Removal of Hardness from Water Samples by a Carbonation Process with a Closed Pressure Reactor
Abstract
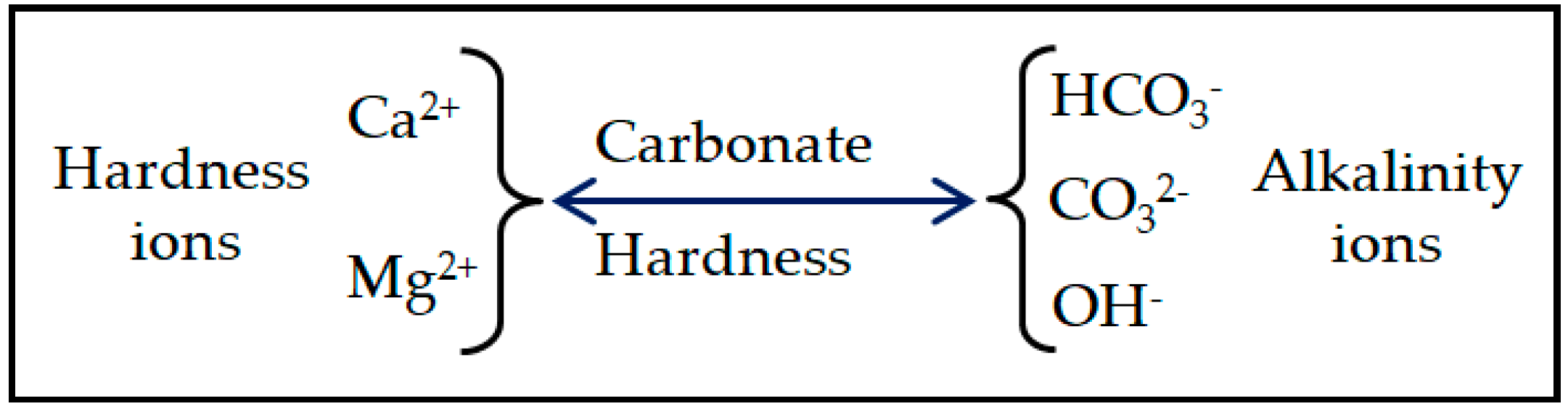
:1. Introduction
2. Materials and Methods
2.1. Artificial Hard Water Samples
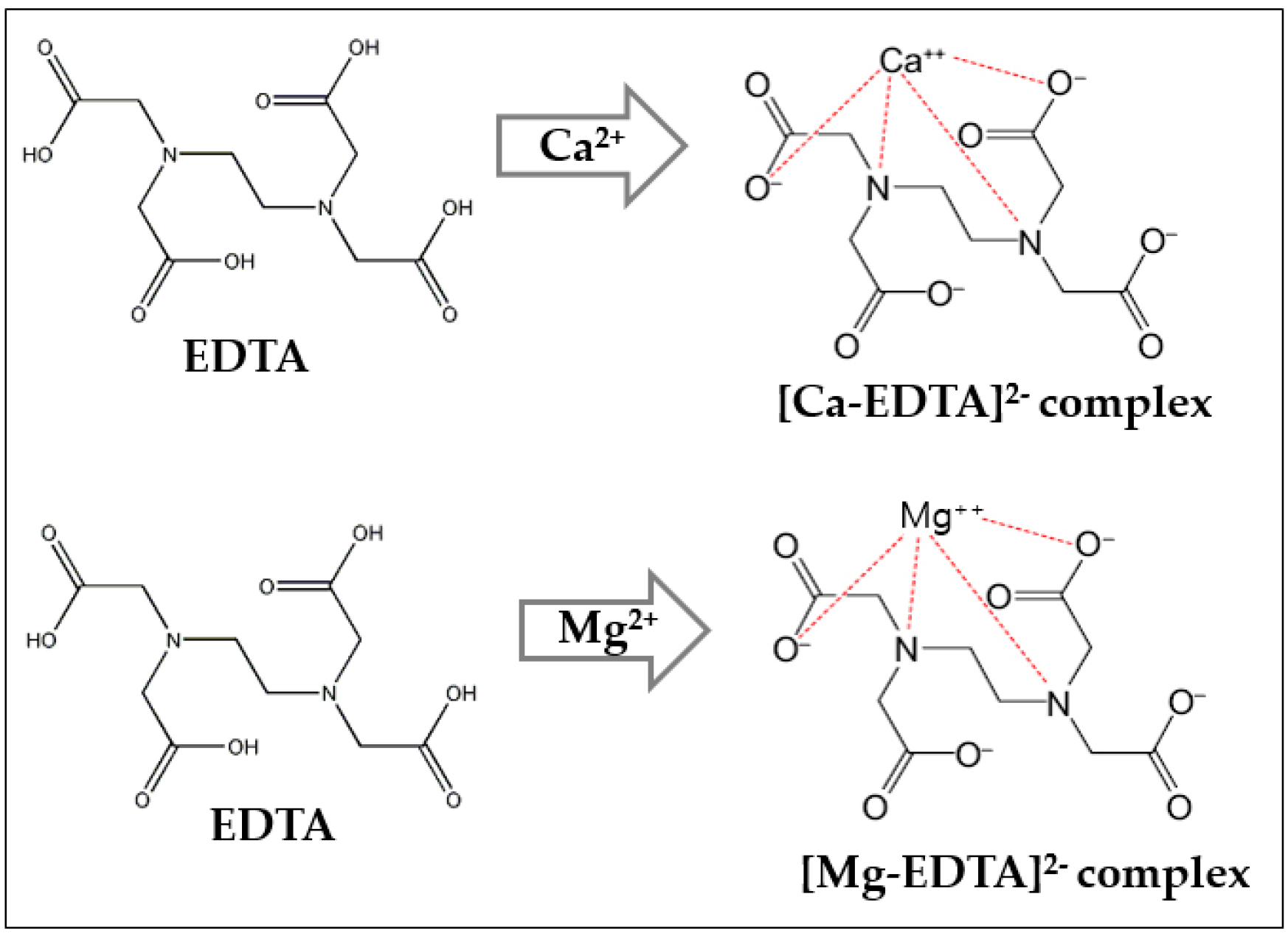
2.2. Water Hardness as Measured by the EDTA Method
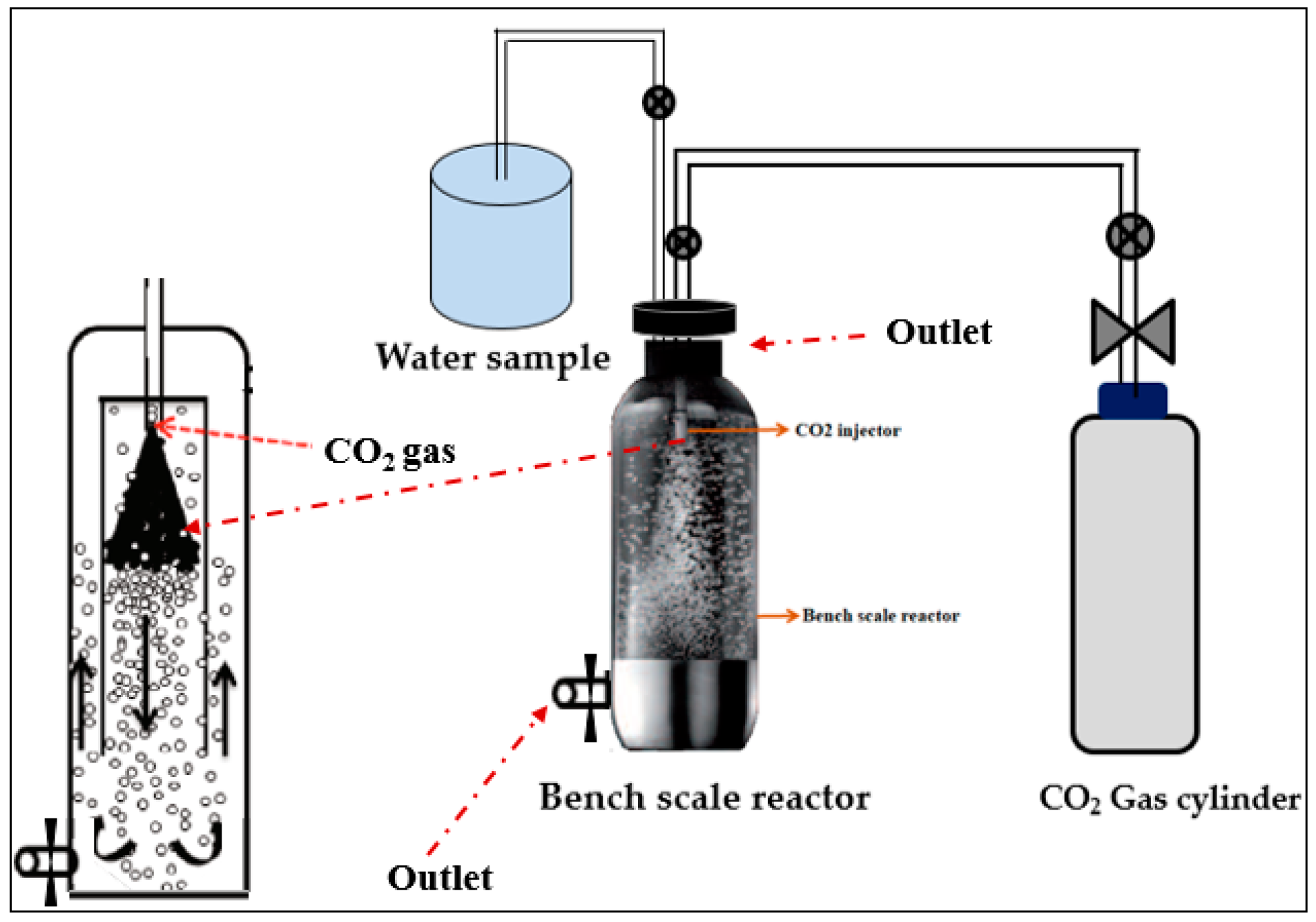
2.3. The KIGAM Closed Pressure Reactor for a Carbonation Process
2.4. Characterization of Crystals by SEM, XRD and EDS
3. Results and Discussion
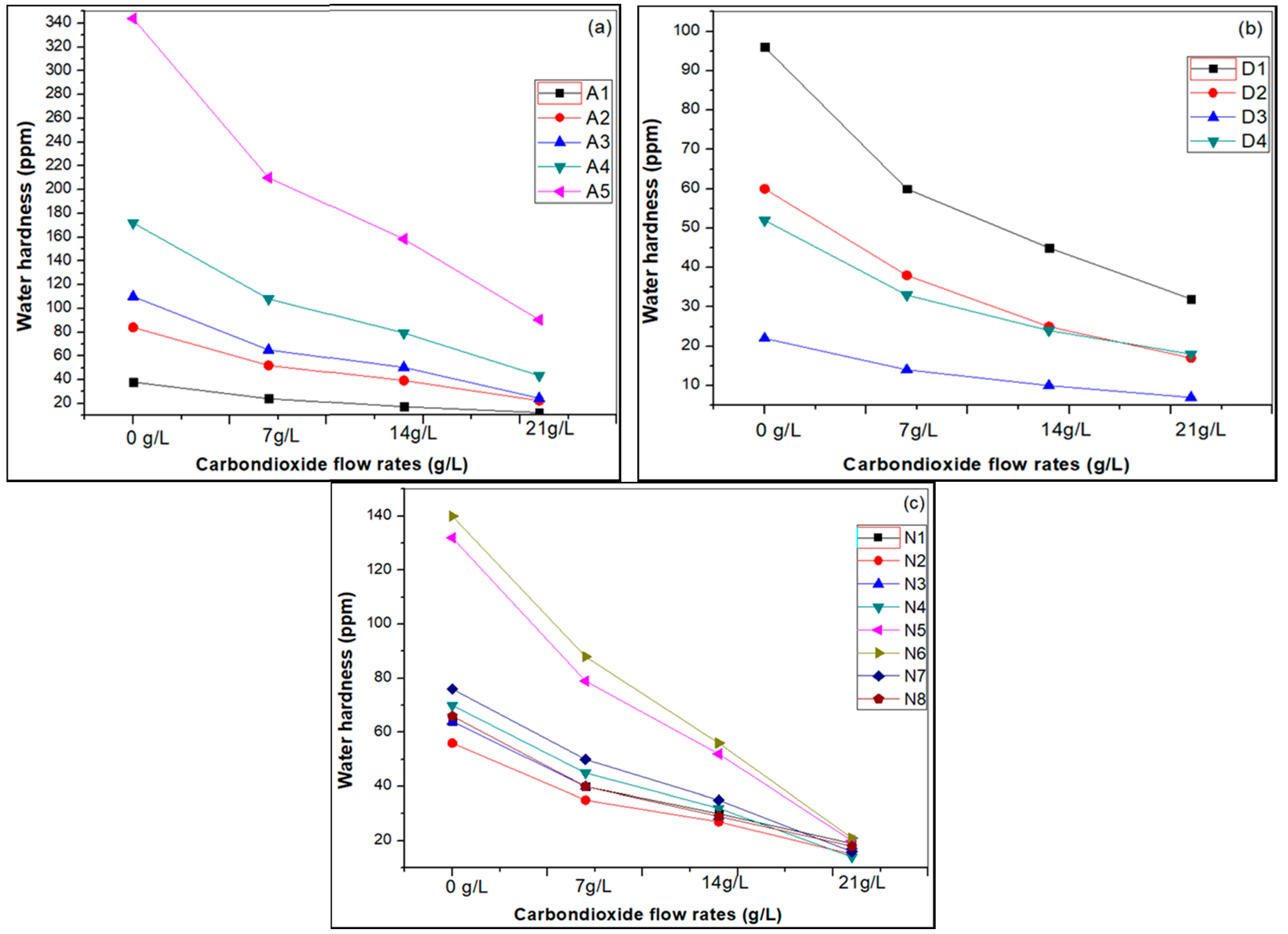
3.1. Effect of Carbon Dioxide Flow Rates
(Dissociation of aqueous CO2 into water)
(Crystal growth occurs spontaneously)
(Finally, calcite formed)
3.2. Effect of Temperature
3.3. pH Effects of Carbonation
3.4. Water Hardness Removal Efficiency of the Carbonation Process
