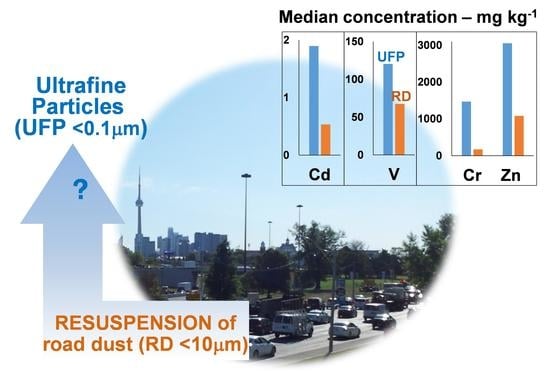
Quantification and Characterization of Metals in Ultrafine Road Dust Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Road Dust Samples
2.2. Particle Size Fractionation of the Resuspended Dust Box
2.3. Element Analysis
2.4. Quality Assurance
2.5. Data Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Basic Properties of the Dust Box Samples
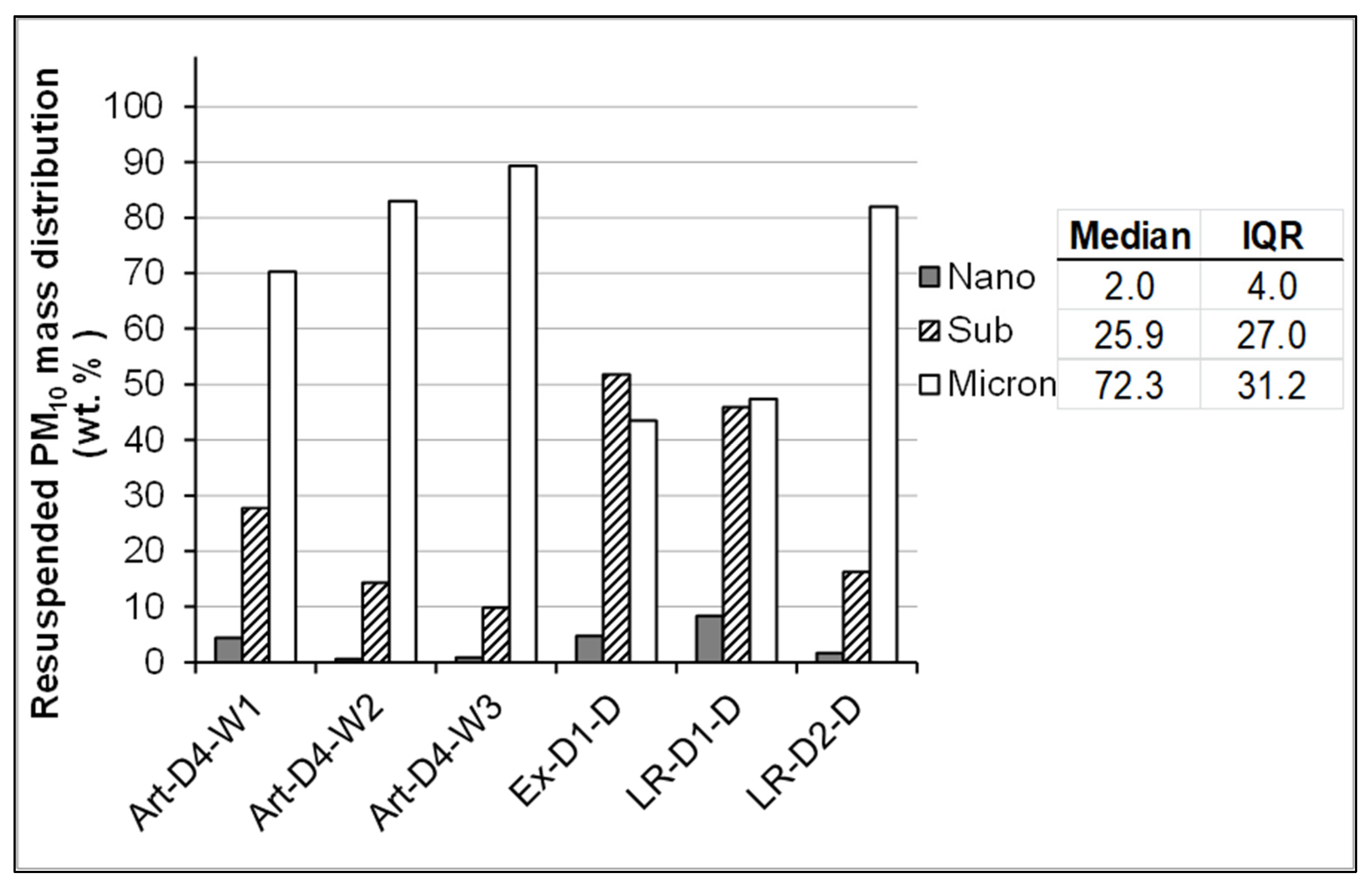
3.2. UFP in Resuspended Road Dust Box Samples
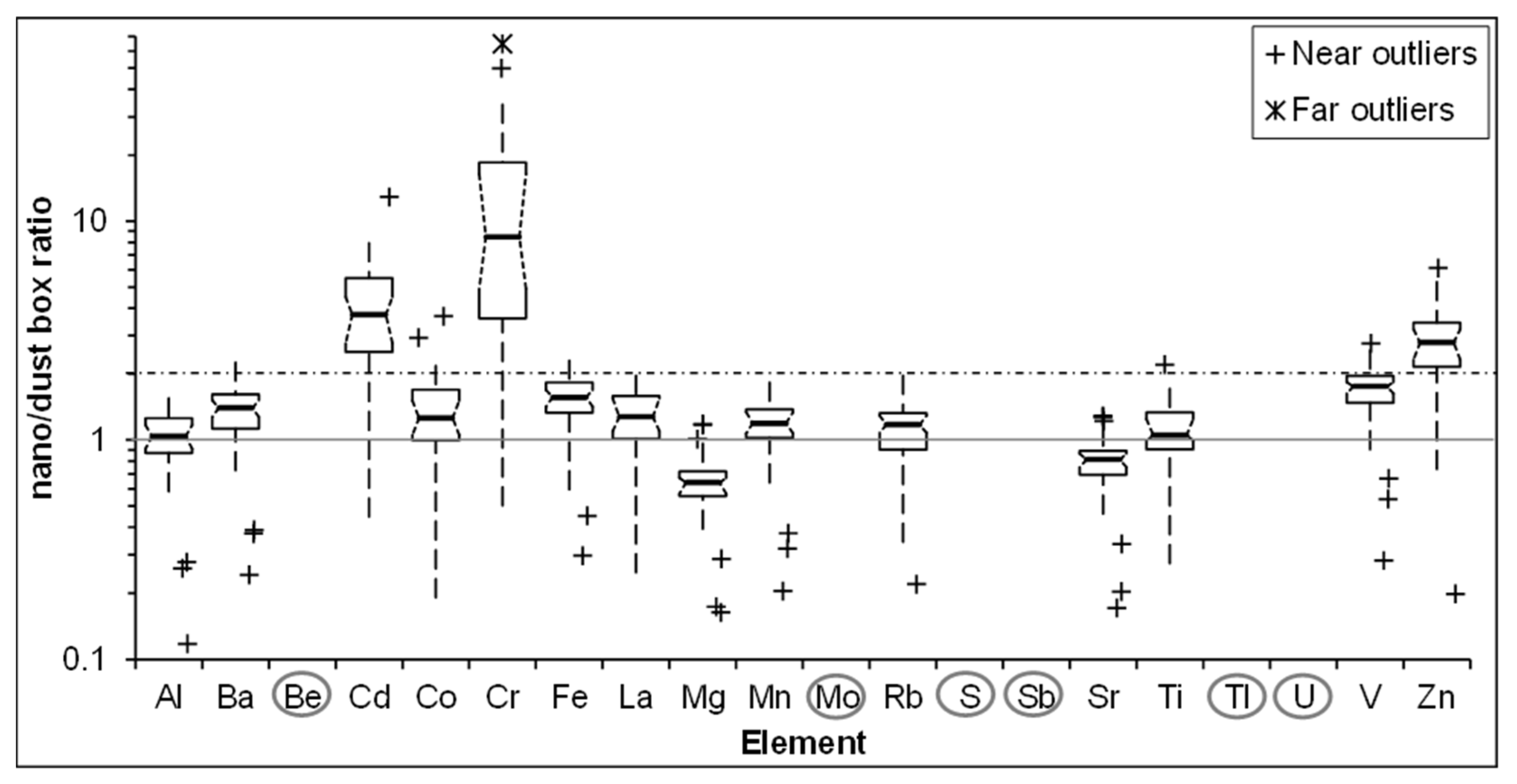
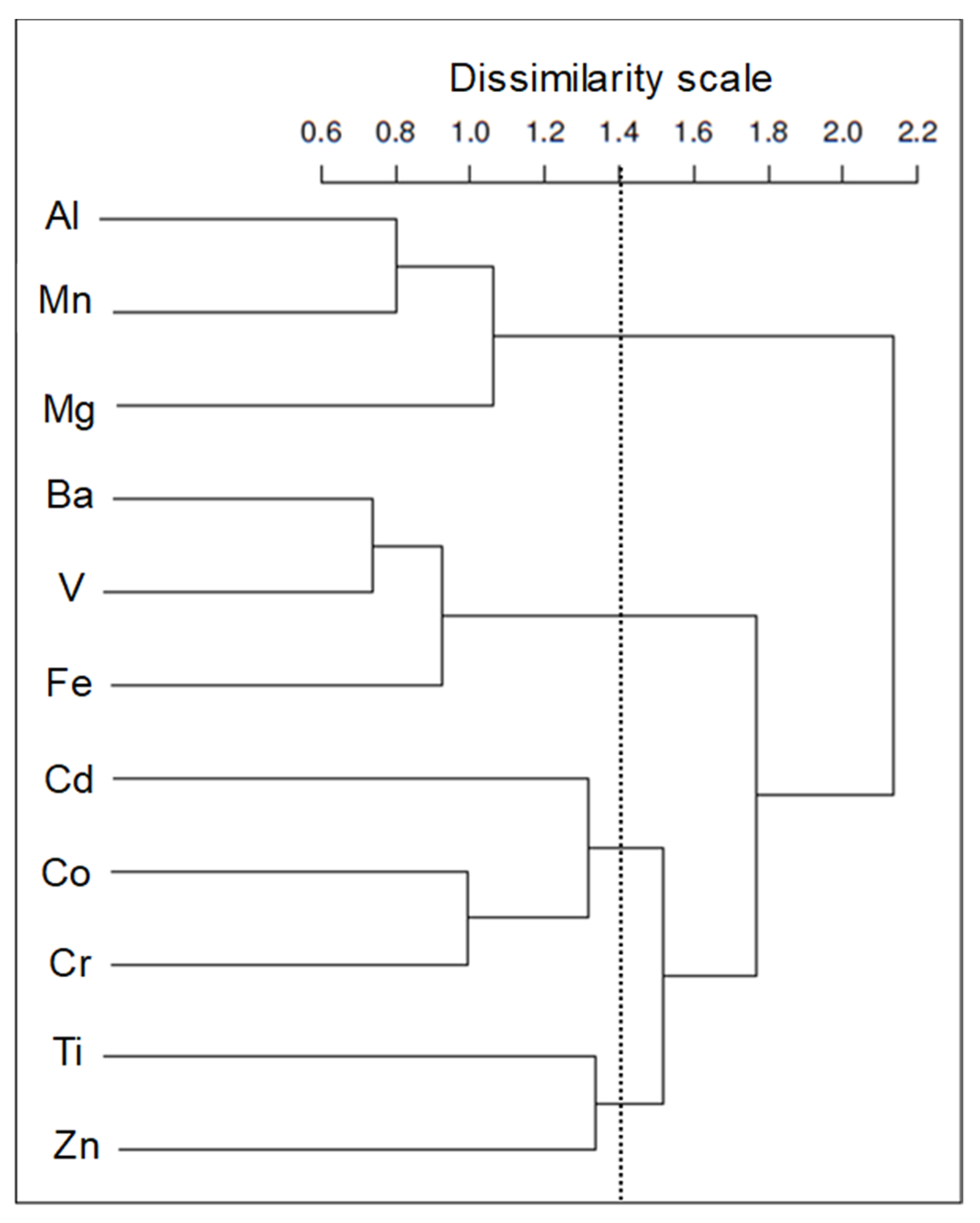
3.3. Metals in UFP
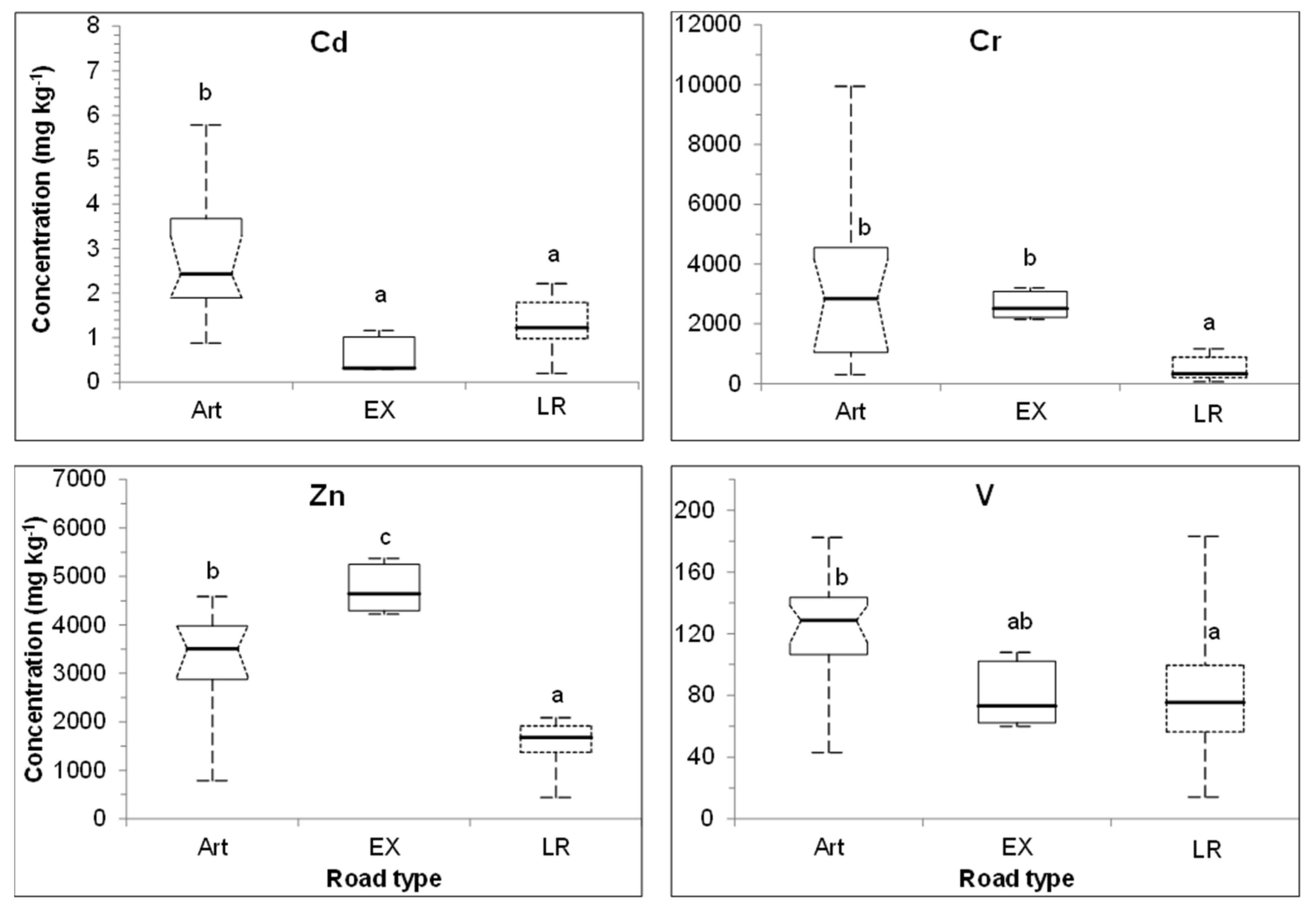
3.4. Impact of Road Type on Key Metal Concentrations in UFP
3.5. Implications for Human Health
3.5.1. Risk of Exposure to UFP and Their Oxidative Potential
3.5.2. Toxicity of Metals in UFP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks; World Health Organization: Geneva, Switzerland, 2016; 147p, ISBN 978 92 4 156519 6. [Google Scholar]
- WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016; 131p, ISBN 978 92 4 151135 3. [Google Scholar]
- Air Quality Expert Group. Ultrafine Particles (UFP) in the UK; Prepared for the Department for Environment, Food and Rural Affairs; Scottish Government; Welsh Government; and Department of the Environment in Northern Ireland; Report No. PB14510; Department for Environment, Food and Rural Affairs: London, UK, 2018. Available online: https://uk-air.defra.gov.uk/library/reports.php?report_id=968 (accessed on 21 April 2020).
- Brown, J.S.; Gordon, T.; Price, O.; Asgharian, B. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol. 2013, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Geiser, M.; Kreyling, W.G. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010, 7, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Sutunkova, M.P.; Katsnelson, B.A.; Privalova, L.I.; Gurvich, V.B.; Konysheva, L.K.; Shur, V.; Shishkina, E.V.; Minigalieva, I.A.; Solovjeva, S.N.; Grebenkina, S.V.; et al. On the contribution of the phagocytosis and the solubilization to the iron oxide nanoparticles retention in and elimination from the lungs under long-term inhalation. Toxicology 2016, 363–364, 19–28. [Google Scholar] [CrossRef]
