RECQ1 Helicase Silencing Decreases the Tumour Growth Rate of U87 Glioblastoma Cell Xenografts in Zebrafish Embryos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
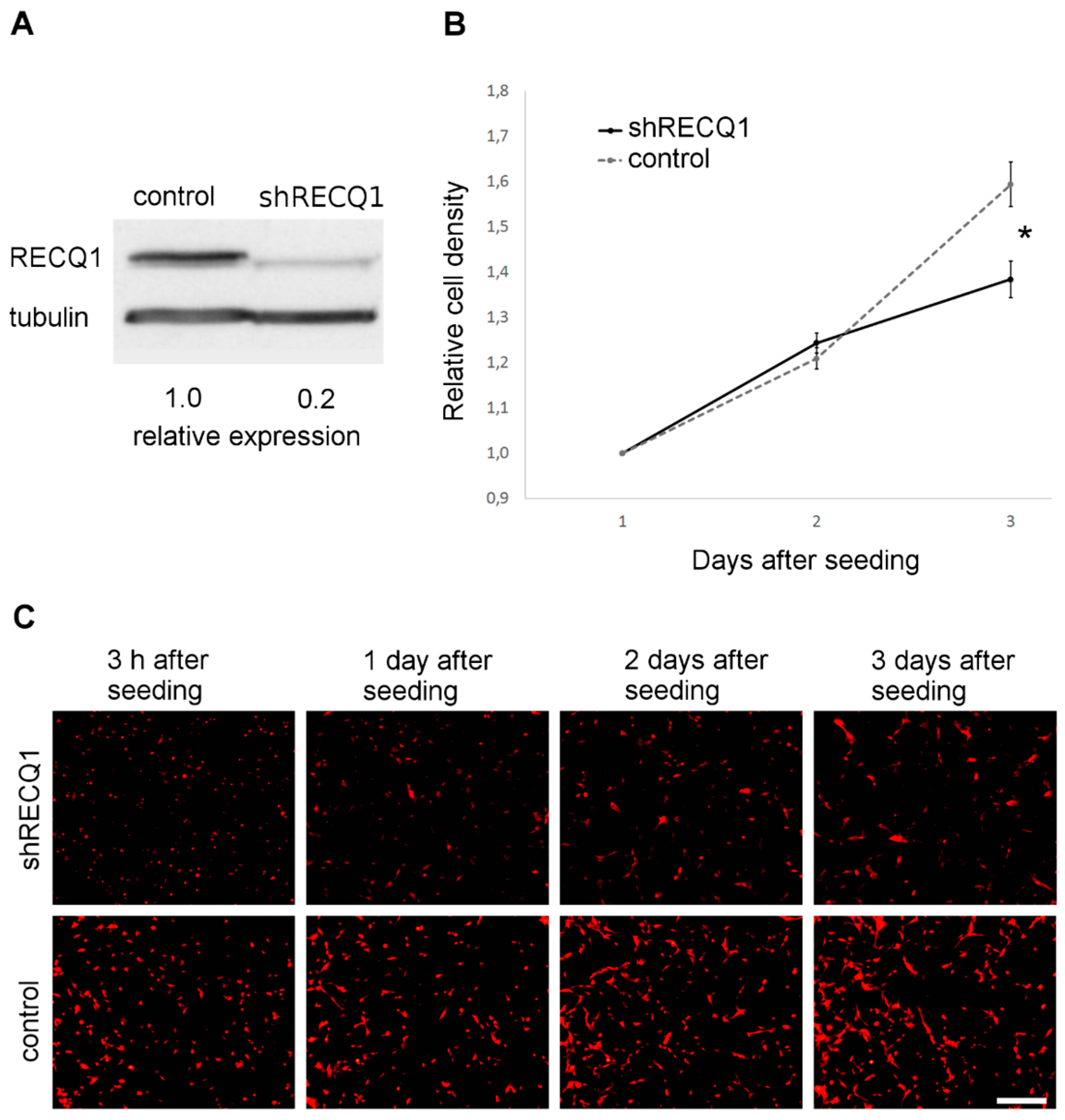
2.2. Preparation of RECQ1-Silenced Fluorescent Glioblastoma Cells
2.3. Analysis of the Rate of Cell Number Increase In Vitro
2.4. Cell Cycle Analysis
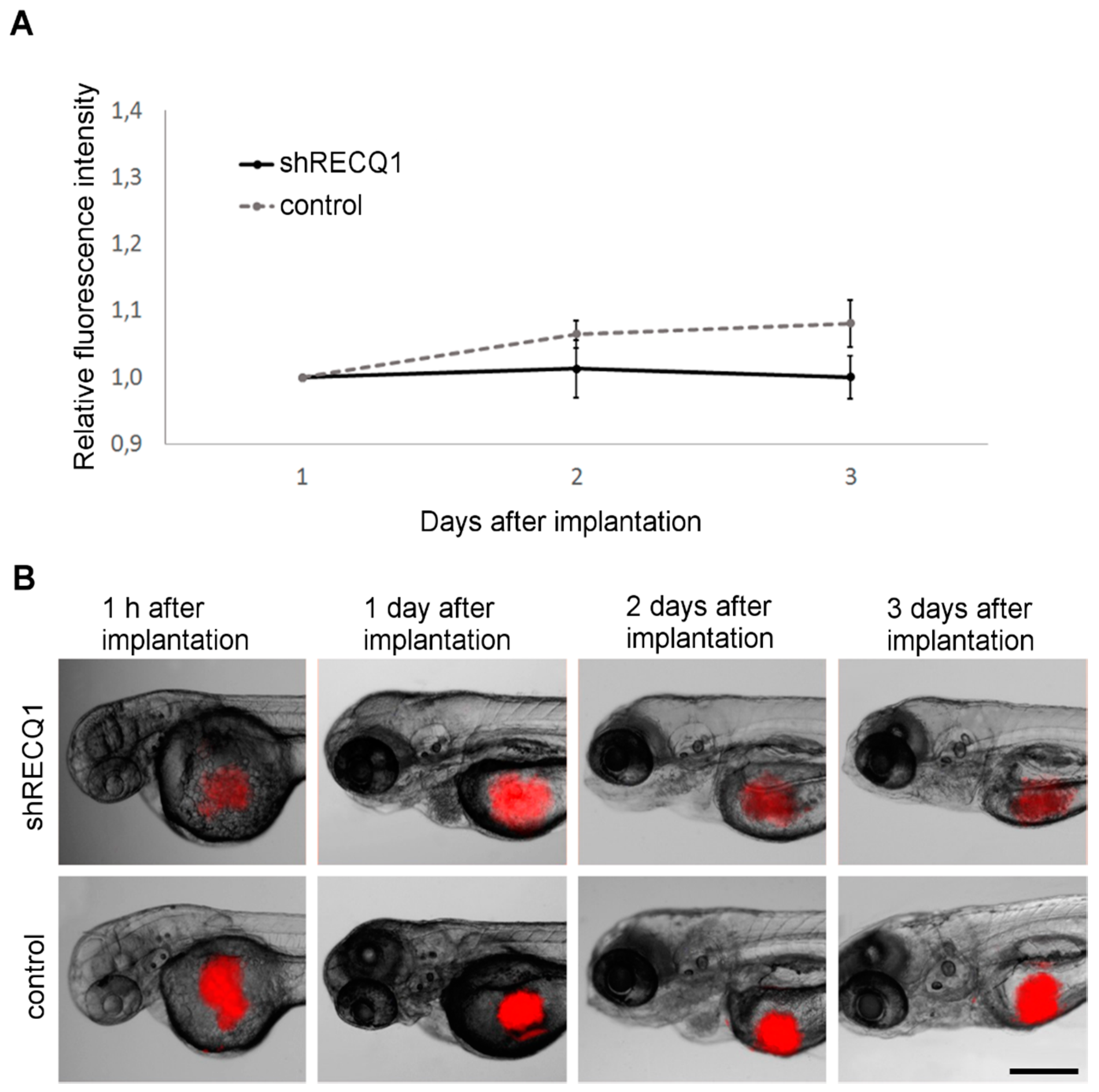
2.5. Analysis of Tumour Growth In Vivo
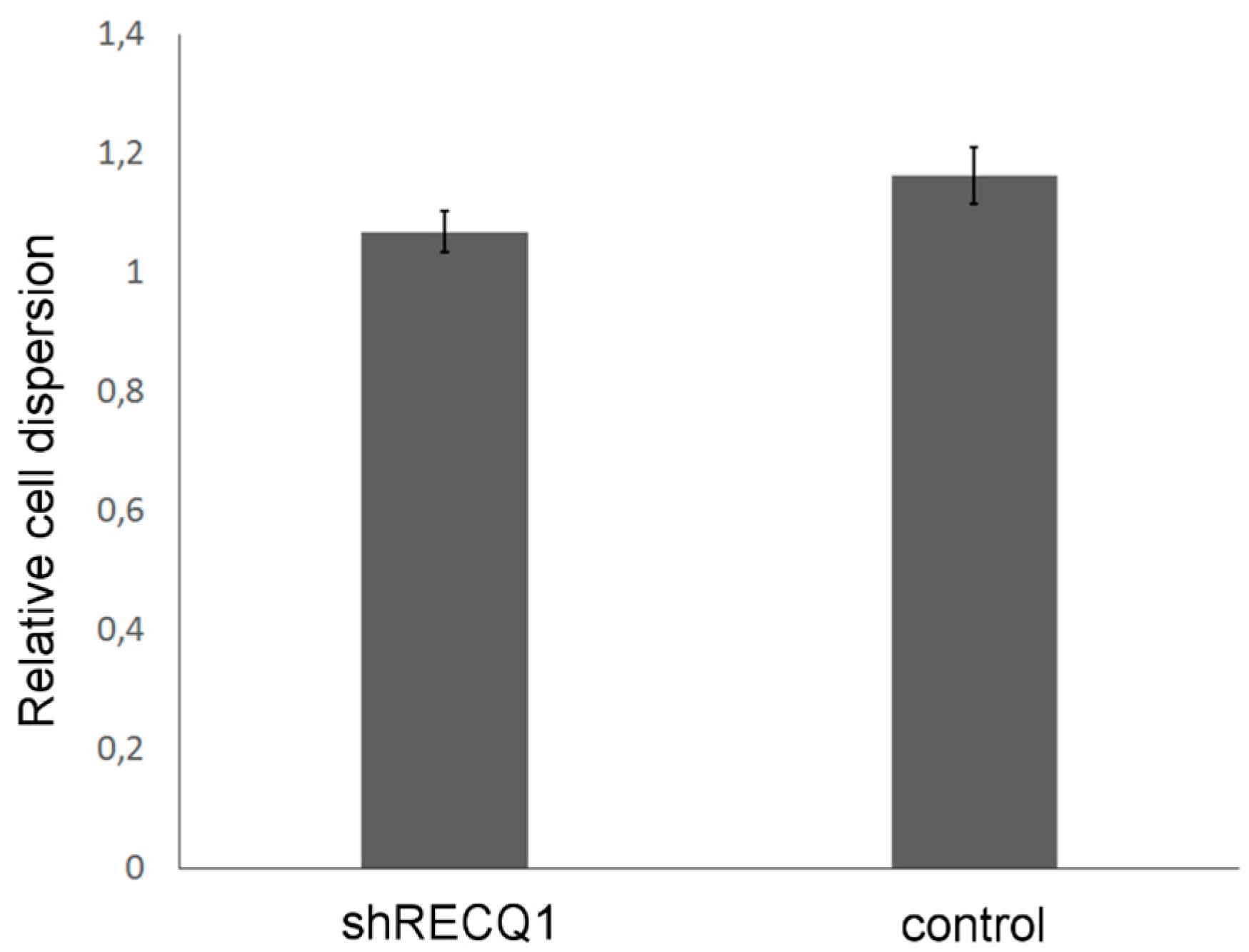
2.6. Analysis of Cell Invasion In Vivo
2.7. Statistical Analyses
3. Results
3.1. Reduced Rate of U87 Cell Number Increase as a Result of RECQ1 Helicase Silencing In Vitro
3.2. Cell-Cycle Perturbation in U87 Cells Resulting from RECQ1 Helicase Silencing
3.3. Absence of Tumour Growth in U87 Cell Xenotransplantsin the Zebrafish Yolk Sac
3.4. In Vivo Observation of Xenotransplant Growth in the Zebrafish Embryonic Brain
3.5. Effects of RECQ1 Silencing on Invasion of U87 Cells in the Embryonic Brain
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, S.; Brosh, R.M. Human RECQ1 is a DNA damage responsive protein required for genotoxic stress resistance and suppression of sister chromatid exchanges. PLoS ONE 2007, 2, e1297. [Google Scholar] [CrossRef] [PubMed]
- Berti, M.; Chaudhuri, A.R.; Thangavel, S.; Gomathinayagam, S.; Kenig, S.; Vujanovic, M.; Odreman, F.; Glatter, T.; Graziano, S.; Mendoza-Maldonado, R.; et al. Human RECQ1 promotes restart of replication forks reversed by DNA topoisomerase I inhibition. Nat. Struct. Mol. Biol. 2013, 20, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Futami, K.; Furuichi, Y. RECQL1 and WRN DNA repair helicases: Potential therapeutic targets and proliferative markers against cancers. Front. Genet. 2015, 5, 441. [Google Scholar] [CrossRef] [PubMed]
