Zinc(II) Adsorption by Low-Carbon Shungite: The Effect of pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Taurit
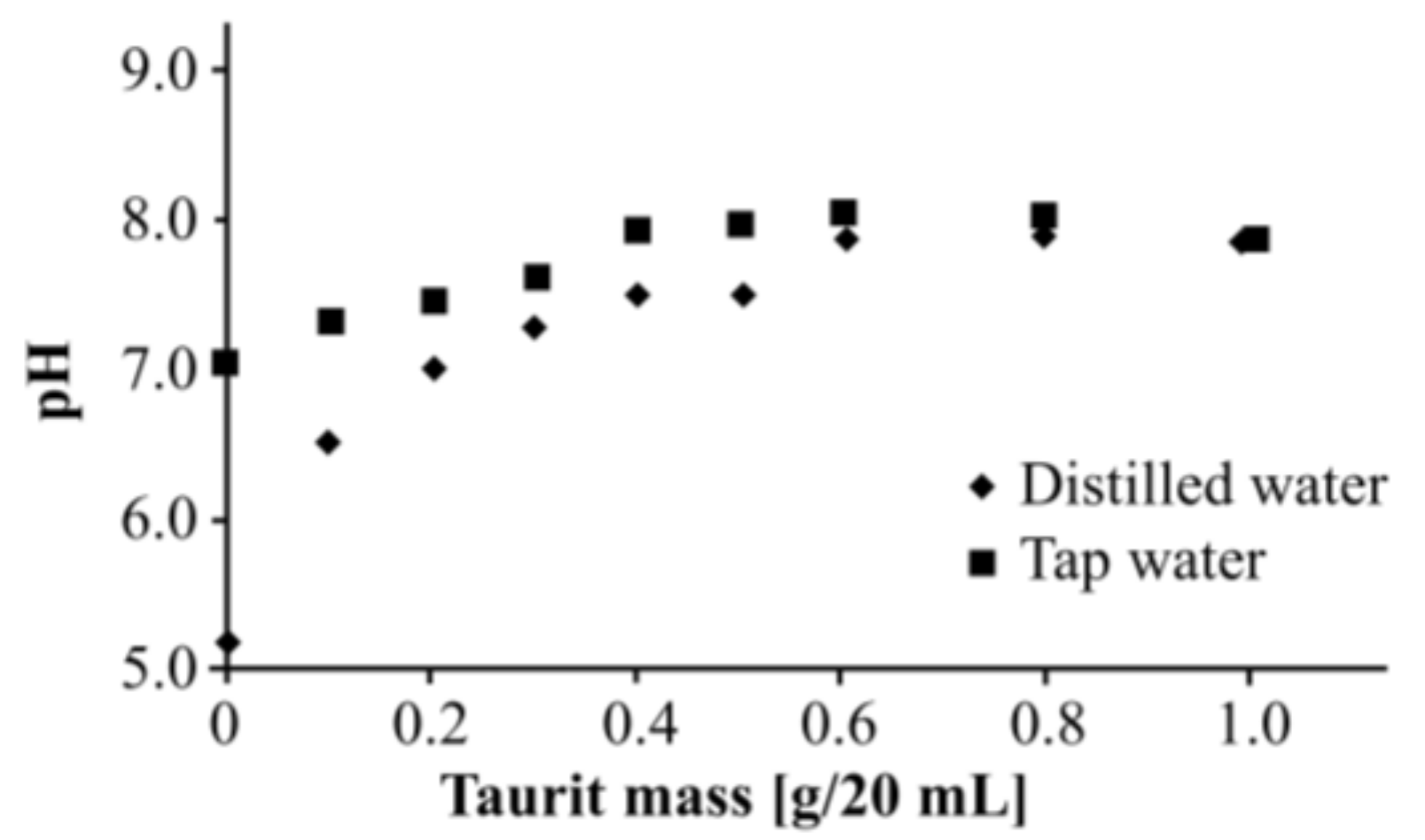
2.2. pH Tests
2.3. Buffer Capacity of Taurit
2.4. Batch Sorption Studies
