Non-Neuronal Functions of the M2 Muscarinic Acetylcholine Receptor
Abstract
:1. Introduction to Acetylcholine and Its Receptors
2. Functions of Muscarinic Acetylcholine Receptors in Non-neuronal Cells
3. Signaling by the Muscarinic Acetylcholine Receptors

4. The M2 Muscarinic Acetylcholine Receptor
4.1. Structural Features of the M2 Receptor

4.2. Signaling of the M2 Muscarinic Acetylcholine Receptor
4.3. Receptor Internalization and Its Connection to Signaling
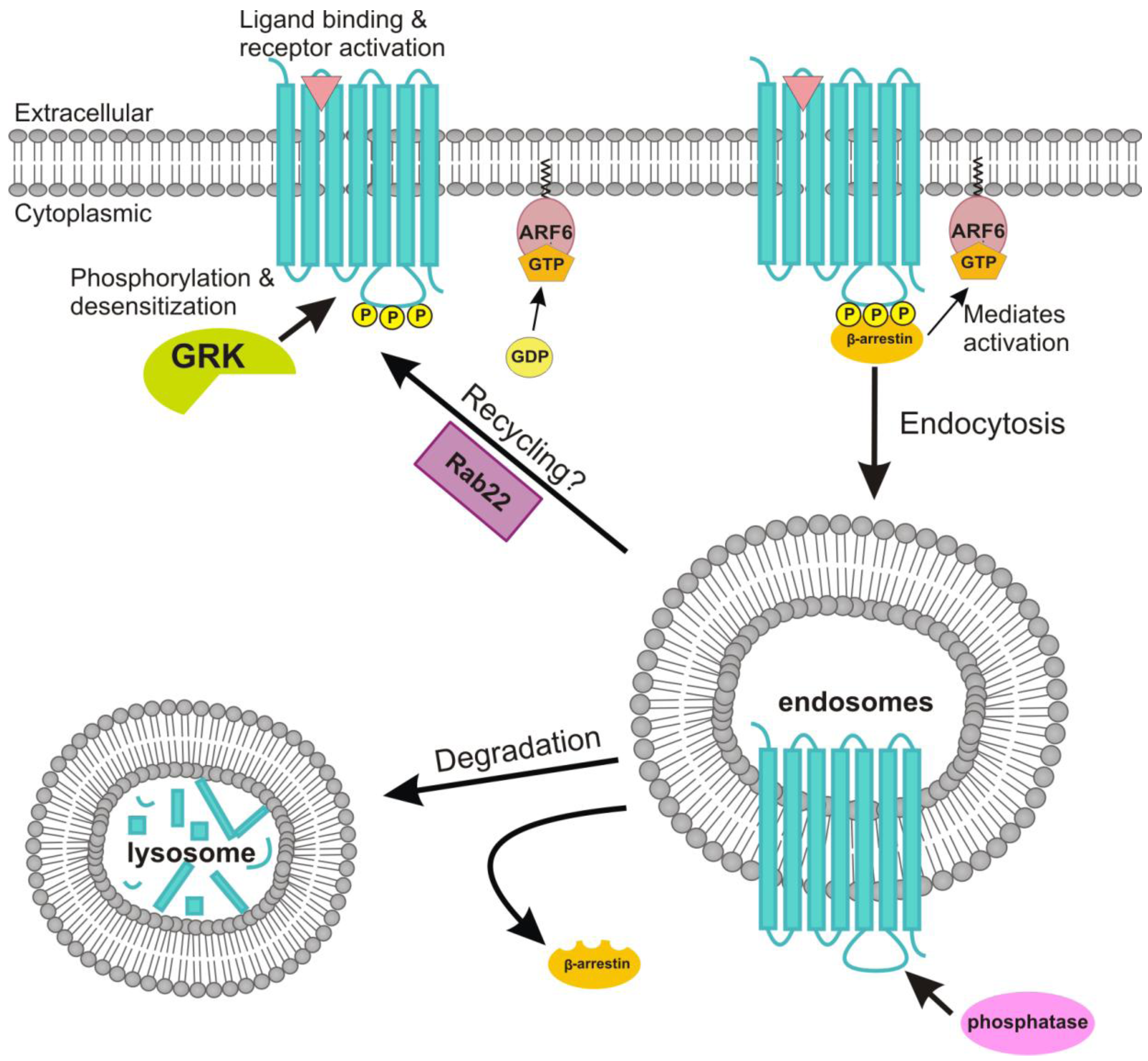
4.4. Role of ADP-Ribosylation Factor 6 in the Endocytosis of the M2 Receptor
5. Muscarinic Acetylcholine Receptors in Human Diseases
6. Conclusions and Future Challenges
Acknowledgments
Conflict of Interest
References and Notes
- Bennett, M.R. The concept of transmitter receptors: 100 years on. Neuropharmacology 2000, 39, 523–546. [Google Scholar] [CrossRef]
- Curtis, D.R.; Ryall, R.W. Nicotinic and Muscarinic Receptors of Renshaw Cells. Nature 1964, 203, 652–653. [Google Scholar] [CrossRef]
- Kurzen, H.; Wessler, I.; Kirkpatrick, C.J.; Kawashima, K.; Grando, S.A. The non-neuronal cholinergic system of human skin. Horm. Metab. Res. 2007, 39, 125–135. [Google Scholar] [CrossRef]
- Bonner, T.I.; Buckley, N.J.; Young, A.C.; Brann, M.R. Identification of a family of muscarinic acetylcholine receptor genes. Science 1987, 237, 527–532. [Google Scholar]
- Caulfield, M.P.; Birdsall, N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998, 50, 279–290. [Google Scholar]
- Hall, J.M.; Caulfield, M.P.; Watson, S.P.; Guard, S. Receptor subtypes or species homologues: relevance to drug discovery. Trends Pharmacol. Sci. 1993, 14, 376–383. [Google Scholar] [CrossRef]
- Jones, S.V.; Heilman, C.J.; Brann, M.R. Functional responses of cloned muscarinic receptors expressed in CHO-K1 cells. Mol. Pharmacol. 1991, 40, 242–247. [Google Scholar]
- Alea, M.P.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Fuxe, K.; Garriga, P. Differential expression of muscarinic acetylcholine receptor subtypes in Jurkat cells and their signaling. J. Neuroimmunol. 2011, 237, 13–22. [Google Scholar] [CrossRef]
- Nahorski, S.R.; Tobin, A.B.; Willars, G.B. Muscarinic M3 receptor coupling and regulation. Life Sci. 1997, 60, 1039–1045. [Google Scholar] [CrossRef]
- Hulme, E.C.; Lu, Z.L.; Saldanha, J.W.; Bee, M.S. Structure and activation of muscarinic acetylcholine receptors. Biochem. Soc. Trans. 2003, 31, 29–34. [Google Scholar]
- Nathanson, N.M. A multiplicity of muscarinic mechanisms: Enough signaling pathways to take your breath away. Proc. Natl. Acad. Sci. USA 2000, 97, 6245–6247. [Google Scholar] [CrossRef]
- Hayashi, M.K.; Haga, T. Palmitoylation of muscarinic acetylcholine receptor m2 subtypes: Reduction in their ability to activate G proteins by mutation of a putative palmitoylation site, cysteine 457, in the carboxyl-terminal tail. Arch. Biochem. Biophys. 1997, 340, 376–382. [Google Scholar] [CrossRef]