- Futami, K.; Ogasawara, S.; Goto, H.; Yano, H.; Furuichi, Y. RecQL1 DNA repair helicase: A potential tumor marker and therapeutic target against hepatocellular carcinoma. Int. J. Mol. Med. 2010, 25, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Lu, X.; Parvathaneni, S.; Bilke, S.; Zhang, H.; Thangavel, S.; Vindigni, A.; Hara, T.; Zhu, Y.; Meltzer, P.S.; et al. Identification of RECQ1-regulated transcriptome uncovers a role of RECQ1 in regulation of cancer cell migration and invasion. Cell Cycle 2014, 13, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Maldonado, R.; Faoro, V.; Bajpai, S.; Berti, M.; Odreman, F.; Vindigni, M.; Ius, T.; Ghasemian, A.; Bonin, S.; Skrap, M.; et al. The human RECQ1 helicase is highly expressed in glioblastoma and plays an important role in tumor cell proliferation. Mol. Cancer 2011, 10, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villano, J.L.; Seery, T.E.; Bressler, L.R. Temozolomide in malignant gliomas: Current use and future targets. Cancer Chemother. Pharmacol. 2009, 64, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Vittori, M.; Motaln, H.; Turnšek, T.L. The study of glioma by xenotransplantation in zebrafish early life stages. J. Histochem. Cytochem. 2015, 63, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Welker, A.M.; Jaros, B.D.; Puduvalli, V.K.; Imitola, J.; Kaur, B.; Beattie, C.E. Standardized orthotopic xenografts in zebrafish reveal glioma cell-line-specific characteristics and tumor cell heterogeneity. Dis. Model. Mech. 2016, 9, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Geiger, G.A.; Fu, W.; Kao, G.D. Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res. 2008, 68, 3396–3404. [Google Scholar] [CrossRef] [PubMed]