3. Results and Discussion
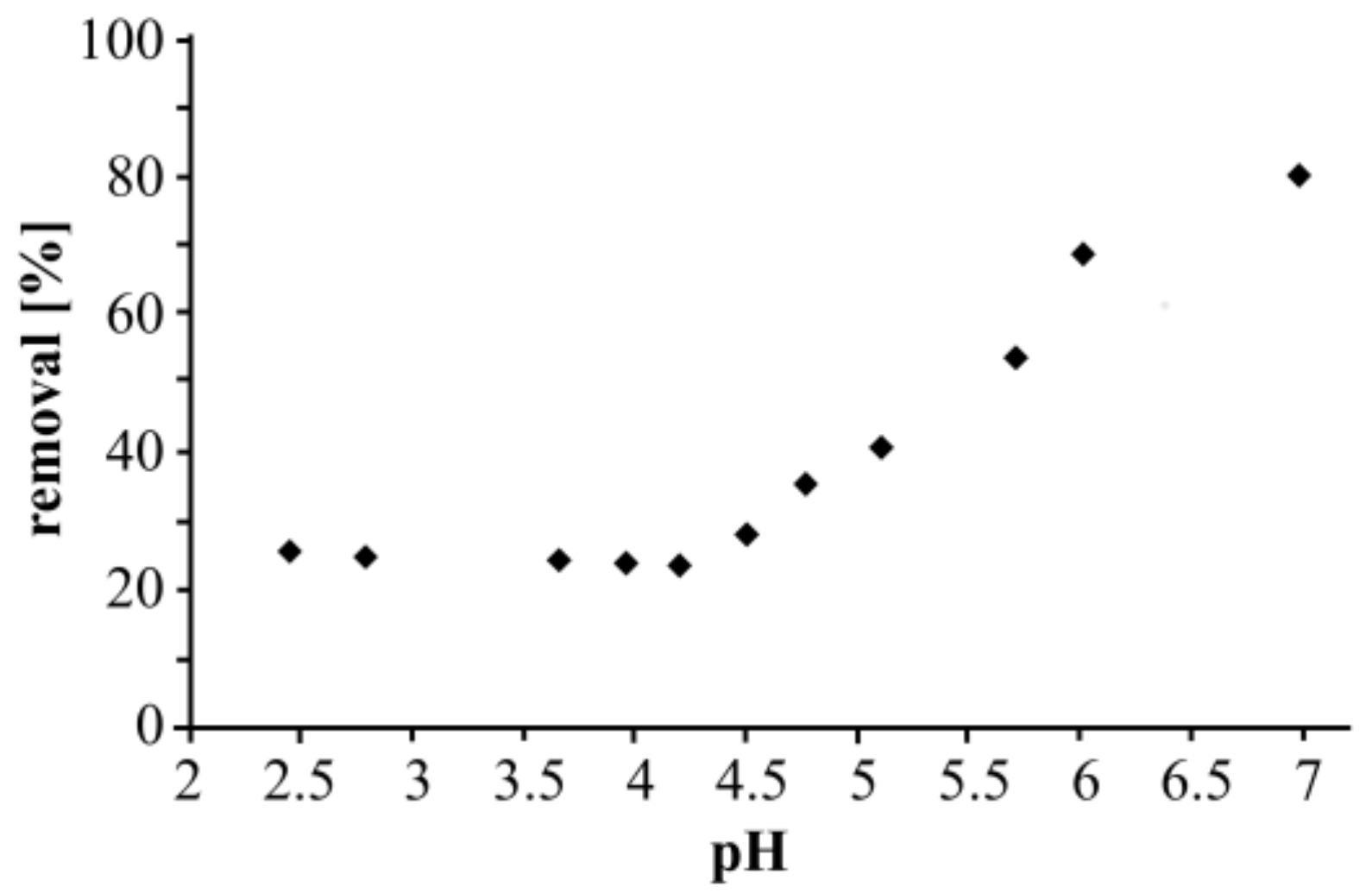
3.1. Influence of pH
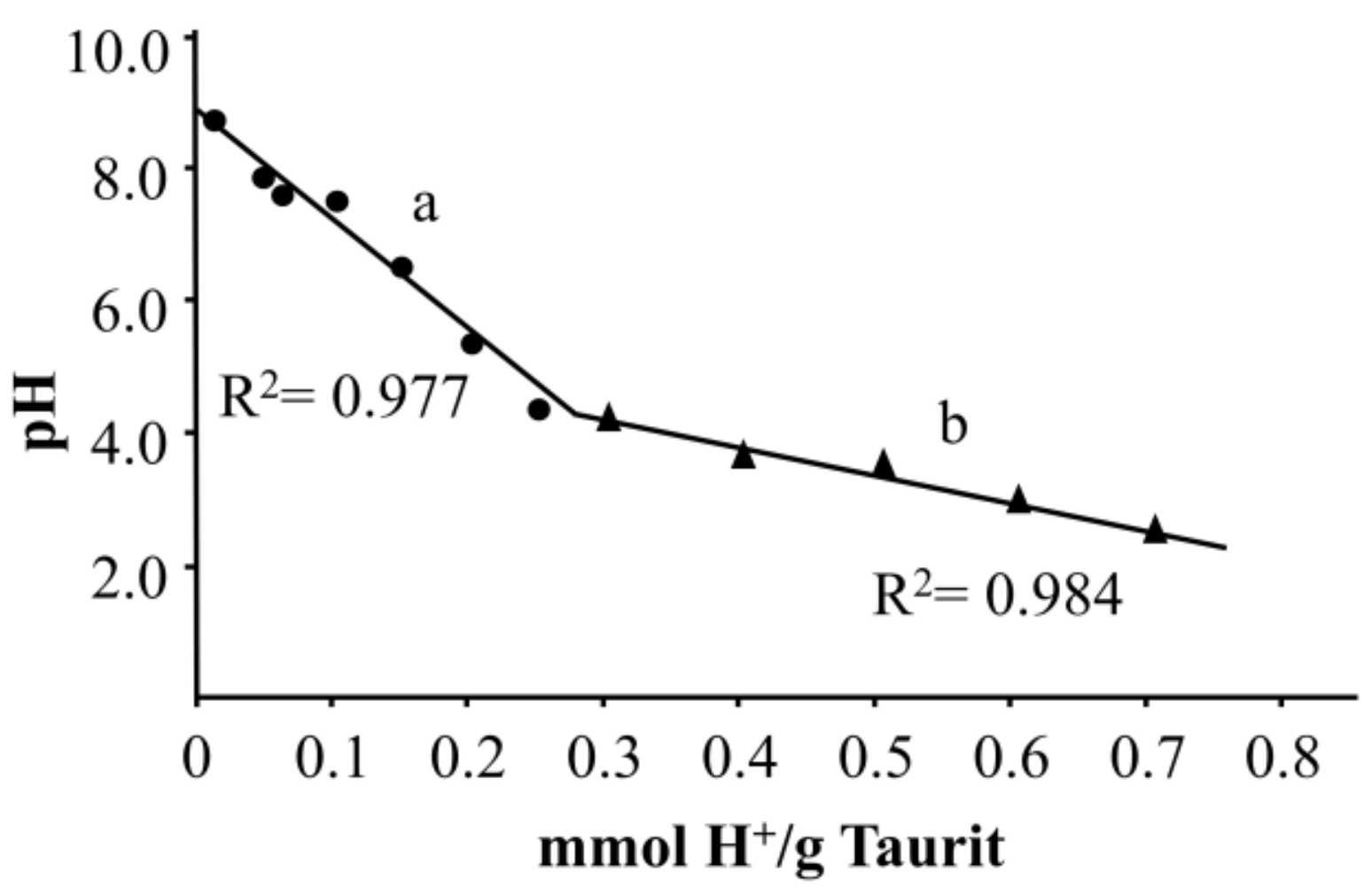
3.2. Acid Neutralization Capacity (ANC)
3.3. Contact Time
3.4. Adsorption Experiments
3.5. Influence of Sulfate Concentration
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IMA List of Minerals, International Mineralogical Association (IMA). Available online: http://nrmima.nrm.se//imalist.htm (accessed on 9 January 2018).
