * |
| SO-JU | 2.999 | 0.176 | 0.002 | 0.02 * |
| SO-YA | 1.801 | 0.107 | 0.009 | 0.09 |
| EA-SA | 2.264 | 0.118 | 0.002 | 0.02 * |
| EA-JU | 2.949 | 0.141 | 0.001 | 0.01 * |
| EA-YA | 2.292 | 0.108 | 0.001 | 0.01 * |
| SA-JU | 3.490 | 0.212 | 0.001 | 0.01 * |
| SA-YA | 2.193 | 0.135 | 0.001 | 0.01 * |
| JU-YA | 2.780 | 0.156 | 0.001 | 0.01 * |
| S | H | D50 | h | v | DO | T | Stream Pattern & Bed Structure | Riparian Condition (VC and VH) |
|---|---|---|---|---|---|---|---|---|
| 0 | 4873 ± 2 | 5 | 0.2–0.8 | 0–0.1 | 6.4 ± 0.2 | 2.4 ± 0.1 | wetland, mud pool | Alpine meadow, VC = 80%, VH = 1–5 cm |
| 1 | 4484 ± 3 | 40 | 0–0.2 | 0.3–0.5 | 8.4 ± 0.2 | 1.3 ± 0.1 | Step-pool developed | Alpine meadow, VC = 100%, VH = 1–5 cm |
| 2 | 4228 ± 2 | 100 | 0.1–0.4 | 0.83 | 8.1 ± 0.1 | 3.5 ± 0.2 | stable bed | Alpine meadow, VC = 100%, VH = 1–5 cm |
| 3 | 4014 ± 3 | 50 | 0–0.25 | 0.3–0.5 | 7.9 ± 0.3 | 4.7 ± 0.1 | braided stream, macro-algae covered bed | Channelized bank, VC < 5% |
| 4 | 3916 ± 4 | 5 | 0–0.15 | 0.1–0.3 | 6.2 ± 0.2 | 10.5 ± 0.2 | wetland linked with channel, rich humus | Alpine meadow, VC = 100%, VH = 5–20 cm |
| 5 | 3901 ± 2 | 5 | 0.2–0.4 | 0.1 | 7.9 ± 0.3 | 3.1 ± 0.2 | barrier lake, sand bed | No vegetation |
| 6 | 3768 ± 2 | 10 | 0–0.3 | 0.3 | 9.6 ± 0.2 | 17.4 ± 0.3 | braided, gravel bed | No vegetation |
| 7 | 3752 ± 4 | 300 | 0.1–0.3 | 0.3–0.5 | 8.5 ± 0.1 | 2.2 ± 0.2 | glacial stream, gravel bed | Shrub, VC = 10% |
| 8 | 3598 ± 5 | 150 | 0–0.5 | 0.3–0.8 | 10.0 ± 0.3 | 10.5 ± 0.2 | braided river, wide valley, gravel bed | Herbaceous vegetation and trees, VC = 100% |
| 9 | 3566 ± 3 | 200 | 0–0.5 | 0.3 | 7.7 ± 0.2 | 12.4 ± 0.3 | braided river, wide valley, gravel-clay bed | No vegetation |
| 10 | 3514 ± 4 | 500 | 0–0.4 | 0.3–0.5 | 8.1 ± 0.4 | 7.2 ± 0.2 | step-pool developed | Channelized bank, VC < 2% |
| 11 | 3237 ± 3 | 1000 | 0.5–1.5 | 0.5–1.5 | 8.0 ± 0.3 | 8.0 ± 0.1 | step-pool developed | No vegetation |
| 12 | 2993 ± 4 | 800 | 0.1–0.5 | 0 | 6.7 ± 0.1 | 13.0 ± 0.2 | river bend, lentic | No vegetation |
| 13 | 2959 ± 3 | 300 | 0.2–0.4 | 1.5–2 | 7.8 ± 0.4 | 8.0 ± 0.3 | gravel bed, lotic | Alpine meadow, VC = 50%, VH = 5–200 cm |
| 14 | 2948 ± 4 | 30 | 0.3–1.0 | 0.3–2.0 | 7.5 ± 0.2 | 15.0 ± 0.2 | wetland, gravel bed | Alpine meadow, VC = 80%, VH = 1–10 cm |
| 15 | 2744 ± 4 | 150 | 0.1–0.5 | 0.5–1.0 | 8.5 ± 0.3 | 10.1 ± 0.1 | stable bed | Herbaceous vegetation, VC < 10% |
| 16 | 2228 ± 5 | 150 | 0–0.2 | 0.3 | 9.6 ± 0.1 | 10.3 ± 0.2 | gravel bed, river bend | Herbaceous vegetation and shrubs, VC > 80%, VH = 20–500 cm |
| 17 | 2208 ± 6 | 5 | 0.2–0.4 | 0.1 | 7.9 ± 0.2 | 13.2 ± 0.2 | barrier lake, sand bed | No vegetation |
| 18 | 1998 ± 5 | 180 | 0–0.4 | 0.3–0.5 | 9.6 ± 0.2 | 11.0 ± 0.2 | gravel and sand bed | Herbaceous vegetation and shrubs, VC > 60%, VH = 10–200 cm |
| 19 | 1100 ± 6 | 50 | 0.1–0.3 | 0.3–0.8 | 9.5 ± 0.3 | 15.0 ± 0.1 | stable bed | Herbaceous vegetation and shrubs, VC > 70%, VH = 20–300 cm |
| 20 | 1001 ± 5 | 120 | 0–0.3 | 0–0.3 | 9.3 ± 0.4 | 14.4 ± 0.2 | Step-pool developed | Herbaceous vegetation and shrubs, VC > 70%, VH = 20–200 cm |
| 21 | 718 ± 7 | 300 | 0–0.5 | 0.3–0.5 | 9.2 ± 0.1 | 13.5 ± 0.3 | river bend, lentic | No vegetation |
| 22 | 705 ± 6 | 200 | 0–0.4 | 0.3–0.5 | 9.5 ± 0.3 | 14.6 ± 0.1 | gravel bed, lotic | No vegetation |
| 23 | 688 ± 8 | 200 | 0–0.4 | 0.4–0.5 | 9.4 ± 0.2 | 14.1 ± 0.2 | river bend, lentic | No vegetation |
| Family | Genus | S0 | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | S17 | S18 | S19 | S20 | S21 | S22 | S23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Planariidae | n.d. | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pratylenchidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Glossiphoniidae | n.d. | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glossiphoniidae | Helobdella | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Piscicolidae | Piscicola geometra | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Branchiobdellidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tubificidae | Limnodrilus sp. | 0 | 0 | 0 | 0 | 5 | 0 | 2 | 2 | 10 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Limnodrilus claparedeianus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 256 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Limnodrilus hoffmeisteri | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 2 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rhyacodrilus stephesoni | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 148 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tubifex tubifex | 15 | 0 | 0 | 0 | 0 | 6 | 1 | 2 | 120 | 38 | 0 | 0 | 2 | 1 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lumbriculidae | Lumbriculus variegatus | 0 | 0 | 0 | 0 | 21 | 0 | 0 | 0 | 102 | 0 | 0 | 0 | 0 | 0 | 65 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Enchytraediae | n.d. | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Naididae | Chaetogaster diaphanus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nais barbata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Nais bretscheri | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nais communis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nais elinguis | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nais pardalis | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Paranais frici | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 