- Konantz, M.; Balci, T.B.; Hartwig, U.F.; Dellaire, G.; André, M.C.; Berman, J.N.; Lengerke, C. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann. N. Y. Acad. Sci. 2012, 1266, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Rampazzo, E.; Persano, L.; Pistollato, F.; Moro, E.; Frasson, C.; Porazzi, P.; Puppa, A.; Bresolin, S.; Battilana, G.; Indraccolo, S.; et al. Wnt activation promotes neuronal differentiation of glioblastoma. Cell Death Dis. 2013, 4, e500. [Google Scholar] [CrossRef] [PubMed]
- Vittori, M.; Breznik, B.; Gredar, T.; Hrovat, K.; Bizjak Mali, L.; Turnšek, T.L. Imaging of human glioblastoma cells and their interactions with mesenchymal stem cells in the zebrafish (Danio rerio) embryonic brain. Radiol. Oncol. 2016, 50, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Stoletov, K.; Montel, V.; Lester, R.D.; Gonias, S.L.; Klemke, R. High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proc. Natl. Acad. Sci. USA 2007, 104, 17406–17411. [Google Scholar] [CrossRef] [PubMed]
- Lally, B.E.; Geiger, G.A.; Kridel, S.; Arcury-Quandt, A.E.; Robbins, M.E.; Kock, N.D.; Wheeler, K.; Peddi, P.; Georgakilas, A.; Kao, G.D.; et al. Identification and biological evaluation of a novel and potent small molecule radiation sensitizer via an unbiased screen of a chemical library. Cancer Res. 2007, 67, 8791–8799. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.W.; Oh, E.S.; Park, S.H.; Chang, Y.T.; Kim, C.H.; Choi, S.Y.; Williams, D.R. A novel zebrafish human tumor xenograft model validated for anti-cancer drug screening. Mol. Biosyst. 2012, 8, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shimada, Y.; Kuroyanagi, J.; Nishimura, Y.; Umemoto, N.; Nomoto, T.; Shintou, T.; Miyazaki, T.; Tanaka, T. Zebrafish xenotransplantation model for cancer stem-like cell study and high-throughput screening of inhibitors. Tumor Biol. 2014, 35, 11861–11869. [Google Scholar] [CrossRef] [PubMed]