- Shah, N.; Khurana, S.; Cheng, K.; Raufman, J.P. Muscarinic receptors and ligands in cancer. Am. J. Physiol. Cell. Physiol. 2009, 296, C221–C232. [Google Scholar]
- Gomeza, J.; Shannon, H.; Kostenis, E.; Felder, C.; Zhang, L.; Brodkin, J.; Grinberg, A.; Sheng, H.; Wess, J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 1692–1697. [Google Scholar] [CrossRef]
- Gomeza, J.; Zhang, L.; Kostenis, E.; Felder, C.; Bymaster, F.; Brodkin, J.; Shannon, H.; Xia, B.; Deng, C.; Wess, J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 10483–10488. [Google Scholar] [CrossRef]
- Miyakawa, T.; Yamada, M.; Duttaroy, A.; Wess, J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J. Neurosci. 2001, 21, 5239–5250. [Google Scholar]
- Yamada, M.; Lamping, K.G.; Duttaroy, A.; Zhang, W.; Cui, Y.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Deng, C.X.; Faraci, F.M.; Wess, J. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. USA 2001, 98, 14096–14101. [Google Scholar] [CrossRef]
- Yamada, M.; Miyakawa, T.; Duttaroy, A.; Yamanaka, A.; Moriguchi, T.; Makita, R.; Ogawa, M.; Chou, C.J.; Xia, B.; Crawley, J.N.; Felder, C.C.; Deng, C.X.; Wess, J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 2001, 410, 207–212. [Google Scholar] [CrossRef]
- Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Wess, J. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem. Res. 2003, 28, 437–442. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. Basic and clinical aspects of non-neuronal acetylcholine: Overview of non-neuronal cholinergic systems and their biological significance. J. Pharmacol. Sci. 2008, 106, 167–173. [Google Scholar] [CrossRef]
- Kummer, W.; Lips, K.S.; Pfeil, U. The epithelial cholinergic system of the airways. Histochem. Cell Biol. 2008, 130, 219–234. [Google Scholar] [CrossRef]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: The non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008, 154, 1558–1571. [Google Scholar] [CrossRef]
- Grando, S.A.; Crosby, A.M.; Zelickson, B.D.; Dahl, M.V. Agarose gel keratinocyte outgrowth system as a model of skin re-epithelization: requirement of endogenous acetylcholine for outgrowth initiation. J. Invest. Dermatol. 1993, 101, 804–810. [Google Scholar]
- Grando, S.A.; Kist, D.A.; Qi, M.; Dahl, M.V. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J. Invest. Dermatol. 1993, 101, 32–36. [Google Scholar]
- Ndoye, A.; Buchli, R.; Greenberg, B.; Nguyen, V.T.; Zia, S.; Rodriguez, J.G.; Webber, R.J.; Lawry, M.A.; Grando, S.A. Identification and mapping of keratinocyte muscarinic acetylcholine receptor subtypes in human epidermis. J. Invest. Dermatol. 1998, 111, 410–416. [Google Scholar] [CrossRef]
