Review of Heavy Metal Adsorption Processes by Several Organic Matters from Wastewaters
Abstract
:1. Introduction
2. Adsorption Efficiency
3. Comparison of Different Adsorption Surfaces
4. Environmental Economics Concepts Related to Practical Application
4.1. Biosorption of Heavy Metals and Water Footprint Calculation through the Example of Algae
- Exact comparative analysis of the water footprints of chemical-based heavy metal adsorption water treatment methods;
- Comparative analysis of the water footprints of heavy metal binding biosorbent planning methods, with additional field comparison;
- Application of water availability indicators for production fields of heavy metal binding biosorbent;
- Application of circular economy value (CEV) indicator for comparing chemical-based and biological-based heavy metal adsorption methods.
4.2. Water Allowance Coefficient—A Tool for Expressing the Water Value of Heavy Metal Adsorption
- WACi = water allowance coefficient, based on algae water footprint changes at region i.
- WFalgae,i = changes of algae water footprint at region i, %.
5. Conclusions, and Additional Scientific Value
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
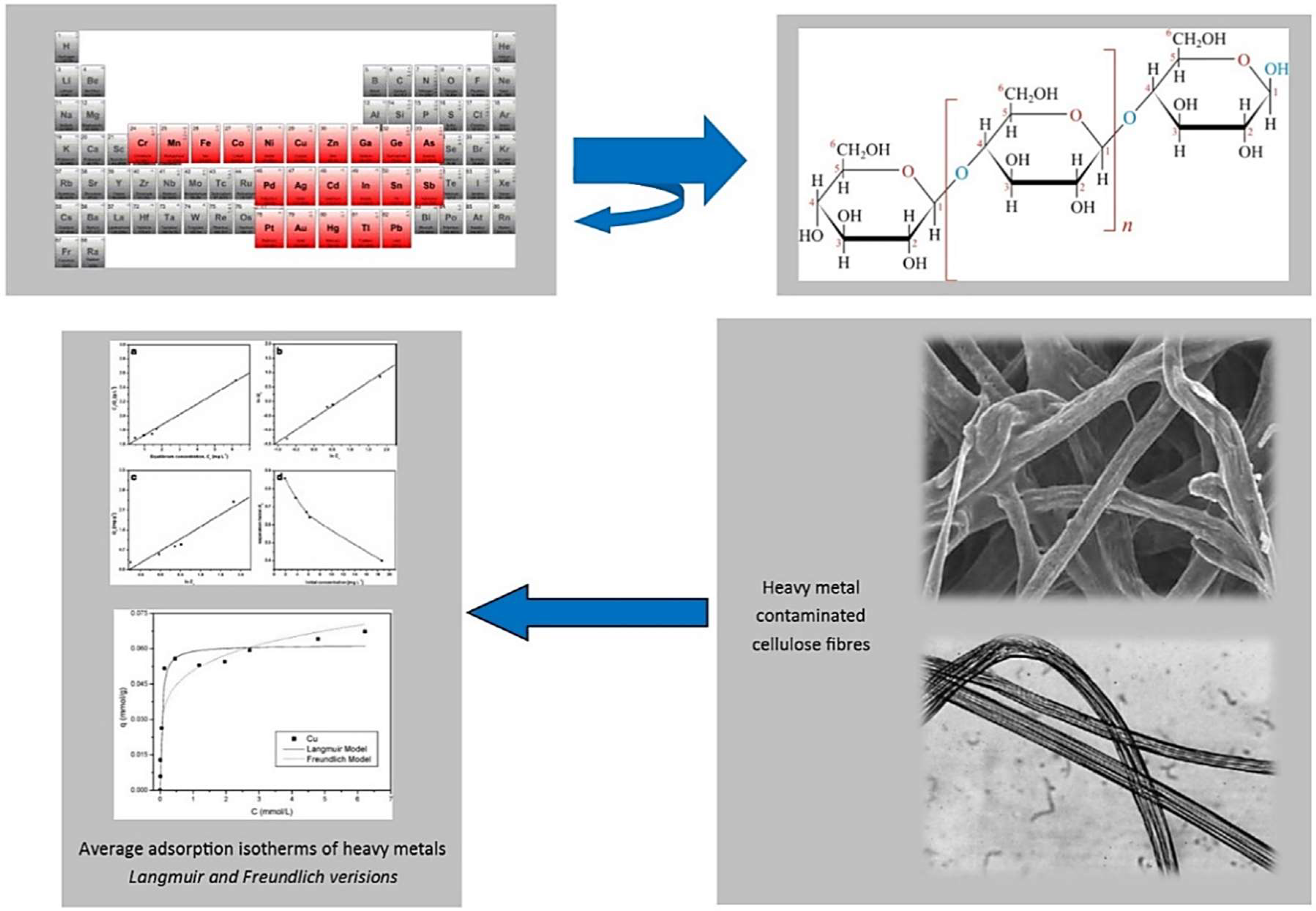
References
- Jeppu, G.P.; Clement, T.P.; Barnett, M.O.; Lee, K.-K. A modified batch reactor system to study equilibrium-reactive transport problems. Sorpt. Transp. Process. Affect. Fate Environ. Pollut. Subsurf. 2012, 129–130, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Jeppu, G.P.; Clement, T.P. A modified Langmuir-Freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol. 2012, 129–130, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.-B.; Yi, A.-H.; Zhang, Z.-Q.; Tang, C.-L.; Zhang, X.-C.; Gao, J.-M. A New Competitive Adsorption Isothermal Model of Heavy Metals in Soils*1 Project supported by the Program for Changjiang Scholars and Innovative Research Team in University of China (No.IRT0749). Pedosphere 2009, 19, 251–257. [Google Scholar] [CrossRef]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Syouf, M.Q.A. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Asuquo, E.; Martin, A.; Nzerem, P.; Siperstein, F.; Fan, X. Adsorption of Cd(II) and Pb(II) ions from aqueous solutions using mesoporous activated carbon adsorbent: Equilibrium, kinetics and characterisation studies. J. Environ. Chem. Eng. 2017, 5, 679–698. [Google Scholar] [CrossRef] [Green Version]
- Kenawy, I.M.; Hafez, M.A.H.; Ismail, M.A.; Hashem, M.A. Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from aqueous single metal solutions by guanyl-modified cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Božić, D.; Gorgievski, M.; Stanković, V.; Štrbac, N.; Šerbula, S.; Petrović, N. Adsorption of heavy metal ions by beech sawdust—Kinetics, mechanism and equilibrium of the process. Ecol. Eng. 2013, 58, 202–206. [Google Scholar] [CrossRef]