- Tian, L.; Shang, Y.; Chen, R.; Bai, R.; Chen, C.; Inthavong, K.; Tu, J. Correlation of regional deposition for inhaled nanoparticles in human and rat olfactory. Part. Fibre Toxicol. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef]
- Yang, Y.; Vance, M.; Tou, F.; Tiwari, A.; Liu, M.; Hochella, M.F., Jr. Nanoparticles in road dust from impervious urban surfaces: Distribution, identification, and environmental implications. Environ. Sci. Nano 2016, 3, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Lenschow, P.; Abraham, H.-J.; Kutzner, K.; Lutz, M.; Preu, J.-D.; Reichenbächer, W. Some ideas about sources of PM10. Atmos. Environ. 2001, 35 (Suppl. S1), S23–S33. [Google Scholar] [CrossRef]
- Kumar, P.; Pirjola, L.; Ketzel, M.; Harrison, R.M. Nanoparticle emissions from 11 non-vehicle exhaust source—A review. Atmos. Environ. 2013, 67, 252–277. [Google Scholar] [CrossRef] [Green Version]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Ramirez, O.; Sánchez de la Campa, A.M.; Amato, F.; Moreno, T.; Silva, L.F.; de la Rosa, J.D. Physicochemical characterization and sources of thoracic fraction of road dust in a Latin American megacity. Sci. Total Environ. 2019, 652, 434–446. [Google Scholar] [CrossRef]
- Wiseman, C.L.S.; Levesque, C.; Rasmussen, P.E. Elemental sources and concentrations in thoracic-sized road dust fractions and their potential for resuspension. Sci. Total Environ. 2021, 786, 147467. [Google Scholar] [CrossRef] [PubMed]