- Buchli, R.; Ndoye, A.; Arredondo, J.; Webber, R.J.; Grando, S.A. Identification and characterization of muscarinic acetylcholine receptor subtypes expressed in human skin melanocytes. Mol. Cell. Biochem. 2001, 228, 57–72. [Google Scholar] [CrossRef]
- Chernyavsky, A.I.; Arredondo, J.; Wess, J.; Karlsson, E.; Grando, S.A. Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J. Cell. Biol. 2004, 166, 261–272. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ndoye, A.; Hall, L.L.; Zia, S.; Arredondo, J.; Chernyavsky, A.I.; Kist, D.A.; Zelickson, B.D.; Lawry, M.A.; Grando, S.A. Programmed cell death of keratinocytes culminates in apoptotic secretion of a humectant upon secretagogue action of acetylcholine. J. Cell. Sci. 2001, 114, 1189–1204. [Google Scholar]
- Bschleipfer, T.; Schukowski, K.; Weidner, W.; Grando, S.A.; Schwantes, U.; Kummer, W.; Lips, K.S. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci. 2007, 80, 2303–2307. [Google Scholar] [CrossRef]
- Chess-Williams, R.; Chapple, C.R.; Yamanishi, T.; Yasuda, K.; Sellers, D.J. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J. Auton. Pharmacol. 2001, 21, 243–248. [Google Scholar] [CrossRef]
- Metzen, J.; Bittinger, F.; Kirkpatrick, C.J.; Kilbinger, H.; Wessler, I. Proliferative effect of acetylcholine on rat trachea epithelial cells is mediated by nicotinic receptors and muscarinic receptors of the M1-subtype. Life Sci. 2003, 72, 2075–2080. [Google Scholar] [CrossRef]
- Acevedo, M. Effect of acetyl choline on ion transport in sheep tracheal epithelium. Pflugers Arch. 1994, 427, 543–546. [Google Scholar]
- Klein, M.K.; Haberberger, R.V.; Hartmann, P.; Faulhammer, P.; Lips, K.S.; Krain, B.; Wess, J.; Kummer, W.; Konig, P. Muscarinic receptor subtypes in cilia-driven transport and airway epithelial development. Eur. Respir. J. 2009, 33, 1113–1121. [Google Scholar] [CrossRef]
- Kilbinger, H.; von Bardeleben, R.S.; Siefken, H.; Wolf, D. Prejunctional muscarinic receptors regulating neurotransmitter release in airways. Life Sci. 1995, 56, 981–987. [Google Scholar] [CrossRef]
- Wang, H.; Han, H.; Zhang, L.; Shi, H.; Schram, G.; Nattel, S.; Wang, Z. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol. Pharmacol. 2001, 59, 1029–1036. [Google Scholar]
- Milner, P.; Kirkpatrick, K.A.; Ralevic, V.; Toothill, V.; Pearson, J.; Burnstock, G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc. Biol. Sci. 1990, 241, 245–248. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front. Biosci. 2004, 9, 2063–2085. [Google Scholar] [CrossRef]
- Kawashima, K.; Yoshikawa, K.; Fujii, Y.X.; Moriwaki, Y.; Misawa, H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007, 80, 2314–2319. [Google Scholar] [CrossRef]
- Qian, J.; Galitovskiy, V.; Chernyavsky, A.I.; Marchenko, S.; Grando, S.A. Plasticity of the murine spleen T-cell cholinergic receptors and their role in in vitro differentiation of naive CD4 T cells toward the Th1, Th2 and Th17 lineages. Genes Immun. 2011, 12, 222–230. [Google Scholar] [CrossRef]