- An, F.-Q.; Wu, R.-Y.; Li, M.; Hu, T.-P.; Gao, J.-F.; Yuan, Z.-G. Adsorption of heavy metal ions by iminodiacetic acid functionalized D301 resin: Kinetics, isotherms and thermodynamics. React. Funct. Polym. 2017, 118, 42–50. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Jalajamony, S.; Sreekumari, S.S. Adsorption of heavy metal ions from aqueous solutions by amine and carboxylate functionalised bentonites. Appl. Clay Sci. 2012, 65–66, 67–71. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.V.; Magroa, J.D. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Adsorption of Pb2+, Ni2+, Cu2+, Co2+ metal ions from aqueous solution by PPI/SiO2 as new high performance adsorbent: Preparation, characterization, isotherm, kinetic, thermodynamic studies. J. Mol. Liq. 2017, 237, 428–436. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar]
- Tovar-Gómez, R.; del Moreno-Virgen, M.; Moreno-Pérez, J.; Bonilla-Petriciolet, A.; Hernández-Montoya, V.; Durán-Valle, C.J. Analysis of synergistic and antagonistic adsorption of heavy metals and acid blue 25 on activated carbon from ternary systems. Chem. Eng. Res. Des. 2015, 93, 755–772. [Google Scholar] [CrossRef]
- Werner, D.; Karapanagioti, H.K.; Sabatini, D.A. Assessing the effect of grain-scale sorption rate limitations on the fate of hydrophobic organic groundwater pollutants. J. Contam. Hydrol. 2012, 129–130, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Guiza, S. Biosorption of heavy metal from aqueous solution using cellulosic waste orange peel. Ecol. Eng. 2017, 99, 134–140. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; de Neto, A.F.; da Silva, M.G.C.; NÃbrega, C.C.; Filho, A.A.M. Characterization and use of in natura and calcined rice husks for biosorption of heavy metals ions from aqueous effluents. Braz. J. Chem. Eng. 2012, 29, 619–634. [Google Scholar] [CrossRef] [Green Version]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; Young, T.M. Clogging influence on metals migration and removal in sub-surface flow constructed wetlands. J. Contam. Hydrol. 2012, 129–130, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, K.; Li, J.; Ying, D.; Wang, Y.; Jia, J. Comparative and competitive adsorption of Pb(II) and Cu(II) using tetraethylenepentamine modified chitosan/CoFe2O4 particles. J. Hazard. Mater. 2017, 326, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, O.; Goodarzi, V.; Saeb, M.R.; Mahmoodi, N.M.; Borja, R. Competitive removal of heavy metal ions from squid oil under isothermal condition by CR11 chelate ion exchanger. J. Hazard. Mater. 2017, 334, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Power, L.M.; Cheng, T.; Rastghalam, Z.S. Cu and Zn adsorption to a heterogeneous natural sediment: Influence of leached cations and natural organic matter. Chemosphere 2016, 144, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Hussain, M.A.; Sher, M.; Irfan, M.I.; Tahir, M.N.; Tremel, W.; Hussain, S.Z.; Hussain, I. Design, characterization and evaluation of hydroxyethylcellulose based novel regenerable supersorbent for heavy metal ions uptake and competitive adsorption. Int. J. Biol. Macromol. 2017, 102, 170–180. [Google Scholar] [CrossRef] [PubMed]
- SReddy, R.; Pandey, N.K.; Mallika, C.; Mudali, U.K. Equilibrium and kinetics of adsorption of ruthenium on activated charcoal from nitric acid solutions. Chem. Eng. Res. Des. 2016, 115, 91–97. [Google Scholar]
- Bulgariu, D.; Bulgariu, L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour. Technol. 2012, 103, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Adamczuk, A.; Kołodyńska, D. Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem. Eng. J. 2015, 274, 200–212. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Erratum to “Transport of biocolloids in water saturated columns packed with sand: Effect of grain size and pore water velocity” [Journal of Contaminant Hydrology 126 (2011) 301–314]. J. Contam. Hydrol. 2012, 129–130, 10. [Google Scholar] [CrossRef]