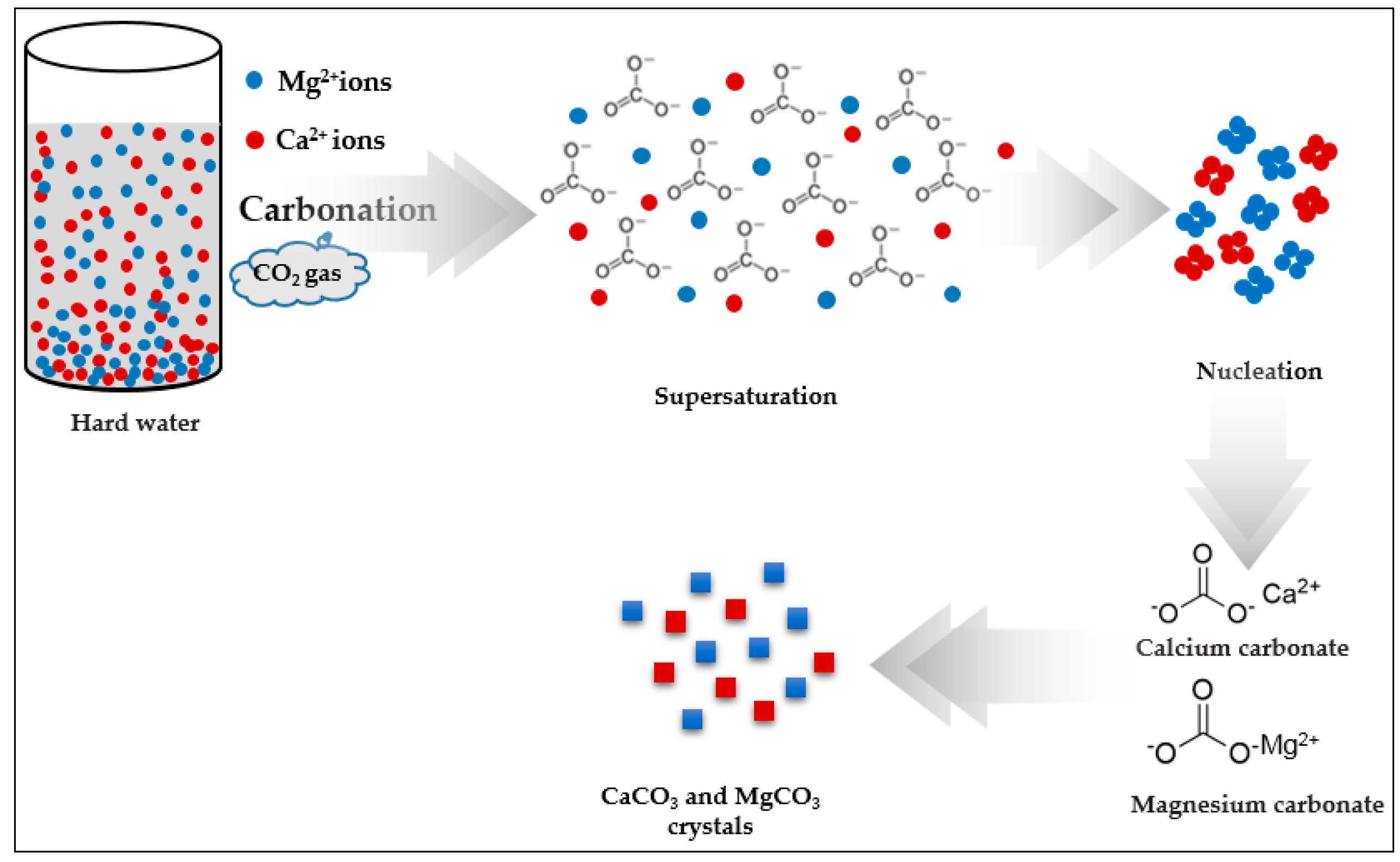
3.5. Crystal Growth Mechanism
4. Conclusions
- (i)
- The carbonation process effectively reduced the hardness in all artificial water samples by approximately (70–80%). At room temperature, a 21 g/L CO2 flow rate for 5 min was enough to remove most of the Ca2+ and Mg2+ ions from the water. The hardness reduction increased with increasing temperature, because carbon dioxide effectively reacts with Ca2+ and Mg2+ ions with increasing temperature. The results indicated that 85% of the hardness was possible to remove by a simple carbonation method.
- (ii)
- In drinking water samples the hardness was also reduced effectively using the carbonation process, with 21 g/L CO2 at room temperature for 5 min reaction reducing the water sample hardness by approximately 60 to 70%.