- Fujii, T.; Watanabe, Y.; Inoue, T.; Kawashima, K. Upregulation of mRNA encoding the M5 muscarinic acetylcholine receptor in human T- and B-lymphocytes during immunological responses. Neurochemical. Res. 2003, 28, 423–429. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 2012, 91, 1027–1032. [Google Scholar] [CrossRef]
- Duttaroy, A.; Zimliki, C.L.; Gautam, D.; Cui, Y.; Mears, D.; Wess, J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes 2004, 53, 1714–1720. [Google Scholar] [CrossRef]
- Horn, F.; van der Wenden, E.M.; Oliveira, L.; AP, I.J.; Vriend, G. Receptors coupling to G proteins: Is there a signal behind the sequence? Proteins 2000, 41, 448–459. [Google Scholar] [CrossRef]
- Albert, P.R.; Robillard, L. G protein specificity: traffic direction required. Cell Signal. 2002, 14, 407–418. [Google Scholar] [CrossRef]
- Rumenapp, U.; Asmus, M.; Schablowski, H.; Woznicki, M.; Han, L.; Jakobs, K.H.; Fahimi-Vahid, M.; Michalek, C.; Wieland, T.; Schmidt, M. The M3 muscarinic acetylcholine receptor expressed in HEK-293 cells signals to phospholipase D via G12 but not Gq-type G proteins: regulators of G proteins as tools to dissect pertussis toxin-resistant G proteins in receptor-effector coupling. J. Biol. Chem. 2001, 276, 2474–2479. [Google Scholar] [CrossRef]
- Meyer zu Heringdorf, D.; Lass, H.; Alemany, R.; Laser, K.T.; Neumann, E.; Zhang, C.; Schmidt, M.; Rauen, U.; Jakobs, K.H.; van Koppen, C.J. Sphingosine kinase-mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 1998, 17, 2830–2837. [Google Scholar] [CrossRef]
- Neubig, R.R.; Siderovski, D.P. Regulators of G-protein signalling as new central nervous system drug targets. Nat. Rev. Drug Discov. 2002, 1, 187–197. [Google Scholar] [CrossRef]
- Logothetis, D.E.; Kurachi, Y.; Galper, J.; Neer, E.J.; Clapham, D.E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 1987, 325, 321–326. [Google Scholar] [CrossRef]
- Jones, S.V. Modulation of the inwardly rectifying potassium channel IRK1 by the m1 muscarinic receptor. Mol. Pharmacol. 1996, 49, 662–667. [Google Scholar]
- Jones, S.V. Muscarinic receptor subtypes: modulation of ion channels. Life Sci. 1993, 52, 457–464. [Google Scholar] [CrossRef]
- Wickman, K.; Clapham, D.E. Ion channel regulation by G proteins. Physiol. Rev. 1995, 75, 865–885. [Google Scholar]
- Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K.; Ciruela, F. Muscarinic acetylcholine receptor-interacting proteins (mAChRIPs): Targeting the receptorsome. Curr. Drug Targets 2012, 13, 53–71. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Correia, P.A.; Romero-Fernandez, W.; Narvaez, M.; Fuxe, K.; Ciruela, F.; Garriga, P. Muscarinic receptor family interacting proteins: role in receptor function. J. Neurosci. Meth. 2011, 195, 161–169. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yang, M.S.; Oh, C.D.; Kim, K.T.; Ha, M.J.; Kang, S.S.; Chun, J.S. Signalling pathway leading to an activation of mitogen-activated protein kinase by stimulating M3 muscarinic receptor. Biochem. J. 1999, 337, 275–280. [Google Scholar] [CrossRef]
