The Systematic Adsorption of Diclofenac onto Waste Red Bricks Functionalized with Iron Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. The Preparation of Adsorbents
2.3. Characterization Techniques
2.4. Adsorption Experiments
3. Results and Discussion
3.1. The Characteristics of PRBP, GCBP, and HCBP
3.2. The Adsorption Kinetics of DCF onto PRBP, GCBP, and HCBP
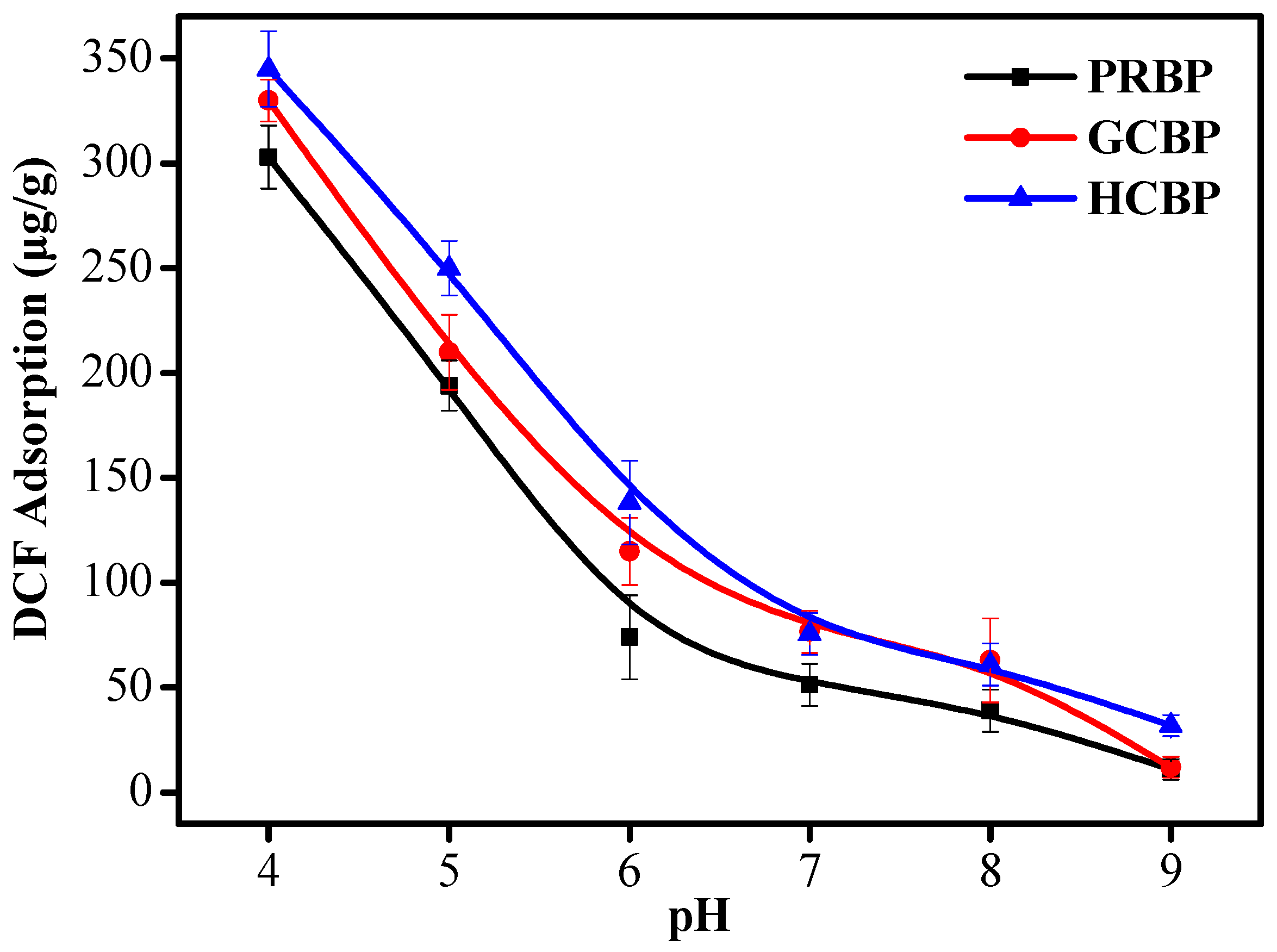
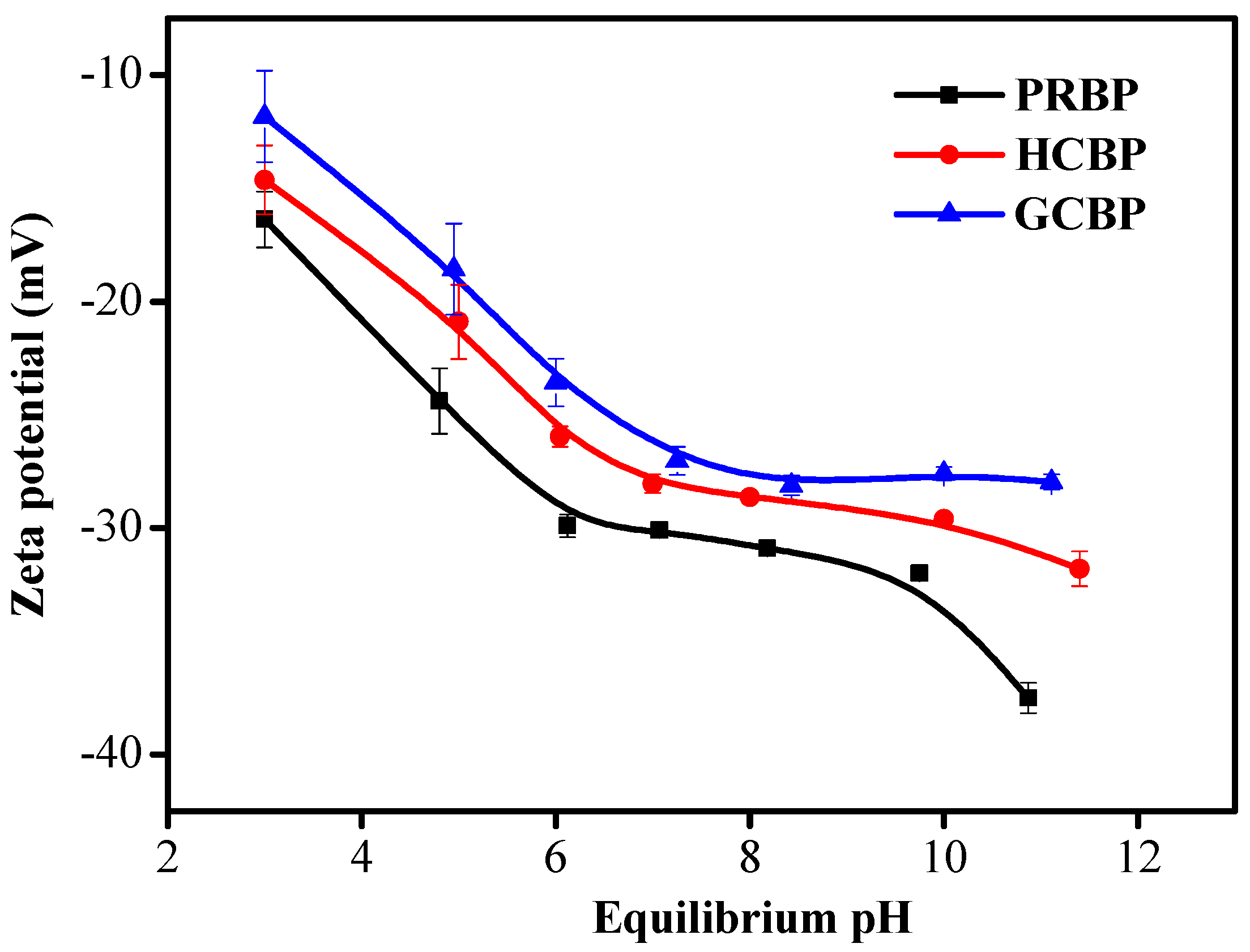
3.3. Effect of pH on the Adsorption of DCF onto PRBP, GCBP, and HCBP
3.4. The Adsorption Isotherms of DCF onto PRBP, GCBP, and HCBP
3.5. Effect of Ionic Strength on DCF Adsorption
3.6. Adsorption Thermodynamics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Li, Y.; Yuan, B.; Shen, C.; Fu, M.; Cui, H.; Sun, W. Large scale preparation of Cu-doped α-FeOOH nanoflowers and their photo-Fenton-like catalytic degradation of diclofenac sodium. Chem. Eng. J. 2016, 291, 174–183. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Lee, G.; Jang, M.; Jang, A.; Her, N.; Son, A.; Yoon, Y. Ultrasonic treatment of endocrine disrupting compounds, pharmaceuticals, and personal care products in water: A review. Chem. Eng. J. 2017, 327, 629–647. [Google Scholar] [CrossRef]
- Melvin, S.D.; Leusch, F.D.L. Removal of trace organic contaminants from domestic wastewater: A meta-analysis comparison of sewage treatment technologies. Environ. Int. 2016, 92–93, 183–188. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Milbeau, C.L.