- Bohli, T.; Ouederni, A.; Fiol, N.; Villaescusa, I. Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. C. R. Chim. 2015, 18, 88–99. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Kolbasov, A.; Sinha-Ray, S.; Yarin, A.L.; Pourdeyhimi, B. Heavy metal adsorption on solution-blown biopolymer nanofiber membranes. J. Membr. Sci. 2017, 530, 250–263. [Google Scholar] [CrossRef]
- Xia, Z.; Baird, L.; Zimmerman, N.; Yeager, M. Heavy metal ion removal by thiol functionalized aluminum oxide hydroxide nanowhiskers. Appl. Surf. Sci. 2017, 416, 565–573. [Google Scholar] [CrossRef]
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atiehef, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Ma, J.; Qin, G.; Zhang, Y.; Sun, J.; Wang, S.; Jiang, L. Heavy metal removal from aqueous solutions by calcium silicate powder from waste coal fly-ash. J. Clean. Prod. 2018, 182, 776–782. [Google Scholar] [CrossRef]
- Vilardi, G.; Palma, L.D.; Verdone, N. Heavy metals adsorption by banana peels micro-powder: Equilibrium modeling by non-linear models. Chin. J. Chem. Eng. 2018, 26, 455–464. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Li, N.; Wang, W.; Nan, J.; Zhao, Z.; Cui, F. Highly efficient removal of bivalent heavy metals from aqueous systems by magnetic porous Fe3O4-MnO2: Adsorption behavior and process study. Chem. Eng. J. 2016, 304, 737–746. [Google Scholar] [CrossRef]
- Misra, R.K.; Jain, S.K.; Khatri, P.K. Iminodiacetic acid functionalized cation exchange resin for adsorptive removal of Cr(VI), Cd(II), Ni(II) and Pb(II) from their aqueous solutions. J. Hazard. Mater. 2011, 185, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hu, E.; Hu, Y. Impact of mineral micropores on transport and fate of organic contaminants: A review. J. Contam. Hydrol. 2012, 129–130, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Putro, J.N.; Santoso, S.P.; Ismadji, S.; Ju, Y.-H. Investigation of heavy metal adsorption in binary system by nanocrystalline cellulose—Bentonite nanocomposite: Improvement on extended Langmuir isotherm model. Microporous Mesoporous Mater. 2017, 246, 166–177. [Google Scholar] [CrossRef]
- Amela, K.; Hassen, M.A.; Kerroum, D. Isotherm and Kinetics Study of Biosorption of Cationic Dye onto Banana Peel. Energy Procedia 2012, 19, 286–295. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, P.; Peng, L.; Lin, Z.; Dang, Z. Kinetics of Heavy Metal Dissociation from Natural Organic Matter: Roles of the Carboxylic and Phenolic Sites. Environ. Sci. Technol. 2016, 50, 10476–10484. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-M.; Werner, D.; Choi, Y.; Luthy, R.G. Long-term monitoring and modeling of the mass transfer of polychlorinated biphenyls in sediment following pilot-scale in-situ amendment with activated carbon. J. Contam. Hydrol. 2012, 129–130, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Šoštarić, T.; Stojanović, M.; Petrović, J.; Mihajlović, M.; Ćosović, A.; Stanković, S. Mechanism of adsorption of Cu2+ and Zn2+ on the corn silk (Zea mays L.). Ecol. Eng. 2017, 99, 83–90. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Negm, N.A.; Hefni, H.H.H.; Wahab, M.M.A. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Can, N.; Ömür, B.C.; Altındal, A. Modeling of heavy metal ion adsorption isotherms onto metallophthalocyanine film. Sens. Actuators B Chem. 2016, 237, 953–961. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Kavitha, N.; Palanivelu, K. Recovery of copper(II) through polymer inclusion membrane with di (2-ethylhexyl) phosphoric acid as carrier from e-waste. J. Membr. Sci. 2012, 415–416, 663–669. [Google Scholar] [CrossRef]
- Lü, L.; Lu, D.; Chen, L.; Luo, F. Removal of Cd(II) by modified lawny grass cellulose adsorbent. Desalination 2010, 259, 120–130. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Bourceanu, G. Removal of chromium(VI) from aqueous solutions using a polyvinyl-chloride inclusion membrane: Experimental study and modelling. Chem. Eng. J. 2013, 220, 24–34. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Sharma, D.C.; Forster, C.F. Removal of hexavalent chromium using sphagnum moss peat. Water Res. 1993, 27, 1201–1208. [Google Scholar] [CrossRef]
- Melita, L.; Popescu, M. Removal of Cr(VI) from industrial water effluents and surface waters using activated composite membranes. J. Membr. Sci. 2008, 312, 157–162. [Google Scholar] [CrossRef]
- Xiyili, H.; Çetintaş, S.; Bingöl, D. Removal of some heavy metals onto mechanically activated fly ash: Modeling approach for optimization, isotherms, kinetics and thermodynamics. Process Saf. Environ. Prot. 2017, 109, 288–300. [Google Scholar] [CrossRef]
- Ko, D.; Lee, J.S.; Patel, H.A.; Jakobsen, M.H.; Hwang, Y.; Yavuz, C.T.; Hansen, H.C.B.; Andersen, H.R. Selective removal of heavy metal ions by disulfide linked polymer networks. J. Hazard. Mater. 2017, 332, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pan, Y.; Cai, P.; Guo, T.; Xiao, H. Single and binary adsorption of heavy metal ions from aqueous solutions using sugarcane cellulose-based adsorbent. Bioresour. Technol. 2017, 241, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Bryant, P.S.; Petersen, J.N.; Lee, J.M.; Brouns, T.M. Sorption of heavy metals by untreated red fir sawdust. Appl. Biochem. Biotechnol. 1992, 34–35, 777–788. [Google Scholar] [CrossRef]
- Höhener, P.; Yu, X. Stable carbon and hydrogen isotope fractionation of dissolved organic groundwater pollutants by equilibrium sorption. J. Contam. Hydrol. 2012, 129–130, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Tebo, B.M. Surface charge properties of and Cu(II) adsorption by spores of the marine Bacillus sp. strain SG-1. Appl. Environ. Microbiol. 1998, 64, 1123–1129. [Google Scholar] [PubMed]
- Kim, N.; Park, M.; Park, D. A new efficient forest biowaste as biosorbent for removal of cationic heavy metals. Bioresour. Technol. 2015, 175, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Groenenberg, J.E.; Lofts, S. The use of assemblage models to describe trace element partitioning, speciation, and fate: A review. Environ. Toxicol. Chem. 2014, 33, 2181–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhre, N.A.; Ibrahim, B.M. The use of new chemically modified cellulose for heavy metal ion adsorption. J. Hazard. Mater. 2018, 343, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, D.