- Kukutschová, J.; Moravec, P.; Tomášek, V.; Matejka, V.; Smolík, J.; Schwarz, J.; Seidlerová, J.; Šafářová, K.; Filip, P. On airborne nano/micro-sized wear particles released from low-metallic automotive brakes. Environ. Pollut. 2011, 159, 998–1006. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S. Characteristics of tire wear particles generated by a tire simulator under various driving conditions. Environ. Sci. Technol. 2018, 52, 12153–12161. [Google Scholar] [CrossRef] [PubMed]
- Ermolin, M.S.; Fedotov, P.S.; Ivaneev, A.I.; Karandashev, V.K.; Fedyunina, N.N.; Eskina, V.V. Isolation and quantitative analysis of road dust nanoparticles. J. Anal. Chem. 2017, 72, 520–532. [Google Scholar] [CrossRef]
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606–6630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cass, G.R.; Hughes, L.A.; Bhave, P.; Kleeman, M.J.; Allen, J.O.; Salmon, L.G. The chemical composition of atmospheric ultrafine particles. Phil. Trans. R. Soc. Lond. A 2000, 358, 2581–2592. [Google Scholar] [CrossRef]
- Godri, K.J.; Harrison, R.M.; Evans, T.; Baker, T.; Dunster, C.; Mudway, I.S.; Kelly, F.J. Increased oxidative burden associated with traffic component of ambient particulate matter at roadside and urban background school sites in London. PLoS ONE 2011, 6, e21961. [Google Scholar] [CrossRef] [Green Version]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of acellular assays of ambient particulate matter oxidative potential: Methods and relationships with composition, sources and health effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Apeagyei, E.; Bank, M.S.; Spengler, J.D. Distribution of heavy metals in road dust along an urban-rural gradient in Massachusetts. Atmos. Environ. 2011, 45, 2310–2323. [Google Scholar] [CrossRef]
- Abdel-Latif, N.M.; Saleh, I.A. Heavy metals contamination in roadside dust along major roads and correlation with urbanization activities in Cairo, Egypt. J. Am. Sci. 2012, 8, 379–389. [Google Scholar]