- Hettich, R.L.; Buseck, P.R. Concerning fullerenes in shungite. Carbon 1996, 34, 685–687. [Google Scholar] [CrossRef]
- Mosin, O.; Ignatov, I. The structure and composition of natural carbonaceous fullerene containing mineral shungite. Int. J. Adv. Sci. Technol. Res. 2013, 3, 9–21. [Google Scholar]
- Kazankapova, M.K.; Bekjanova, A.J.; Efremov, S.A.; Nurtaeva, A.K.; Nauryzbaev, M.K. Treatment of oil-containing wastewater using microorganisms immobilized on shungite. Int. J. Biol. Chem. 2013, 5, 104–110. [Google Scholar]
- Oh, H.-M.; Ku, Y.-H.; Ahn, K.-H.; Kwon, G.-S.; Kho, Y.-H.; Mheen, T.-I.; Yoon, B.-D. Phenolic wastewater treatment by a mixed culture GE2 immobilized on activated carbon. J. Microb. Biot. 1996, 6, 116–119. [Google Scholar]
- Ibragimova, N.; (Kazakh-German University, Almaty, Kazakhstan). Personal communication, 2015.
- Kenzhebayev, N.; Ibragimova, N.; Biyasheva, Z.; Sennik, A. Assessment of quality of water from open water sources in the Almaty HPS-2 influence area. J. Biotechnol. 2014, 185, S23. [Google Scholar] [CrossRef]
- Zwain, H.M.; Vakili, M.; Dahlan, I. Waste material adsorbents for zinc removal from wastewater: A comprehensive review. Int. J. Chem. Eng. 2014, 2014. [Google Scholar] [CrossRef]
- Rowell, D.L. Bodenkunde: Untersuchungsmethoden und ihre Anwendungen; Springer: Heidelberg, Germany, 1997; pp. 288–289. ISBN 978-3-642-59093-1. [Google Scholar]
- Peric, J.; Trgo, M.; Vukojevic Medvidovic, N. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Minceva, M.; Fajgar, R.; Markovska, L.; Meshko, V. Comparative study of Zn2+, Cd2+, and Pb2+ removal from water solution using natural clinoptilolitic zeolite and commercial granulated activated carbon. Equilibrium of adsorption. Sep. Sci. Technol. 2008, 43, 2117–2143. [Google Scholar] [CrossRef]
- Al-Tohami, F.; Ackacha, M.A.; Belaid, R.A.; Hamaadi, M. Adsorption of Zn (II) ions from aqueous solutions by novel adsorbent: Ngella sativa seeds. APCBEE Proc. 2013, 5, 400–404. [Google Scholar] [CrossRef]
- Shavandi, M.A.; Haddadian, Z.; Ismail, M.H.S.; Abdullah, N.; Abidin, Z.Z. Removal of Fe(III), Mn(II) and Zn(II) from palm oil mill effluent (POME) by natural zeolite. J. Taiwan Inst. Chem. Eng. 2012, 43, 750–759. [Google Scholar] [CrossRef]
- Gonzalez, P.G.; Pliego-Cuervo, Y.B. Adsorption of Cd(II), Hg(II) and Zn(II) from aqueous solution using mesoporous activated carbon produced from Bambusa vulgaris striata. Chem. Eng. Res. Des. 2014, 92, 2715–2724. [Google Scholar] [CrossRef]
- Khademi, Z.; Ramavandi, B.; Ghaneian, M.T. The behaviors and characteristics of a mesoporous activated carbon prepared from Tamarix hispida for Zn(II) adsorption from wastewater. J. Environ. Chem. Eng. 2015, 3, 2057–2067. [Google Scholar] [CrossRef]
- Amuda, O.S.; Giwa, A.A.; Bello, I.A. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Sreejalekshmi, K.G.; Vimexen, V.; Dev, V.V. Evaluation of adsorption properties of sulphurised activated carbon for the effective and economically viable removal of Zn(II) from aqueous solutions. Ecotox. Environ. Saf. 2016, 124, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Reichle, R.A.; McCurdy, K.G.; Hepler, L.G. Zinc hydroxide: Solubility product and hydroxy-complex stability constants from 12.5–75 °C. Can. J. Chem. 1975, 53, 3841–3845. [Google Scholar] [CrossRef]