-J. Thermodynamic parameters for adsorption equilibrium of heavy metals and dyes from wastewaters. Bioresour. Technol. 2014, 160, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Syngouna, V.I.; Chrysikopoulos, C.V. Transport of biocolloids in water saturated columns packed with sand: Effect of grain size and pore water velocity. J. Contam. Hydrol. 2012, 129–130, 11–24. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kong, H.; Jang, J. Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles. J. Colloid Interface Sci. 2011, 359, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Sillanpää, M. Heavy metals adsorption by novel EDTA-modified chitosan–silica hybrid materials. J. Colloid Interface Sci. 2011, 358, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zaini, M.A.A.; Amano, Y.; Machida, M. Adsorption of heavy metals onto activated carbons derived from polyacrylonitrile fiber. J. Hazard. Mater. 2010, 180, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-Y.; Hong, J.-Y.; Jang, J. Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles: Isotherms and kinetic study. J. Hazard. Mater. 2011, 190, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Tofighy, M.A.; Mohammadi, T. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Visa, M.; Bogatu, C.; Duta, A. Simultaneous adsorption of dyes and heavy metals from multicomponent solutions using fly ash. Appl. Surf. Sci. 2010, 256, 5486–5491. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.; Ang, H.M. Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus sheathiana bark: Kinetics, mechanism and equilibrium study. Process Saf. Environ. Prot. 2016, 102, 336–352. [Google Scholar] [CrossRef]
- Zheng, X.; Li, B.; Zhu, B.; Kuang, R.; Xu, B.; Ma, M. Crayfish Carapace Micro-powder (CCM): A Novel and Efficient Adsorbent for Heavy Metal Ion Removal from Wastewater. Water 2010, 2, 257–272. [Google Scholar] [CrossRef] [Green Version]
- Niuniavaite, D.; Baltakys, K.; Dambrauskas, T. The Adsorption Kinetic Parameters of Co2+ Ions by α-C2SH. Buildings 2018, 8, 10. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. Int. J. Environ. Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Ziames, G.G.; Khanna, V. Microalgal biomass production pathways: Evaluation of life cycle environmental impacts. Biotechnol. Biofules 2014, 88, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Munoz, R.; Gonzalez-Fernandez, C. Microalgae-Based Biofuels and Bioproducts; Woodhead Publishing: Cambridge, UK, 2017; p. 560. ISBN 978-0-081-01023-5. [Google Scholar]
- Feng, P.-Z.; Zhu, L.; Qin, X.-X.; Li, Z.-H. Water Footprint of Biodiesel Production from Microalgae Cultivated in Photobioreactors. J. Environ. Eng. 2016, 142, 1–5. [Google Scholar] [CrossRef]
- Gerbens-Leenes, P.W.; Xu, L.; de Vries, G.J.; Hoekstra, A.Y. The blue water footrpint and land use of biofuels from algae. Water Resour. Res. 2014, 50, 8549–8563. [Google Scholar] [CrossRef]
- Junior, E.N.; Kumar, M.; Pankratz, S.; Oyedun, A.O.; Kumar, A. Development of life cycle water footprints for the production of fuels and chemicals from algae biomass. Water Res. 2018, 140, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Batan, L.; Quinn, J.C.; Bradley, T.H. Analysis of water footprint of a photobioreactor microalgae biofuel production from blue, green and lifecycle perspectives. Algal Res. 2013, 3, 196–203. [Google Scholar] [CrossRef]