- Wiseman, C.L.S.; Hassan Pour, Z.; Zereini, F. Platinum group element and cerium concentrations in roadside environments in Toronto, Canada. Chemosphere 2016, 145, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Miazgowicz, A.; Krennhuber, K.; Lanzerstorfer, C. Metals concentrations in road dust from high traffic and low traffic area: A size dependent comparison. Int. J. Environ. Sci. Technol. 2020, 17, 3365–3372. [Google Scholar] [CrossRef] [Green Version]
- Acosta, J.A.; Faz, A.; Kalbitz, K.; Jansen, B.; Martínez-Martínez, S. Heavy metal concentrations in particle size fractions from street dust of Murcia (Spain) as the basis for risk assessment. J. Environ. Monit. 2011, 13, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Padoan, E.; Romè, C.; Ajmone-Marsan, F. Bioaccessibility and size distribution of metals in road dust and roadside soils along a peri-urban transect. Sci. Total Environ. 2017, 601–602, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, C.L.S.; Niu, J.; Levesque, C.; Chénier, M.; Rasmussen, P.E. An assessment of the inhalation bioaccessibility of platinum group elements in road dust using a simulated lung fluid. Environ. Pollut. 2018, 241, 1009–1017. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Heavy metals in the finest size fractions of road-deposited sediments. Environ. Pollut. 2018, 239, 522–531. [Google Scholar] [CrossRef]
- Lanzerstorfer, C.; Logiewa, A. The upper size limit of the dust samples in road dust heavy metal studies: Benefits of a combined sieving and air classification sample preparation procedure. Environ. Pollut. 2019, 245, 1079–1085. [Google Scholar] [CrossRef]
- Levesque, L.; Wiseman, C.L.S.; Beauchemin, S.; Rasmussen, P.E. Thoracic fraction (PM10) of resuspended urban dust: Geochemistry, particle size distribution and lung bioaccessibility. Geosciences 2021, 11, 87. [Google Scholar] [CrossRef]
- Statistics Canada. Census Profile by Toronto Community Areas. 2016. Available online: https://www.toronto.ca/city-government/data-research-maps/neighbourhoods-communities/community-council-area-profiles/ (accessed on 15 April 2020).