- Pillat, M.M.; Oliveira, M.N.; Motaln, H.; Breznik, B.; Glaser, T.; Lah, T.T.; Ulrich, H. Glioblastoma-mesenchymal stem cell communication modulates expression patterns of kinin receptors: Possible involvement of bradykinin in information flow. Cytom. A 2016, 89, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Podergajs, N.; Motaln, H.; Rajčević, U.; Verbovšek, U.; Koršič, M.; Obad, N.; Espedal, H.; Vittori, M.; Herold-Mende, C.; Miletic, H.; et al. Transmembrane protein CD9 is glioblastoma biomarker, relevant for maintenance of glioblastoma stem cells. Oncotarget 2016, 7, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Valente, L.J.; Gray, D.H.; Michalak, E.M.; Pinon-Hofbauer, J.; Egle, A.; Scott, C.L.; Janic, A.; Strasser, A. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep. 2013, 3, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shen, X.; Chen, X.; Liang, R.; Azares, A.R.; Liu, Y. Celf1 regulates cell cycle and is partially responsible for defective myoblast differentiation in myotonic dystrophy RNA toxicity. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Cooperation and Development. Test No. 236. Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for Testing of Chemicals; OECD Publishing: Paris, France, 2013; ISBN 9789264203709. [Google Scholar] [CrossRef]
- ISO 7346-3. Water Quality—Determination of the Acute Lethal Toxicity of Substances to a Freshwater Fish Brachydanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)—Part 3: Flow-Through Method; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- Short, S.C.; Martindale, C.; Bourne, S.; Brand, G.; Woodcock, M.; Johnston, P. DNA repair after irradiation in glioma cells and normal human astrocytes. Neuro-Oncology 2007, 9, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Faoro, V.; Kenig, S.; Storici, P.; Vindigni, A. The