- BSubhadra, G.; Edwards, M. Coproduct market analysis and water footprint of simulated commercial algal biorefineries. Appl. Energy 2010, 88, 3515–3523. [Google Scholar] [CrossRef]
- Utomo, H.D.; Tan, K.X.D.; Choong, Z.Y.D.; Yu, J.J.; Ong, J.J.; Lim, Z.B. Biosorption of heavy metal by algae biomass in surface water. J. Environ. Prot. 2016, 7, 1547–1560. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, H.W.; Kao, P.C.; Pan, J.L.; Chang, J.S. Biosorption of cadmium by CO2-fixing microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Park, S.; Hong, H.J.; Choi, Y.E.; Yang, J.W. Biosorption of chromium (Cr(III)/Cr(VI)) on the residual microalga Nannochloris oculata after lipid extraction for biodiesel production. Bioresour. Technol. 2011, 102, 11155–11160. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.; Muller, A.; Csogor, Z.; Frimmel, F.H.; Posten, C. The adsorption kinetics of metal ions onto different microalgae and siliceous earth. Water Res. 2001, 35, 779–785. [Google Scholar] [CrossRef]
- Torres, E.; Mera, R.; Herrero, C.; Abalde, J. Isotherm studies for the determination of Cd (II) ions removal capacity in living biomass of a microalga with high tolerance to cadmium toxicity. Environ. Sci. Pollut. Res. Int. 2014, 21, 12616–12628. [Google Scholar] [CrossRef] [PubMed]
- Folgar, S.; Torres, E.; Perez-Rama, M.; Cid, A.; Herrero, C.; Abalde, J. Dunaliella salina as marine microalga highly tolerant to but a poor remover of cadmium. J. Hazard. Mater. 2009, 165, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Rama, M.; Torres, E.; Suárez, C.; Herrero, C.C.; Abalde, J. Sorption isotherm studies of Cd(II) ions using living cells of the marine microalga Tetraselmis suecica (Kylin) Butch. J. Environ. Manag. 2010, 91, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dong, F.; Kang, W.; Sun, S.; Wei, H.; Zhang, W.; Nie, X.; Guo, Y.; Huang, T.; Liu, Y. Biosorption of Strontium from Simulated Nuclear Wastewater by Scenedesmus spinosus under Culture Conditions: Adsorption and Bioaccumulation Processes and Models. Int. J. Environ. Res. Public Health 2014, 11, 6099–6118. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; He, J.; Huang, H.; Miller, T.R.; Christakos, G.; Reichwaldt, E.S.; Ghadouani, A.; Lin, S.; Xu, X.; Shi, J. A novel single-parameter approach for forecasting algal blooms. Water Res. 2017, 108, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. The Influence of Salt Anions on Heavy Metal Ion Adsorption on the Example of Nickel. Materials 2018, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castañeda, M.E.; Medina, D.I. Use of Surfactant-Modified Zeolites and Clays for the Removal of Heavy Metals from Water. Water 2017, 9, 235. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.-H.; Wu, J. Organic-Inorganic Hybrid Polymers as Adsorbents for Removal of Heavy Metal Ions from Solutions: A Review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kőnigné Péter, A. Heavy Metal Adsorption Properties on Several Biosorbents (Nehézfém Adszorpció Jellemzése Különböző Bioszorbenseken). Ph.D. Dissertation, University of Pécs, Pécs, Hungary, 2014. Available online: http://kemia.ttk.pte.hu/pages/kemiadi/files/doc/KonigAniko-hazivedes.pdf (accessed on 20 August 2018).