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323 Pt A, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat to drinking water? Trend Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Hajj-Mohamad, M.; Darwano, H.; Duy, S.V.; Sauve, S.; Prevost, M.; Arp, H.P.; Dorner, S. The distribution dynamics and desorption behaviour of mobile pharmaceuticals and caffeine to combined sewer sediments. Water Res. 2017, 108, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Balbi, T.; Montagna, M.; Fabbri, R.; Carbone, C.; Franzellitti, S.; Fabbri, E.; Canesi, L. Diclofenac affects early embryo development in the marine bivalve Mytilus galloprovincialis. Sci. Total Environ. 2018, 642, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Thelusmond, J.R.; Kawka, E.; Strathmann, T.J.; Cupples, A.M. Diclofenac, carbamazepine and triclocarban biodegradation in agricultural soils and the microorganisms and metabolic pathways affected. Sci. Total Environ. 2018, 640, 1393–1410. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.M.; Jassby, D.; Song, S.K.; Zhou, X.F.; Zhao, H.Y.; Filip, J.; Petala, E.; Zhang, Y.L. Enhanced Oxidative and Adsorptive Removal of Diclofenac in Heterogeneous Fenton-like Reaction with Sulfide Modified Nanoscale Zerovalent Iron. Environ. Sci. Technol. 2018, 52, 6466–6475. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Li, X.K.; Tang, Y.M.; Zhou, J.L.; Wu, D.; Wu, Y.; Li, L.S. Mechanism insight of pollutant degradation and bromate inhibition by Fe-Cu-MCM-41 catalyzed ozonation. J. Hazard. Mater. 