- Crespo, P.; Xu, N.; Daniotti, J.L.; Troppmair, J.; Rapp, U.R.; Gutkind, J.S. Signaling through transforming G protein-coupled receptors in NIH 3T3 cells involves c-Raf activation. Evidence for a protein kinase C-independent pathway. J. Biol. Chem. 1994, 269, 21103–21109. [Google Scholar]
- Hawes, B.E.; van Biesen, T.; Koch, W.J.; Luttrell, L.M.; Lefkowitz, R.J. Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J. Biol. Chem. 1995, 270, 17148–17153. [Google Scholar]
- Crespo, P.; Xu, N.; Simonds, W.F.; Gutkind, J.S. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature 1994, 369, 418–420. [Google Scholar] [CrossRef]
- Rosenblum, K.; Futter, M.; Jones, M.; Hulme, E.C.; Bliss, T.V. ERKI/II regulation by the muscarinic acetylcholine receptors in neurons. J. Neurosci. 2000, 20, 977–985. [Google Scholar]
- Berkeley, J.L.; Levey, A.I. Muscarinic activation of mitogen-activated protein kinase in PC12 cells. J. Neurochem. 2000, 75, 487–493. [Google Scholar] [CrossRef]
- van Biesen, T.; Hawes, B.E.; Raymond, J.R.; Luttrell, L.M.; Koch, W.J.; Lefkowitz, R.J. G(o)-protein alpha-subunits activate mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. J. Biol. Chem. 1996, 271, 1266–1269. [Google Scholar]
- Lopez-Ilasaca, M.; Crespo, P.; Pellici, P.G.; Gutkind, J.S.; Wetzker, R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science 1997, 275, 394–397. [Google Scholar] [CrossRef]
- Coso, O.A.; Teramoto, H.; Simonds, W.F.; Gutkind, J.S. Signaling from G protein-coupled receptors to c-Jun kinase involves beta gamma subunits of heterotrimeric G proteins acting on a Ras and Rac1-dependent pathway. J. Biol. Chem. 1996, 271, 3963–3966. [Google Scholar]
- Nagao, M.; Yamauchi, J.; Kaziro, Y.; Itoh, H. Involvement of protein kinase C and Src family tyrosine kinase in Galphaq/11-induced activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. J. Biol. Chem. 1998, 273, 22892–22898. [Google Scholar] [CrossRef]
- Wylie, P.G.; Challiss, R.A.; Blank, J.L. Regulation of extracellular-signal regulated kinase and c-Jun N-terminal kinase by G-protein-linked muscarinic acetylcholine receptors. Biochem. J. 1999, 338, 619–628. [Google Scholar] [CrossRef]
- Yamauchi, J.; Nagao, M.; Kaziro, Y.; Itoh, H. Activation of p38 mitogen-activated protein kinase by signaling through G protein-coupled receptors. Involvement of Gbetagamma and Galphaq/11 subunits. J. Biol. Chem. 1997, 272, 27771–27777. [Google Scholar] [CrossRef]
- Hosey, M.M.; Benovic, J.L.; DebBurman, S.K.; Richardson, R.M. Multiple mechanisms involving protein phosphorylation are linked to desensitization of muscarinic receptors. Life Sci. 1995, 56, 951–955. [Google Scholar] [CrossRef]