- Gupta, S.K.; Malik, A.; Bux, F. Algal Biofuels: Recent Advances and Future Prospects; Springer International Publishing: Berlin, Germany, 2017; p. 466. Available online: https://www.springer.com/gb/book/9783319510095 (accessed on 20 August 2018).
- Kim, S.K.; Lee, C.G. Marine Bioenergy: Trends and Developments; Taylor and Francis Group: New York, NY, USA, 2015; p. 769. ISBN 978-1-4822-2238-8. [Google Scholar]
- Arshad, M. Perspectives on Water Usage for Biofuels Production; Springer: Berlin, Germany, 2017; p. 121. ISBN 978-3-319-66407-1. [Google Scholar]
- Tu, Q.; Lu, M.; Thiansathit, W.; Keener, T.C. Review of Water Consumption and Water Conservation Technologies in the Algal Biofuel Process. Water Environ. Res. 2016, 88, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Fogarassy, C.; Horvath, B.; Kovacs, A.; Szoke, L.; Takacs-Gyorgy, K. A Circular Evaluation Tool for Sustainable Event Management—An Olympic Case Study. Acta Polytech. Hung. 2017, 14, 161–177. [Google Scholar]
- Fogarassy, C.; Kovacs, A.; Horvath, B.; Borocz, M. The development of a circular evaluation (CEV) tool–case study for the 2024 Budapest Olympics. Hung. Agric. Eng. 2017, 31, 10–20. [Google Scholar] [CrossRef]
- Fogarassy, C.; Neubauer, É.; Bakosné, M.B.; Zsarnóczai, J.S.; Molnár, S. Water footprint based Water Allowance Coefficient. Water Resour. Ind. 2014, 7–8, 1–8. [Google Scholar] [CrossRef] [Green Version]

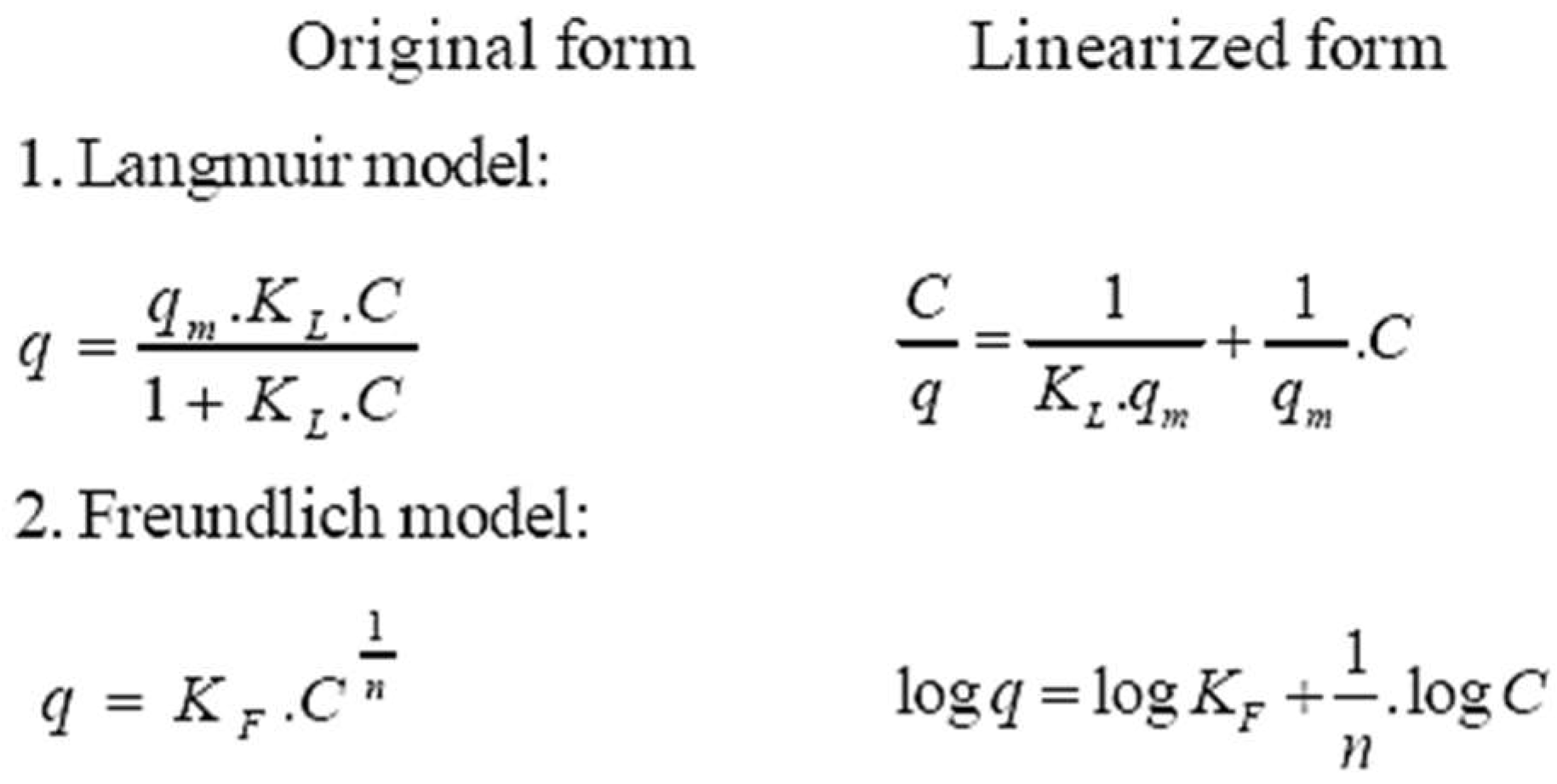
| Research Fields of Adsorption Techniques | Number of Relevant Cited Literatures | Listed Literatures (With Citation Numbers—Listed in “References” Section) |
|---|---|---|
| Studies of adsorption kinetics (isotherms, modelling, process analyses and thermodynamics) | 22 | [1,2,3,7,9,10,13,23,26,28,36,37,41,42,58,61,67,69,75,78,79,80] |