2018, 346, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zambianchi, M.; Durso, M.; Liscio, A.; Treossi, E.; Bettini, C.; Capobianco, M.L.; Aluigi, A.; Kovtun, A.; Ruani, G.; Corticelli, F.; et al. Graphene oxide doped polysulfone membrane adsorbers for the removal of organic contaminants from water. Chem. Eng. J. 2017, 326, 130–140. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Leusch, F.D.L.; Roddick, F.; McAdam, E.J.; Magram, S.F.; Nghiem, L.D. Continuous biotransformation of bisphenol A and diclofenac by laccase in an enzymatic membrane reactor. Int. Biodeterior. Biodegrad. 2014, 95, 25–32. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Qin, X. Adsorption of diclofenac onto goethite: Adsorption kinetics and effects of pH. Chemosphere 2017, 180, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Jung, C.; Li, H.; Yu, M.; Flora, J.R.V.; Boateng, L.K.; Her, N.; Zoh, K.D.; Yoon, Y. Adsorption characteristics of diclofenac and sulfamethoxazole to graphene oxide in aqueous solution. Chemosphere 2015, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Yang, L.; Huang, T. Adsorption characteristics of construction waste for heavy metals from urban stormwater runoff. Chin. J. Chem. Eng. 2015, 23, 1542–1550. [Google Scholar] [CrossRef]