- Pitcher, J.A.; Touhara, K.; Payne, E.S.; Lefkowitz, R.J. Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. J. Biol. Chem. 1995, 270, 11707–11710. [Google Scholar] [CrossRef]
- Elorza, A.; Sarnago, S.; Mayor, F., Jr. Agonist-dependent modulation of G protein-coupled receptor kinase 2 by mitogen-activated protein kinases. Mol. Pharmacol. 2000, 57, 778–783. [Google Scholar]
- Pitcher, J.A.; Tesmer, J.J.; Freeman, J.L.; Capel, W.D.; Stone, W.C.; Lefkowitz, R.J. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J. Biol. Chem. 1999, 274, 34531–34534. [Google Scholar]
- Vogler, O.; Nolte, B.; Voss, M.; Schmidt, M.; Jakobs, K.H.; van Koppen, C.J. Regulation of muscarinic acetylcholine receptor sequestration and function by beta-arrestin. J. Biol. Chem. 1999, 274, 12333–12338. [Google Scholar]
- Edwardson, J.M.; Szekeres, P.G. Endocytosis and recycling of muscarinic receptors. Life Sci. 1999, 64, 487–494. [Google Scholar] [CrossRef]
- van Koppen, C.J. Multiple pathways for the dynamin-regulated internalization of muscarinic acetylcholine receptors. Biochem. Soc. Trans. 2001, 29, 505–508. [Google Scholar] [CrossRef]
- Vogler, O.; Bogatkewitsch, G.S.; Wriske, C.; Krummenerl, P.; Jakobs, K.H.; van Koppen, C.J. Receptor subtype-specific regulation of muscarinic acetylcholine receptor sequestration by dynamin. Distinct sequestration of m2 receptors. J. Biol. Chem. 1998, 273, 12155–12160. [Google Scholar]
- Haga, K.; Kruse, A.C.; Asada, H.; Yurugi-Kobayashi, T.; Shiroishi, M.; Zhang, C.; Weis, W.I.; Okada, T.; Kobilka, B.K.; Haga, T.; Kobayashi, T. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 2012, 482, 547–551. [Google Scholar] [CrossRef]
- Ichiyama, S.; Oka, Y.; Haga, K.; Kojima, S.; Tateishi, Y.; Shirakawa, M.; Haga, T. The structure of the third intracellular loop of the muscarinic acetylcholine receptor M2 subtype. FEBS Lett. 2006, 580, 23–26. [Google Scholar] [CrossRef]
- Hawes, B.E.; Luttrell, L.M.; Exum, S.T.; Lefkowitz, R.J. Inhibition of G protein-coupled receptor signaling by expression of cytoplasmic domains of the receptor. J. Biol. Chem. 1994, 269, 15776–15785. [Google Scholar]
- Schlador, M.L.; Grubbs, R.D.; Nathanson, N.M. Multiple topological domains mediate subtype-specific internalization of the M2 muscarinic acetylcholine receptor. J. Biol. Chem. 2000, 275, 23295–23302. [Google Scholar] [CrossRef]
- Tsuga, H.; Kameyama, K.; Haga, T.; Honma, T.; Lameh, J.; Sadee, W. Internalization and down-regulation of human muscarinic acetylcholine receptor m2 subtypes. Role of third intracellular m2 loop and G protein-coupled receptor kinase 2. J. Biol. Chem. 1998, 273, 5323–5330. [Google Scholar]
- Wu, G.; Bogatkevich, G.S.; Mukhin, Y.V.; Benovic, J.L.; Hildebrandt, J.D.; Lanier, S.M. Identification of Gbetagamma binding sites in the third intracellular loop of the M(3)-muscarinic receptor and their role in receptor regulation. J. Biol. Chem. 2000, 275, 9026–9034. [Google Scholar]