- Rasmussen, P.E.; Gardner, H.D.; Jianjun, J. Buoyancy-corrected gravimetric analysis of lightly loaded filters. J. Air Waste Manag. Assoc. 2010, 60, 1065–1077. [Google Scholar] [CrossRef]
- Niu, J.; Rasmussen, P.E.; Chénier, M. Ultrasonic dissolution for ICP-MS determination of trace elements in lightly loaded airborne PM filters. Intern. J. Environ. Anal. Chem. 2013, 93, 661–678. [Google Scholar] [CrossRef]
- Morrison, J.M.; Goldhaber, M.B.; Lee, L.; Holloway, J.M.; Wanty, R.B.; Wolf, R.E.; Ranville, J.F. A regional-scale study of chromium and nickel in soils of northern California, USA. Appl. Geochem. 2009, 24, 1500–1511. [Google Scholar] [CrossRef]
- Huggins, F.E.; Huffman, G.P.; Robertson, J.D. Speciation of elements in NIST particulate matter SRMs 1648 and 1650. J. Hazar. Mater. 2000, 74, 1–23. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 2nd ed.; HarperCollins Publishers: New York, NY, USA, 1989. [Google Scholar]
- Analytical Methods Committee. What Should Be Done with Results below the Detection Limit? Mentioning the Unmentionable; AMC Technical Brief. No. 5; Royal Society of Chemistry: London, UK, 2001; Available online: https://www.rsc.org/images/results-below-detection-limit-technical-brief-5_tcm18-214854.pdf (accessed on 26 September 2019).
- Helsel, D.R. Less than obvious. Statistical treatment of data below detection limit. Environ. Sci. Technol. 1990, 24, 1767–1774. [Google Scholar] [CrossRef]
- USEPA. Guidance for Data Quality Assessment. Practical Methods for Data Analysis; EPA QA/G-9. QA00 Update; United States Environmental Protection Agency: Washington, DC, USA, 2000. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/g9-final.pdf (accessed on 15 October 2019).
- NIST/SEMATECH. e-Handbook of Statistical Methods; Section 7.1.6; U.S. Department of Commerce: Washington, DC, USA, 2012. Available online: http://www.itl.nist.gov/div898/handbook/ (accessed on 26 September 2019).