| Adsorption by biosorbent materials | 22 | [4,17,18,19,22,25,27,29,30,36,45,46,49,50,53,56,60,70,71,81,82] |
| Algal biosorbent adsorptions | 19 | [19,27,46,49,69,83,84,85,86,87,88,89,90,91,92,93,94,95,96] |
| Adsorption processes in microbiological ways | 5 | [65,66,76,77,97] |
| Physical and chemical quality of several adsorbents | 14 | [5,24,37,38,39,40,43,47,48,55,63,64,77,79] |
| Unique adsorbent materials (such as industrial waste and bentonites) * | 24 | [6,7,8,9,10,11,12,13,14,15,16,20,21,30,33,34,35,42,64,68,71,73,98,99] |
| Biopolymers, nanomaterials and composite adsorbents | 14 | [31,32,41,44,51,52,54,57,59,72,74,76,100,101] |
| Adsorption Efficiency of Each Adsorbents [in %] | Algal Biosorbent | Microbiological Adsorption Processes | Biopolymer Adsorbents | Unique Adsorbents (Artificial Materials) |
|---|---|---|---|---|
| 60–70% | 60%– | |||
| 70–80% | 70%– | 70%– | ||
| 80–90% | 90% | |||
| 90–95% | 90%– | −94–95% | −94–95% | |
| 95–(~)99% | −98–99% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czikkely, M.; Neubauer, E.; Fekete, I.; Ymeri, P.; Fogarassy, C. Review of Heavy Metal Adsorption Processes by Several Organic Matters from Wastewaters. Water 2018, 10, 1377. https://doi.org/10.3390/w10101377
Czikkely M, Neubauer E, Fekete I, Ymeri P, Fogarassy C. Review of Heavy Metal Adsorption Processes by Several Organic Matters from Wastewaters. Water. 2018; 10(10):1377. https://doi.org/10.3390/w10101377
Chicago/Turabian StyleCzikkely, Marton, Eva Neubauer, Ilona Fekete, Prespa Ymeri, and Csaba Fogarassy. 2018. "Review of Heavy Metal Adsorption Processes by Several Organic Matters from Wastewaters" Water 10, no. 10: 1377. https://doi.org/10.3390/w10101377