- Michal, P.; Rudajev, V.; El-Fakahany, E.E.; Dolezal, V. Membrane cholesterol content influences binding properties of muscarinic M2 receptors and differentially impacts activation of second messenger pathways. Eur. J. Pharmacol. 2009, 606, 50–60. [Google Scholar] [CrossRef]
- Chmelar, R.S.; Nathanson, N.M. Identification of a novel apical sorting motif and mechanism of targeting of the M2 muscarinic acetylcholine receptor. J. Biol. Chem. 2006, 281, 35381–35396. [Google Scholar] [CrossRef]
- van Koppen, C.J.; Nathanson, N.M. Site-directed mutagenesis of the m2 muscarinic acetylcholine receptor. Analysis of the role of N-glycosylation in receptor expression and function. J. Biol. Chem. 1990, 265, 20887–20892. [Google Scholar]
- Park, P.; Sum, C.S.; Hampson, D.R.; Van Tol, H.H.; Wells, J.W. Nature of the oligomers formed by muscarinic m2 acetylcholine receptors in Sf9 cells. Eur. J. Pharmacol. 2001, 421, 11–22. [Google Scholar] [CrossRef]
- Pisterzi, L.F.; Jansma, D.B.; Georgiou, J.; Woodside, M.J.; Chou, J.T.; Angers, S.; Raicu, V.; Wells, J.W. Oligomeric size of the m2 muscarinic receptor in live cells as determined by quantitative fluorescence resonance energy transfer. J. Biol. Chem. 2010, 285, 16723–16738. [Google Scholar] [CrossRef]
- Goin, J.C.; Nathanson, N.M. Quantitative analysis of muscarinic acetylcholine receptor homo- and heterodimerization in live cells: regulation of receptor down-regulation by heterodimerization. J. Biol. Chem. 2006, 281, 5416–5425. [Google Scholar] [CrossRef]
- Maggio, R.; Barbier, P.; Fornai, F.; Corsini, G.U. Functional role of the third cytoplasmic loop in muscarinic receptor dimerization. J. Biol. Chem. 1996, 271, 31055–31060. [Google Scholar] [CrossRef]
- Michal, P.; El-Fakahany, E.E.; Dolezal, V. Muscarinic M2 receptors directly activate Gq/11 and Gs G-proteins. J. Pharmacol. Exp. Ther. 2007, 320, 607–614. [Google Scholar]
- Mochizuki, N.; Ohba, Y.; Kiyokawa, E.; Kurata, T.; Murakami, T.; Ozaki, T.; Kitabatake, A.; Nagashima, K.; Matsuda, M. Activation of the ERK/MAPK pathway by an isoform of rap1GAP associated with G alpha(i). Nature 1999, 400, 891–894. [Google Scholar] [CrossRef]
- Daub, H.; Wallasch, C.; Lankenau, A.; Herrlich, A.; Ullrich, A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997, 16, 7032–7044. [Google Scholar] [CrossRef]
- Gschwind, A.; Zwick, E.; Prenzel, N.; Leserer, M.; Ullrich, A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 2001, 20, 1594–1600. [Google Scholar] [CrossRef]
- Prenzel, N.; Zwick, E.; Daub, H.; Leserer, M.; Abraham, R.; Wallasch, C.; Ullrich, A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 1999, 402, 884–888. [Google Scholar]