- Hart, A. Mann-Whitney test is not just a test of medians: Differences in spread can be important. Br. Med. J. 2001, 323, 391–393. [Google Scholar] [CrossRef] [Green Version]
- Van Dongen, S.; Enright, A.J. Metric distances derived from cosine similarity and Pearson and Spearman correlations. arXiv 2012, arXiv:1208.3145v1. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; Version 4.0.0; Released 24 April 2020; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 2 June 2020).
- McKeague, J.A.; Desjardins, J.G.; Wolynetz, M.S. Minor Elements in Canadian Soils; Land Resource Research Institute Contribution No. LRRI 27; Agriculture and Agri-Food Canada: Edmonton, AB, Canada, 1979; 75p. [Google Scholar]
- Rasmussen, P.E.; Subramanian, K.S.; Jessiman, B.J. A multi-element profile of house dust in relation to exterior dust and soils in the city of Ottawa, Canada. Sci. Total Environ. 2001, 267, 125–140. [Google Scholar] [CrossRef]
- Ciacci, L.; Reck, B.K.; Nassar, N.T.; Graedel, T.E. Lost by design. Environ. Sci. Technol. 2015, 49, 9443–9451. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-H.; Traub, A.; Huang, A.; Hilker, N.; Wang, J.M.; Herod, D.; Dabek-Zlotorynska, E.; Celo, V.; Evans, G.J. Long-term analysis of PM2.5 from 2004 to 2007 in Toronto: Composition, sources, and oxidative potential. Environ. Pollut. 2020, 263, 114652. [Google Scholar] [CrossRef]
- Kong, S.; Lu, B.; Ji, Y.; Zhao, X.; Bai, Z.; Xu, Y.; Liu, Y.; Jiang, H. Risk assessment of heavy metals in road and soil dusts within PM2.5, PM10 and PM100 fractions in Dongying city, Shandong Province, China. J. Environ. Monit. 2012, 14, 791–803. [Google Scholar] [CrossRef]
- Padoan, E.; Malandrino, M.; Giacomino, A.; Grosa, M.M.; Lollobrigida, F.; Martini, S.; Abollino, O. Spatial distribution and potential sources of traces elements in PM10 monitored in urban and rural sites of Piedmont region. Chemospere 2016, 145, 495–507. [Google Scholar] [CrossRef]
- WHO. World Health Organization Air Quality Guidelines for Europe, 2nd ed.; CD ROM Version; World Health Organization: Geneva, Switzerland, 2000; Available online: https://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/pre2009/who-air-quality-guidelines-for-europe,-2nd-edition,-2000-cd-rom-version (accessed on 12 October 2020).
- Canadian Council of Ministers of the Environment (CCME). Canadian soil quality guidelines for the protection of environmental and human health: Cadmium (1999). In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999. [Google Scholar]
- Canadian Council of Ministers of the Environment (CCME). Canadian water quality guidelines for the protection of aquatic life: Chromium—Hexavalent chromium and trivalent chromium. In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999. [Google Scholar]
- Canadian Council of Ministers of the Environment (CCME). Canadian soil quality guidelines for the protection of environmental and human health: Zinc (2018). In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999. [Google Scholar]
- Dabek-Zlotorzynska, E.; Celo, V.; Ding, L.; Herod, D.; Jeong, C.-H.; Evans, G.; Hilker, N. Characteristics and sources of PM2.5 and reactive gases near roadways in two metropolitan areas in Canada. Atmos. Environ. 2019, 218, 116980. [Google Scholar] [CrossRef]
- WHO. Asphalt (Bitumen); Concise International Chemical Assessment Document 59; World Health Organization: Geneva, Switzerland, 2004; ISBN 92 4 153059 6. [Google Scholar]
- Canadian Council of Ministers of the Environment (CCME). Canadian soil quality guidelines for the protection of environmental and human health: Vanadium (1997). In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999. [Google Scholar]
- Canada. Screening Assessment Cobalt and Cobalt-Containing Substances; Cat. No.: EN14-273/2017E-PDF; Environment and Climate Change Canada: Gatineau, QC, Canada; Health Canada: Tunney’s Pasture, ON, Canada, 2017. Available online: https://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=DCEB359C-1 (accessed on 18 June 2020).