- Dehou, S.C.; Wartel, M.; Recourt, P.; Revel, B.; Mabingui, J.; Montiel, A.; Boughriet, A. Physicochemical, crystalline and morphological characteristics of bricks used for ground waters purification in Bangui region (Central African Republic). Appl. Clay Sci. 2012, 59, 69–75. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, Z.; Chai, X.; Weng, Z.; Ding, R.; Dong, L. Fabrication of hematite (α-Fe2O3) nanoparticles using electrochemical deposition. Appl. Surf. Sci. 2016, 368, 303–308. [Google Scholar] [CrossRef]
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesized magnetic nanoparticles coated zeolite for the adsorption of pharmaceutical compounds from aqueous solution using batch and column studies. Chemosphere 2013, 93, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory Preparation and Characterization; Wiley: Weinhein, Germany, 2000. [Google Scholar]
- Ghanizadeh, G.; Ehrampoush, M.H.; Ghaneian, M.T. Application of iron impregnated activated carbon forremoval of arsenic from water. Iran. J. Environ. Health Sci. Eng. 2010, 7, 145–156. [Google Scholar]
- Li, W.; Wang, L.; Liu, F.; Liang, X.; Feng, X.; Tan, W.; Zheng, L.; Yin, H. Effects of Al3+ doping on the structure and properties of goethite and its adsorption behavior towards phosphate. J. Environ. Sci. 2016, 45, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Shi, Y.; Wang, X.; Li, Z. Sorption and retention of diclofenac on zeolite in the presence of cationic surfactant. J. Hazard. Mater. 2017, 323 Pt A, 584–592. [Google Scholar] [CrossRef]