- (iii)
- For the natural water samples collected from different areas of South Korea, 21 g/L of CO2 carbonation for 5 min at room temperature reduced the hardness by 70 to 85%. The results revealed that the carbonation process in a closed pressure reactor effectively control the water hardness of different kinds of water by relatively simple means.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Water Review, Consumer Report. A Publication of the Water Quality; The South Korean Research Council: Chungcheongnam-do, Korea, 1990; Volume 5.
- Padmapriya, R.; Saranya, T.; Thirunalasundari, T. Phyllanthus emblica-A Biopotential for hard water treatment. Int. J. Pure Appl. Biosci. 2015, 3, 291–295. [Google Scholar]
- Neri, L.C.; Johansen, H.L. Water hardness and cardiovascular mortality. N. Y. Acad. Sci. 1978, 304, 203–221. [Google Scholar] [CrossRef]
- Sengupta, P. Potential health impacts of Hard Water. Int. J. Prev. Med. 2014, 4, 866–875. [Google Scholar]
- Comstock, G.W. Water hardness and cardiovascular disease. Am. J. Epidemiol. 1979, 110, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, L.C.L.; Nascimento, L.; Cavalcanti, B.F. Water hardness removal for industrial use: Application of the electrolysis process. Open Access Sci. Rep. 2012, 1, 460–465. [Google Scholar]
- Smith, E.J.; Davison, W.; Hamilton-Taylor, J. Methods for preparing synthetic freshwaters. Water Res. 2002, 36, 1286–1296. [Google Scholar] [CrossRef]
- Marking, L.L.; Dawson, V.K. Methods for assessment of toxicity or efficacy of mixtures of chemicals. In Investigations in Fish Control; Series Number 67; U.S. Fish and Wildlife Service (USFWS): La Crosse, WI, USA, 1975; pp. 1–8. [Google Scholar]
- Cash, D. Analysis of Calcium in a Supplement tablet; Analysis of Magnesium in Epsom Salt; Hardness of Water; Mohawk College of Applied Arts and Technology: Hamilton, ON, Canada, 2008. [Google Scholar]
- Kakisako, M.; Nishikawa, K.; Nakano, M.; Harada, K.S.; Tatsuoka, T.; Koga, N. Stepwise inquiry into hard water in a high school chemistry laboratory. J. Chem. Educ. 2016, 93, 1923–1928. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water. U.S Geological Survey Water Supply Paper 2254, 1-272; United States Government Printing Office, 1985. Available online: https://pubs.usgs.gov/wsp/wsp2254/pdf/wsp2254a.pdf (accessed on 15 August 2017).