- Datta, J.; Kosiorek, P.; Wloch, M. Effect of high loading of titanium dioxide particles on the morphology, mechanical and thermo-mechanical properties of the natural rubber-based composites. Iran. Polym. J. 2016, 25, 1021–1035. [Google Scholar] [CrossRef] [Green Version]
- Canadian Council of Ministers of the Environment (CCME). Canadian soil quality guidelines for the protection of environmental and human health: Barium. In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2013. [Google Scholar]
- Jeong, C.-H.; Wang, J.M.; Hilker, N.; Debosz, J.; Sofowote, U.; Su, Y.; Noble, M.; Healy, R.M.; Munoz, T.; Dabek-Zlotorzynska, E.; et al. Temporal and spatial variability of traffic-related PM2.5 sources: Comparison of exhaust and non-exhaust emissions. Atmos. Environ. 2019, 198, 55–69. [Google Scholar] [CrossRef]
- Khan, R.K.; Strand, M.A. Road dust and its effect on human health: A literate review. Epidemiol. Health 2018, 40, e2018013. [Google Scholar] [CrossRef] [PubMed]
- Nosko, O.; Vanhanen, J.; Olofsson, U. Emission of 1.3–10 nm airborne particles from brake materials. Aerosol Sci. Technol. 2017, 51, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Hata, M.; Zhang, T.; Bao, L.; Otani, Y.; Bai, Y.; Furuuchi, M. Characteristics of the nanoparticles in a road tunnel. Aerosol Air Qual. Res. 2013, 13, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Wessels, A.; Birmili, W.; Albrecht, C.; Hellack, B.; Jermann, E.; Wick, G.; Harrison, R.M.; Schins, R.P.F. Oxidant generation and toxicity of size-fractionated ambient particles in human lung epithelial cells. Environ. Sci. Technol. 2010, 44, 3539–3545. [Google Scholar] [CrossRef]
- Carex Canada. Priority Carcinogens; Faculty of Health Sciences, Simon Fraser University: Vancouver, BC, Canada, 2020; Available online: https://www.carexcanada.ca (accessed on 15 October 2020).
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrei, S.; Lin, S.; Wang, X.; Liao, Y.-P.; et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef]
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bushell, M.; Beauchemin, S.; Kunc, F.; Gardner, D.; Ovens, J.; Toll, F.; Kennedy, D.; Nguyen, K.; Vladisavljevic, D.; Rasmussen, P.; et al. Characterization of commercial metal oxide nanomaterials: Crystalline phase, particle size and specific surface area. Nanomaterials 2020, 10, 1812. [Google Scholar] [CrossRef]
- Boyadzhiev, A.; Avramescu, M.-L.; Wu, D.; Williams, A.; Rasmussen, P.; Halappanavar, S. Impact of copper oxide particle solubility on lung epithelial cell toxicity: Response characterization using global transcriptional analysis. Nanotoxicology 2021, 15, 380–399. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Beauchemin, S.; Nugent, M.; Dugandzic, R.; Lanouette, M.; Chénier, M. Influence of matrix composition on the bioaccessibility of copper, zinc, and nickel in urban residential dust and soil. Hum. Ecol. Risk Assess. 2008, 14, 351–371. [Google Scholar] [CrossRef]
- Barrett, J.E.S.; Taylor, K.G.; Hudson-Edwards, K.A.; Charnock, J.M. Solid-phase speciation of Zn in road dust sediment. Mineral. Mag. 2011, 75, 2611–2629. [Google Scholar] [CrossRef]
- Beauchemin, S.; Rasmussen, P.E.; Mackinnon, T.; Chénier, M.; Boros, K. Zinc in house dust: Speciation, bioaccessibility, and impact of humidity. Environ. Sci. Technol. 2014, 48, 9022–9029. [Google Scholar] [CrossRef] [PubMed]
- Gonet, T.; Maher, B.A. Airborne, vehicle-derived Fe-bearing nanoparticles in the urban environment: A review. Environ. Sci. Technol. 2019, 53, 9970–9991. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.O.; Pinto, D.; Neckel, A.; Oliveira, M.L.S. An analysis of vehicular exhaust derived nanoparticles and historial Belgium fortress building interfaces. Geosci. Front. 2020, 11, 2053–2060. [Google Scholar] [CrossRef]
| Nano MOUDI Nominal Cut-Point | Particle Size on Filter | Classes |
|---|---|---|
| nm | nm | |
| 10,000 | >10,000 | - |
| 5600 | <10,000 >5600 | Micron 1–10 µm (PM10-1) |
| 3200 | <5600 >3200 | |
| 1800 | <3200 >1800 | |
| 1000 | <1800 >1000 | |
| 560 | <1000 >560 | Sub-micron 0.1–1 µm (PM1-0.1) |
| 320 | <560 >320 | |
| 180 | <320 >180 | |
| 100 | <180 >100 | |