- Lim, H.K.; Teng, T.T.; Ibrahim, M.H.; Ahmad, A.; Chee, H.T. Adsorption and removal of zinc (II) from aqueous solution using powdered fish bones. APCBEE Proc. 2012, 1, 96–102. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Rodríguez, A.; Álvarez, S.; García, J. Removal of caffeine and diclofenac on activated carbon in fixed bed column. Chem. Eng. Res. Des. 2012, 90, 967–974. [Google Scholar] [CrossRef]
- Shrestha, S.; Son, G.; Lee, S.H.; Lee, T.G. Isotherm and thermodynamic studies of Zn (II) adsorption on lignite and coconut shell-based activated carbon fiber. Chemosphere 2013, 92, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.P.C.; Satyaveni, S.; Ramesh, A.; Seshaiah, K.; Murthy, K.S.N.; Choudary, N.V. Sorption of cadmium and zinc from aqueous solutions by zeolite 4A, zeolite 13X and bentonite. J. Environ. Manag. 2006, 81, 265–272. [Google Scholar]
- Simantiraki, F.; Gidarakos, E. Comparative assessment of compost and zeolite utilisation for the simultaneous removal of BTEX, Cd and Zn from the aqueous phase: Batch and continuous flow study. J. Environ. Manag. 2015, 159, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Depci, T.; Kul, A.R.; Önal, Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: Study in single- and multi-solute systems. Chem. Eng. J. 2012, 200–202, 224–236. [Google Scholar] [CrossRef]
- Ramos, R.L.; Jacome, L.A.B.; Barron, J.M.; Rubio, L.F.; Coronado, R.M.G. Adsorption of zinc(II) from an aqueous solution onto activated carbon. J. Hazard. Mater. 2002, B90, 27–38. [Google Scholar] [CrossRef]
- Jakubczyk, P.; (GHI Company, Pirna, Germany). Personal communication, 2018.


| pH 7.0 | pH 8.6 | pH 9.6 | pH 10.9 |
|---|---|---|---|
| 54.4 mg L−1 | 0.34 mg L−1 | 0.02 mg L−1 | 0.06 mg L−1 |
| Sand | Clay | Humus | Peat |
|---|---|---|---|
| 16 | 80 | 80 | 128 |
| Authors | Adsorbent | BET (m2 g−1) | kf (mg Zn/gads)/(mg/L) | n |
|---|---|---|---|---|
| [24] | Zeolite 4A | - | 1.141 | 2.200 |
| [24] | Zeolite 13X | - | 0.801 | 2.315 |
| [24] | Bentonite | - | 0.627 | 2.377 |
| [13] | Zeolite | 30–60 | 0.015 | 3.058 |
| [25] | Zeolite | 26 | 0.924 | 1.89 |
| [14] | Sulfurated AC | - | 20.7 | 0.32 |
| [15] | AC | 608 | 0.16 | 1.0 |
| [17] | AC | 1006 | 41.63 | 3.214 |
| [22] | Coconut AC | - | 0.957 | 43 |
| [26] | AC | - | 2.41 | 2.22 |
| [27] | AC | 768 | 10.05 | 5.54 |
| [27] | AC (F-400) | 987 | 1.022 | 2.05 |
| [27] | AC (F-300) | 863 | 1.827 | 3.38 |
| This study | Taurit/dist H2O | 13.4 | 2.4 | 4.0 |
| This study | Taurit/tap H2O | 13.4 | 1.5 | 2.5 |
| Taurit Mass (g) | 24 h | 7 Days |
|---|---|---|
| 0.1 | 3.7 | 9.2 |
| 0.2 | 3.9 | 9.2 |
| 0.3 | 3.1 | 5.9 |
| 0.4 | 2.0 | 2.9 |
| 0.5 | 3.3 | 2.0 |
| 0.6 | 1.1 | 3.9 |
| 0.8 | 1.2 | 3.9 |
| 1.0 | 0 | 1.3 |
| 1.1 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.R.; Sgolik, L.; Kreller, A.; Dornack, C. Zinc(II) Adsorption by Low-Carbon Shungite: The Effect of pH. Water 2018, 10, 422. https://doi.org/10.3390/w10040422
Fischer AR, Sgolik L, Kreller A, Dornack C. Zinc(II) Adsorption by Low-Carbon Shungite: The Effect of pH. Water. 2018; 10(4):422. https://doi.org/10.3390/w10040422
Chicago/Turabian StyleFischer, Axel R., Luisa Sgolik, André Kreller, and Christina Dornack. 2018. "Zinc(II) Adsorption by Low-Carbon Shungite: The Effect of pH" Water 10, no. 4: 422. https://doi.org/10.3390/w10040422