human RECQ1 helicase is highly expressed in glioblastoma and plays an important role in tumor cell proliferation. In Cross-Border Italy-Slovenia Biomedical Research: Are We Ready for Horizon 2020? Proceedings of the Cross-Border Biomedical Conference, Trieste, Italy, 27 February 2014; Passamonti, S., Gustincich, S., Lah Turnšek, T., Peterlin, B., Pišot, R., Storici, P., Eds.; Università di Trieste: Trieste, Italy, 2014; pp. 256–260. [Google Scholar]
- Kenig, S.; Faoro, V.; Bourkoula, E.; Podergajs, N.; Ius, T.; Vindigni, M.; Skrap, M.; Lah, T.; Cesselli, D.; Storici, P.; et al. Topoisomerase IIβ mediates the resistance of glioblastoma stem cells to replication stress-inducing drugs. Cancer Cell Int. 2016, 16, 58. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vittori, M.; Breznik, B.; Hrovat, K.; Kenig, S.; Lah, T.T. RECQ1 Helicase Silencing Decreases the Tumour Growth Rate of U87 Glioblastoma Cell Xenografts in Zebrafish Embryos. Genes 2017, 8, 222. https://doi.org/10.3390/genes8090222
Vittori M, Breznik B, Hrovat K, Kenig S, Lah TT. RECQ1 Helicase Silencing Decreases the Tumour Growth Rate of U87 Glioblastoma Cell Xenografts in Zebrafish Embryos. Genes. 2017; 8(9):222. https://doi.org/10.3390/genes8090222
Chicago/Turabian StyleVittori, Miloš, Barbara Breznik, Katja Hrovat, Saša Kenig, and Tamara T. Lah. 2017. "RECQ1 Helicase Silencing Decreases the Tumour Growth Rate of U87 Glioblastoma Cell Xenografts in Zebrafish Embryos" Genes 8, no. 9: 222. https://doi.org/10.3390/genes8090222
APA StyleVittori, M., Breznik, B., Hrovat, K., Kenig, S., & Lah, T. T. (2017). RECQ1 Helicase Silencing Decreases the Tumour Growth Rate of U87 Glioblastoma Cell Xenografts in Zebrafish Embryos. Genes, 8(9), 222. https://doi.org/10.3390/genes8090222