| 56 | <100 >56 | Nano <100 nm (PM0.1 or UFP) |
| 32 | <56 >32 | |
| 18 | <32 >18 | |
| 10 | <18 >10 |
| Units | Art-D4-W1 | Art-D4-W2 | Art-D4-W3 | Ex-D1-D | LR-D1-D | LR-D2-D | Soil b | Ottawa Dust c | |
|---|---|---|---|---|---|---|---|---|---|
| pH | 7.94 | 7.55 | 7.90 | 8.08 | 8.01 | 8.58 | n.d. d | n.d. | |
| OC e | wt.% | 7.5 | 4.8 | 8.6 | 8.7 | 3.8 | n.d. | n.d. | n.d. |
| Si | wt.% | 7.23 | 7.84 | 8.97 | 12.02 | 8.05 | 9.45 | n.d. | n.d. |
| Fe | wt.% | 3.07 | 3.15 | 3.58 | 3.69 | 2.37 | 3.10 | 2.4 | 1.89 |
| Ca | wt.% | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.5 | 9.68 |
| Mg | wt.% | 2.99 | 2.96 | 3.36 | 2.93 | 2.82 | 3.98 | 0.70 | 1.58 |
| Al | wt.% | 2.67 | 2.85 | 3.30 | 4.24 | 2.86 | 3.36 | 6.1 | 4.75 |
| S | wt.% | 1.17 | 1.33 | 1.09 | 0.32 | 0.38 | 0.39 | n.d. | n.d. |
| Ti | mg kg−1 | 1279 | 1437 | 1781 | 2045 | 1111 | 1332 | 4400 | n.d. |
| Zn | mg kg−1 | 1258 | 1112 | 1310 | 881 | 597 | 1051 | 41 | 112 |
| Mn | mg kg−1 | 1017 | 1113 | 1016 | 931 | 1183 | 1120 | 636 | 431 |
| Sr | mg kg−1 | 530 | 538 | 522 | 485 | 289 | 332 | 283 | 459 |
| Ba | mg kg−1 | 466 | 483 | 527 | 575 | 335 | 499 | n.d. | 576 |
| Cu | mg kg−1 | 235 | 219 | 254 | 235 | 129 | 194 | 16 | 66 |
| Pb | mg kg−1 | 192 | 227 | 171 | 107 | 156 | 131 | 23 | 39 |
| Cr | mg kg−1 | 168 | 173 | 173 | 221 | 120 | 223 | 48 | 43 |
| Sample | Size Fraction | Replicate 1 | Replicate 2 | Replicate 3 | Median | Mean | ±SD a | Fractions | Sum of Means |
|---|---|---|---|---|---|---|---|---|---|
| nm | __________________% of total nano mass_______________ | nm | % of total nano mass | ||||||
| Art-D4-W1 | <18 >10 | 13 | 15 | 26 | 15 | 18 | 7 | ||
| <32 >18 | 18 | 32 | 0 | 18 | 17 | 16 | <32 >10 | 35 | |
| <56 >32 | 25 | 39 | 28 | 28 | 30 | 8 | |||
| <100 >56 | 44 | 14 | 46 | 44 | 35 | 18 | <100 >32 | 65 | |
| Art-D4-W2 | <18 >10 | 9 | . b | 6 | 6 | 7 | 4 | ||
| <32 >18 | 8 | . | 13 | 13 | 11 | 10 | <32 >10 | 18 | |
| <56 >32 | 48 | . | 34 | 48 | 41 | 19 | |||
| <100 >56 | 36 | . | 46 | 41 | 41 | 7 | <100 >32 | 82 | |
| Art-D4-W3 | <18 >10 | 2 | 2 | 28 | 2 | 11 | 15 | ||
| <32 >18 | 9 | 18 | 0 | 9 | 9 | 9 | <32 >10 | 20 | |
| <56 >32 | 36 | 43 | 21 | 36 | 33 | 11 | |||
| <100 >56 | 53 | 38 | 51 | 51 | 47 | 8 | <100 >32 | 80 | |
| Ex-D1-D | <18 >10 | 9 | 0 | 26 | 9 | 11 | 13 | ||
| <32 >18 | 0 | 0 | 28 | 0 | 9 | 16 | <32 >10 | 21 | |
| <56 >32 | 46 | 7 | 23 | 23 | 25 | 20 | |||
| <100 >56 | 45 | 93 | 23 | 45 | 54 | 36 | <100 >32 | 79 | |
| LR-D1-D | <18 >10 | 29 | 0 | 9 | 9 | 13 | 15 | ||
| <32 >18 | 12 | 21 | 3 | 12 | 12 | 9 | <32 >10 | 25 | |
| <56 >32 | 38 | 37 | 62 | 38 | 46 | 14 | |||
| <100 >56 | 21 | 42 | 25 | 25 | 29 | 11 | <100 >32 | 75 | |
| LR-D2-D | <18 >10 | 15 | 0 | . | 8 | 8 | 11 | ||
| <32 >18 | 6 | 5 | . | 5 | 5 | 1 | <32 >10 | 13 | |
| <56 >32 | 62 | 41 | . | 52 | 52 | 15 | |||
| <100 >56 | 17 | 53 | . | 35 | 35 | 26 | <100 >32 | 87 | |
| Sources a | Cd | Cr | Zn | V | Co | Ti | Ba | Fe | Al | Mg | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| brakes | x | x | x | x | x | ||||||
| tires, rubber | x | x | x | x | x | ||||||
| fossil fuel combustion (industrial, vehicle exhaust) | x | x | x | x | x | x | |||||
| pigments, paints, plastic | x | x | x | x | x | x | |||||
| automobile parts, steel and alloys in transport | x | x | x | x | x | x | x | x | |||
| galvanic protection | x | x | |||||||||
| cement | x | x | x | ||||||||
| asphalt (bitumen) | x | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beauchemin, S.; Levesque, C.; Wiseman, C.L.S.; Rasmussen, P.E. Quantification and Characterization of Metals in Ultrafine Road Dust Particles. Atmosphere 2021, 12, 1564. https://doi.org/10.3390/atmos12121564
Beauchemin S, Levesque C, Wiseman CLS, Rasmussen PE. Quantification and Characterization of Metals in Ultrafine Road Dust Particles. Atmosphere. 2021; 12(12):1564. https://doi.org/10.3390/atmos12121564
Chicago/Turabian StyleBeauchemin, Suzanne, Christine Levesque, Clare L. S. Wiseman, and Pat E. Rasmussen. 2021. "Quantification and Characterization of Metals in Ultrafine Road Dust Particles" Atmosphere 12, no. 12: 1564. https://doi.org/10.3390/atmos12121564