- Kim, Y.Y.; Schenk, A.S.; Ihli, J.; Kulak, A.N.; Hetherington, N.B.J.; Tang, C.C.; Schmahl, W.W.; Griesshaber, E.; Hyett, G.; Meldrum, F.C. A critical analysis of calcium carbonate mesocrystals. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mahvi, A.H.; Shafiee, F.; Naddafi, K. Feasibility study of crystallization process for water softening in pellet reactor. Int. J. Environ. Sci. Technol. 2005, 1, 301–304. [Google Scholar] [CrossRef]





| Artificial Hard Water | Drinking Water | Natural Water | |||
|---|---|---|---|---|---|
| Sample Name | Number | Sample Name | Number | Sample Name | Number |
| Artificial Soft water | A-(1) | CHOICE water | D-(1) | Softener pass water | N-(1) |
| Artificial Slightly Hard water | A-(2) | ViO water | D-(2) | Seoul Arisu water | N-(2) |
| Artificial Moderately Hard water | A-(3) | Sam Da Soo water | D-(3) | Seoul Park Tap water | N-(3) |
| Artificial Very Hard water | A-(4) | O-EAU water | D-(4) | Seoul Outdoor Swimming pool (1) water | N-(4) |
| Seoul Outdoor Swimming pool (2) water | N-(5) | ||||
| Seoul Outdoor Swimming pool Toilet water | N-(6) | ||||
| (Korea Institute of Geoscience and Mineral Resources(KIGAM)-Waste water | N-(7) | ||||
| Water Samples | Reagent Added (mg/L) | Approximate Final Water Quality | ||||
|---|---|---|---|---|---|---|
| NaHCO3 | CaSO4·2H2O | MgSO4 | KCl | pH | Hardness (mg/L) | |
| Soft | 12.0 | 7.5 | 7.5 | 0.5 | 6.4–6.8 | 10–13 |
| Slightly hard | 48.0 | 30.0 | 30.0 | 2.0 | 7.2–7.6 | 40–48 |
| Moderately hard | 96.0 | 60.0 | 60.0 | 4.0 | 7.4–7.8 | 80–100 |
| Hard | 192.0 | 120.0 | 120.0 | 8.0 | 7.6–8.0 | 160–180 |
| Very hard | 384.0 | 240.0 | 240.0 | 16.0 | 8.0–8.4 | 280–320 |
| Sample Name | Water Hardness-before Carbonation (ppm) | Water Hardness after Carbonation (mg/L) | ||
|---|---|---|---|---|
| 7 g/L CO2 for 3 min | 14 g/L CO2 for 4 min | 21 g/L CO2 for 5 min | ||
| I. Artificial hard water | ||||
| A-(1) | 38 | 24 | 17 | 12 |
| A-(2) | 84 | 52 | 39 | 22 |
| A-(3) | 110 | 65 | 50 | 24 |
| A-(4) | 172 | 108 | 79 | 43 |
| A-(5) | 344 | 210 | 158 | 90 |
| II. Drinking water samples | ||||
| D-(1) | 96 | 60 | 45 | 32 |
| D-(2) | 60 | 38 | 25 | 17 |
| D-(3) | 22 | 14 | 10 | 7 |
| D-(4) | 52 | 33 | 24 | 18 |
| III. Natural water samples | ||||
| N-(1) | 64 | 40 | 30 | 19 |
| N-(2) | 56 | 35 | 27 | 15 |
| N-(3) | 64 | 40 | 29 | 18 |
| N-(4) | 70 | 45 | 32 | 14 |
| N-(5) | 132 | 79 | 52 | 20 |
| N-(6) | 140 | 88 | 56 | 21 |
| N-(7) | 76 | 50 | 35 | 16 |
| N-(8) | 66 | 40 | 29 | 18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, M.K.; Chilakala, R.; Han, C.; Thenepalli, T. Removal of Hardness from Water Samples by a Carbonation Process with a Closed Pressure Reactor. Water 2018, 10, 54. https://doi.org/10.3390/w10010054
Ahn MK, Chilakala R, Han C, Thenepalli T. Removal of Hardness from Water Samples by a Carbonation Process with a Closed Pressure Reactor. Water. 2018; 10(1):54. https://doi.org/10.3390/w10010054
Chicago/Turabian StyleAhn, Min Kyung, Ramakrishna Chilakala, Choon Han, and Thriveni Thenepalli. 2018. "Removal of Hardness from Water Samples by a Carbonation Process with a Closed Pressure Reactor" Water 10, no. 1: 54. https://doi.org/10.3390/w10010054