- Muthukumaran, C.; Sivakumar, V.M.; Thirumarimurugan, M. Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J. Taiwan Inst. Chem. Eng. 2016, 63, 354–362. [Google Scholar] [CrossRef]
- Huang, B.; Xiong, D.; Zhao, T.; He, H.; Pan, X. Adsorptive removal of PPCPs by biomorphic HAP templated from cotton. Water Sci. Technol. 2016, 74, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Thanhmingliana; Tiwari, D. Efficient use of hybrid materials in the remediation of aquatic environment contaminated with micro-pollutant diclofenac sodium. Chem. Eng. J. 2015, 263, 364–373. [Google Scholar] [CrossRef]
- Shu, J.; Wang, Z.; Huang, Y.; Huang, N.; Ren, C.; Zhang, W. Adsorption removal of Congo red from aqueous solution by polyhedral Cu2O nanoparticles: Kinetics, isotherms, thermodynamics and mechanism analysis. J. Alloys Compd. 2015, 633, 338–346. [Google Scholar] [CrossRef]
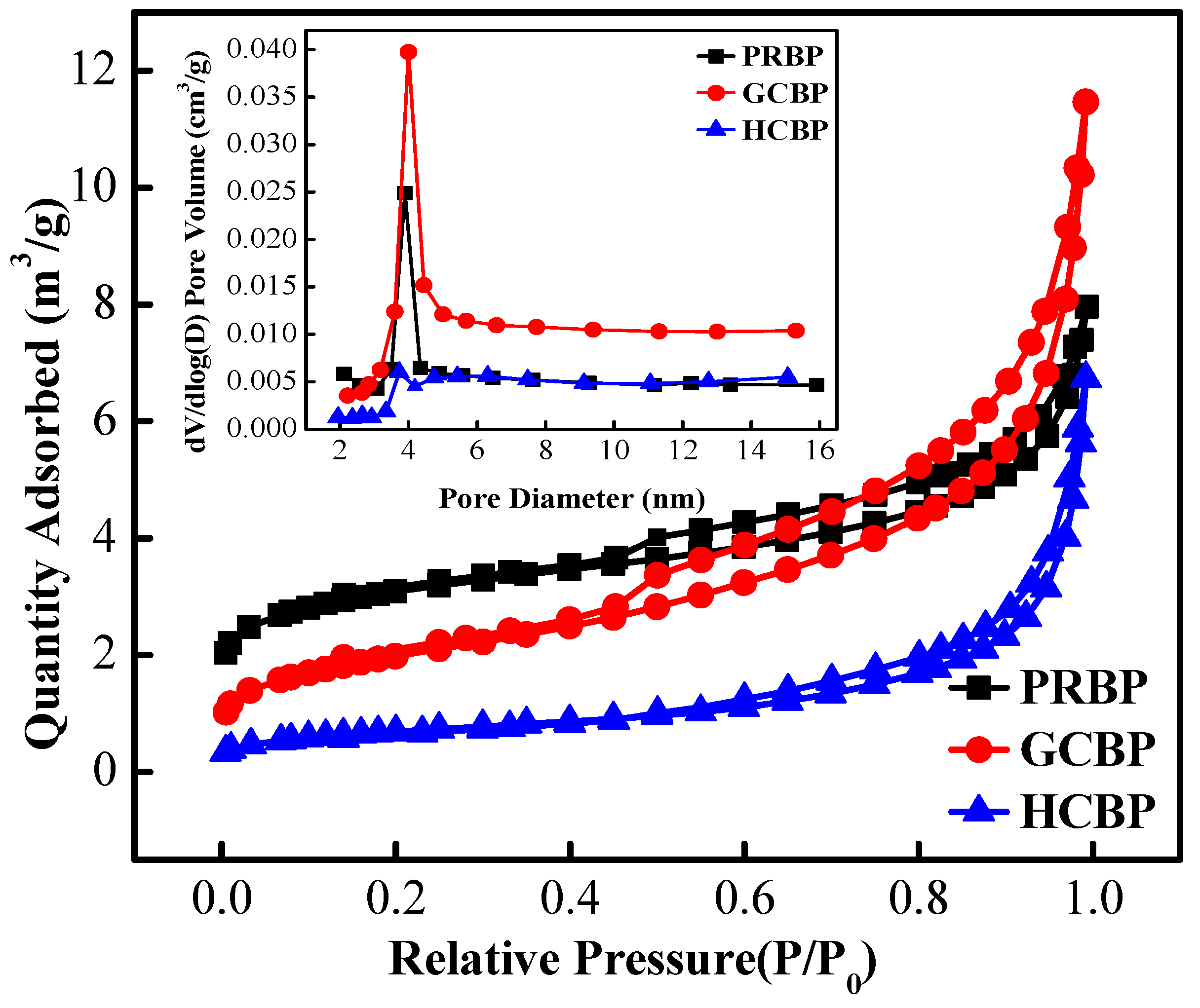
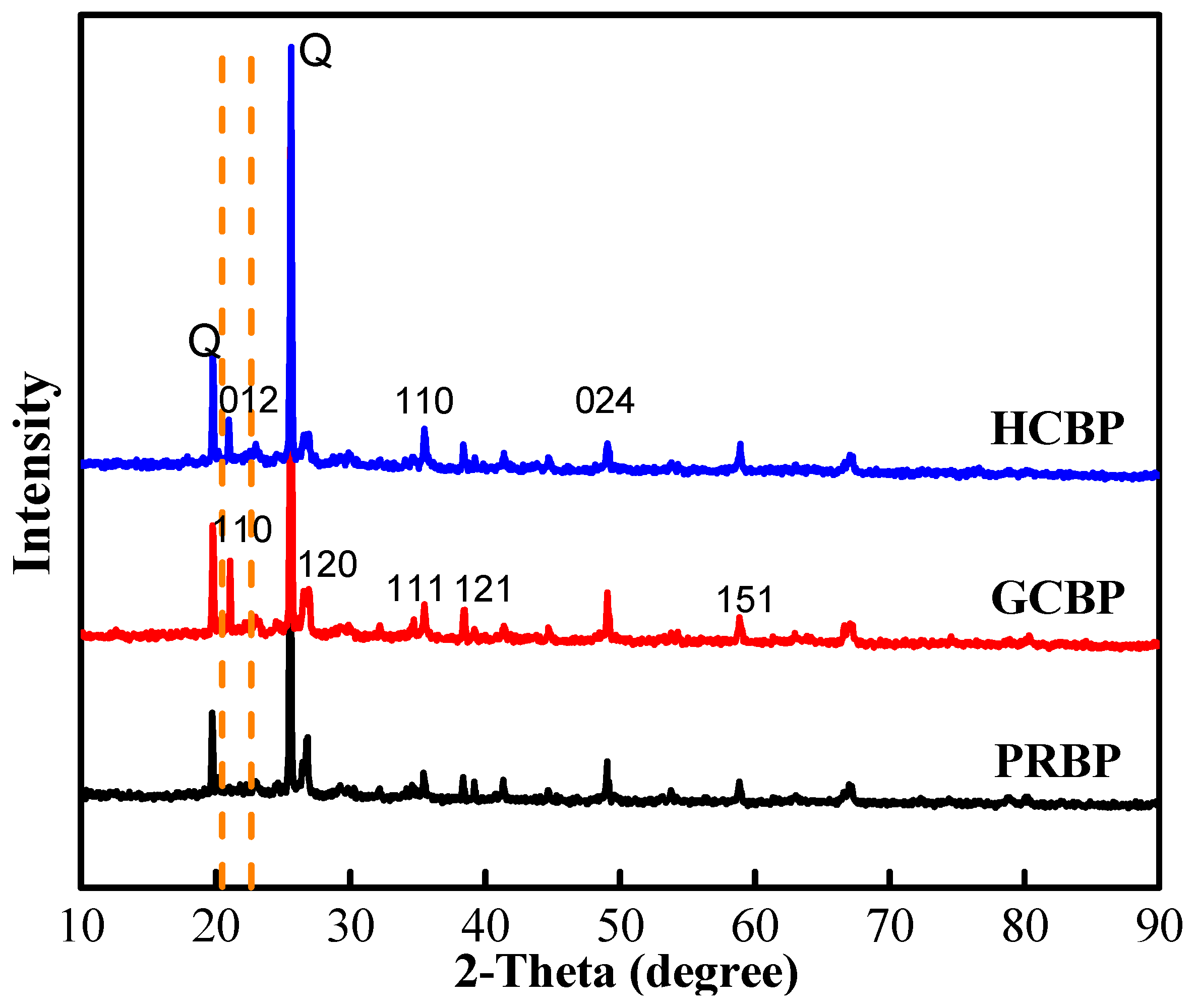





| Adsorbents | Element Content (%) XRF | BET (m2/g) | Pore Size (nm) | Coated Fe (mg/g) | |||
|---|---|---|---|---|---|---|---|
| Si2O | Al2O3 | Fe2O3 | CaO | ||||
| PRBP | 72.57 | 13.97 | 4.12 | 1.53 | 10.42 | 4.52 | - |
| GCBP | 73.02 | 13.67 | 4.17 | 1.33 | 6.93 | 5.35 | 3.18 |
| HCBP | 71.88 | 14.12 | 4.20 | 1.46 | 2.43 | 5.80 | 2.29 |
| Adsorbents | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| K1 (μg/mg/h) | qe (μg/g) | r2 | K2 (μg/h) | qe (μg/g) | r2 | |