9 | 274 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Stylaria lacustris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Uncinais uncinata | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lymnaeidae | Radix sp1 | 0 | 0 | 0 | 0 | 3 | 0 | 16 | 0 | 53 | 2 | 0 | 20 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Radix sp2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 2 | 0 | 19 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Planorbidae | Gyraulus sp. | 0 | 0 | 0 | 0 | 36 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hippeutis sp. | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Physidae | Physa sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 133 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ampullariidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sphaeriidae | n.d. | 46 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gammaridae | n.d. | 0 | 1 | 0 | 0 | 0 | 0 | 55 | 0 | 22 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Clausidiidae | 57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Hydrachnidae | n.d. | 0 | 253 | 0 | 47 | 0 | 0 | 7 | 0 | 78 | 10 | 220 | 4 | 22 | 0 | 47 | 8 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Entomobryomorpha | n.d. | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elmidae | n.d. | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Chrysomelidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ptilodactylidae | Stenocolus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Dryopidae | Elmoparnus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ceratopogonidae | n.d. | 0 | 0 | 0 | 0 | 29 | 1 | 0 | 2 | 0 | 0 | 13 | 4 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chironomidae | Alotanypus sp. | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brillia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cardiocladius sp. | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 69 | 0 | 0 | 54 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Chaetodadius sp. | 0 | 0 | 0 | 133 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Conclvapelopia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cricotopus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Diamesa sp. | 0 | 45 | 0 | 0 | 0 | 168 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dicrotendipes sp. | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Eukiefferiella sp. | 0 | 4 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 52 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Heleniella sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Heterotrissodadius sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Kiefferulus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Larsia sp. | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Limnphyes sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Metriocnemus sp. | 35 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Micropsectra sp. | 0 | 11 | 0 | 0 | 0 | 0 | 15 | 0 | 3 | 136 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Monodiamesa sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 42 | 1 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Orthocladius sp. | 0 | 36 | 2 | 0 | 0 | 0 | 12 | 0 | 20 | 110 | 4 | 7 | 0 | 1 | 7 | 18 | 3 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | |
| Pagastia sp. | 0 | 19 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Paracladopelma sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Paradadopelma sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Parakiefferiella sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Parametriocnemus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Paratanytarsus sp. | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Paratendipes sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1156 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Paratrichodadius sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Polypedilum sp. | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 21 | 54 | 0 | 0 | 236 | 0 | 0 | 39 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Porilla sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Psectrodadius sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 308 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pseudodiamesa sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pseudorthocladius sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Radotanypus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rheocricotopus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Stictochironomus sp. | 0 | 0 | 0 | 0 | 62 | 0 | 58 | 0 | 22 | 578 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sympothastia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 45 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tanypus sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tanytarsus sp. | 3 | 0 | 0 | 0 | 0 | 0 | 11 | 19 | 0 | 0 | 0 | 9 | 5 | 4 | 10 | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tendipus sp. | 32 | 0 | 0 | 0 | 0 | 0 | 102 | 0 | 3 | 1170 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |
| Tvetenia sp. | 0 | 0 | 0 | 125 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Simuliidae | n.d. | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 8 | 10 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| Psychodidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dolichopodidae | n.d. | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Empididae | n.d. | 0 | 0 | 13 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stratiomyiidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tipulidae | Antocha sp. | 0 | 1 | 9 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 11 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hexatoma sp. | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 34 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Pedicia sp. | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 64 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tipula sp. | 1 | 2 | 2 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Blephariceridae | n.d. | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscidae | n.d. | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Syrphidae | n.d. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Baetidae | Baetiella sp. | 0 | 46 | 35 | 1 | 0 | 2 | 0 | 0 | 1 | 0 | 87 | 7 | 0 | 1 | 23 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Baetis sp. | 0 | 46 | 69 | 1 | 0 | 3 | 0 | 21 | 2 | 0 | 128 | 7 | 1 | 1 | 24 | 20 | 7 | 0 | 2 | 14 | 6 | 0 | 0 | 0 | |
| Ephemeridae | Ephemera sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Ecdyuridae | Cinygmina sp. | 0 | 0 | 79 | 0 | 0 | 0 | 0 | 20 | 0 | 0 | 64 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Iron sp. | 0 | 0 | 160 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 100 | 0 | 0 | 1 | 0 | 4 | 2 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | |
| Ephemerellidae | Drunella sp. | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Serratella sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Leptophlebiidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 72 | 0 | 0 | 0 | 1 | 0 | 240 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Siphlonuridae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Corixidae | n.d. | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Naucoridae | Gestroiella sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Hydropsychidae | n.d. | 0 | 38 | 0 | 16 | 0 | 0 | 1 | 0 | 1 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 104 | 14 | 0 | 0 | 0 |
| Rhyacophilidae | Himalopsyche sp. | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rhyacophila sp. | 0 | 0 | 16 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 59 | 4 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Limnophilidae | n.d. | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leptoceridae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 25 | 59 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydropsychidae | n.d. | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psychomyiidae | n.d. | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Molannidae | n.d. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brachycentridae | n.d. | 0 | 20 | 165 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hydrobiosidae | n.d. | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glossosomatidae | n.d. | 0 | 0 | 31 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 225 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philopotamidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Libellulidae | n.d. | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coenagrionidae | n.d. | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gomphus | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Aeshnidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sisyridae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Perlidae | n.d. | 0 | 37 | 0 | 1 | 0 | 0 | 1 | 10 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Chloroperlidae | n.d. | 0 | 47 | 30 | 0 | 0 | 0 | 0 | 37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nemouridae | n.d. | 0 | 0 | 13 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 70 | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Capniidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 0 | 0 | 69 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pteronarcidae | n.d. | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Perlodidae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 36 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 3 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Styloperlidae | Cerconychia sp. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Leuctridae | n.d. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhao, N.; Zhou, X.; Pan, B.; Liu, W.; Tian, S.; Wang, Z. Macroinvertebrate Biodiversity Trends and Habitat Relationships within Headwater Rivers of the Qinghai-Tibet Plateau. Water 2018, 10, 1214. https://doi.org/10.3390/w10091214
Xu M, Zhao N, Zhou X, Pan B, Liu W, Tian S, Wang Z. Macroinvertebrate Biodiversity Trends and Habitat Relationships within Headwater Rivers of the Qinghai-Tibet Plateau. Water. 2018; 10(9):1214. https://doi.org/10.3390/w10091214
Chicago/Turabian StyleXu, Mengzhen, Na Zhao, Xiongdong Zhou, Baozhu Pan, Wei Liu, Shimin Tian, and Zhaoyin Wang. 2018. "Macroinvertebrate Biodiversity Trends and Habitat Relationships within Headwater Rivers of the Qinghai-Tibet Plateau" Water 10, no. 9: 1214. https://doi.org/10.3390/w10091214
APA StyleXu, M., Zhao, N., Zhou, X., Pan, B., Liu, W., Tian, S., & Wang, Z. (2018). Macroinvertebrate Biodiversity Trends and Habitat Relationships within Headwater Rivers of the Qinghai-Tibet Plateau. Water, 10(9), 1214. https://doi.org/10.3390/w10091214