| PRBP | 43.6 | 114.8 | 0.988 | 33.1 | 137.2 | 0.988 |
| GCBP | 59.0 | 124.5 | 0.967 | 49.2 | 142.4 | 0.982 |
| HCBP | 75.9 | 127.7 | 0.927 | 63.0 | 144.0 | 0.965 |
| Adsorbents | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| B (L/mg) | Qmax (μg/g) | r2 | Kf (μg/g) | n | r2 | |
| PRBP | 0.097 | 187.9 | 0.97 | 29.0 | 2.17 | 0.90 |
| GCBP | 0.078 | 220.5 | 0.98 | 29.8 | 2.06 | 0.97 |
| HCBP | 0.056 | 292.3 | 0.99 | 32.0 | 1.99 | 0.98 |
| Adsorbents | T (K) | Kd (L/g) | ΔH (KJ/g) | ΔS (J/g/K) |
|---|---|---|---|---|
| PRBP | 298 | 0.097 | −28.64 | −128.53 |
| 308 | 0.094 | |||
| 318 | 0.086 | |||
| GCBP | 298 | 0.078 | −34.70 | −146.49 |
| 308 | 0.075 | |||
| 318 | 0.055 | |||
| HCBP | 298 | 0.090 | −17.98 | −90.62 |
| 308 | 0.089 | |||
| 318 | 0.080 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, Y.; Chen, H.; Zhang, X.; Li, H. The Systematic Adsorption of Diclofenac onto Waste Red Bricks Functionalized with Iron Oxides. Water 2018, 10, 1343. https://doi.org/10.3390/w10101343
Zhang Z, Li Y, Chen H, Zhang X, Li H. The Systematic Adsorption of Diclofenac onto Waste Red Bricks Functionalized with Iron Oxides. Water. 2018; 10(10):1343. https://doi.org/10.3390/w10101343
Chicago/Turabian StyleZhang, Ziyang, Yuxiu Li, Hongrui Chen, Xiaoran Zhang, and Haiyan Li. 2018. "The Systematic Adsorption of Diclofenac onto Waste Red Bricks Functionalized with Iron Oxides" Water 10, no. 10: 1343. https://doi.org/10.3390/w10101343