- McCole, D.F.; Keely, S.J.; Coffey, R.J.; Barrett, K.E. Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-alpha. J. Biol. Chem. 2002, 277, 42603–42612. [Google Scholar]
- Tsai, W.; Morielli, A.D.; Peralta, E.G. The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate ion channel activity. EMBO J. 1997, 16, 4597–4605. [Google Scholar] [CrossRef]
- Stirnweiss, J.; Valkova, C.; Ziesche, E.; Drube, S.; Liebmann, C. Muscarinic M2 receptors mediate transactivation of EGF receptor through Fyn kinase and without matrix metalloproteases. Cell. Signal. 2006, 18, 1338–1349. [Google Scholar] [CrossRef]
- DeFea, K.A.; Zalevsky, J.; Thoma, M.S.; Dery, O.; Mullins, R.D.; Bunnett, N.W. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell. Biol 2000, 148, 1267–1281. [Google Scholar] [CrossRef]
- Tohgo, A.; Pierce, K.L.; Choy, E.W.; Lefkowitz, R.J.; Luttrell, L.M. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem. 2002, 277, 9429–9436. [Google Scholar]
- Jones, K.T.; Echeverry, M.; Mosser, V.A.; Gates, A.; Jackson, D.A. Agonist mediated internalization of M2 mAChR is beta-arrestin-dependent. J. Mol. Signal. 2006, 1, 7. [Google Scholar] [CrossRef]
- Pitcher, J.A.; Freedman, N.J.; Lefkowitz, R.J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998, 67, 653–692. [Google Scholar] [CrossRef]
- Nakata, H.; Kameyama, K.; Haga, K.; Haga, T. Location of agonist-dependent-phosphorylation sites in the third intracellular loop of muscarinic acetylcholine receptors (m2 subtype). Eur. J. Biochem. 1994, 220, 29–36. [Google Scholar] [CrossRef]
- Pals-Rylaarsdam, R.; Xu, Y.; Witt-Enderby, P.; Benovic, J.L.; Hosey, M.M. Desensitization and internalization of the m2 muscarinic acetylcholine receptor are directed by independent mechanisms. J. Biol. Chem. 1995, 270, 29004–29011. [Google Scholar]
- Pals-Rylaarsdam, R.; Gurevich, V.V.; Lee, K.B.; Ptasienski, J.A.; Benovic, J.L.; Hosey, M.M. Internalization of the m2 muscarinic acetylcholine receptor. Arrestin-independent and -dependent pathways. J. Biol. Chem. 1997, 272, 23682–23689. [Google Scholar]
- Lee, K.B.; Ptasienski, J.A.; Bunemann, M.; Hosey, M.M. Acidic amino acids flanking phosphorylation sites in the M2 muscarinic receptor regulate receptor phosphorylation, internalization, and interaction with arrestins. J. Biol. Chem. 2000, 275, 35767–35777. [Google Scholar]
- Lee, K.B.; Ptasienski, J.A.; Pals-Rylaarsdam, R.; Gurevich, V.V.; Hosey, M.M. Arrestin binding to the M(2) muscarinic acetylcholine receptor is precluded by an inhibitory element in the third intracellular loop of the receptor. J. Biol. Chem. 2000, 275, 9284–9289. [Google Scholar]
- Krueger, K.M.; Daaka, Y.; Pitcher, J.A.; Lefkowitz, R.J. The role of sequestration in G protein-coupled receptor resensitization. Regulation of beta2-adrenergic receptor dephosphorylation by vesicular acidification. J. Biol. Chem. 1997, 272, 5–8. [Google Scholar] [CrossRef]
- Thangaraju, A.; Sawyer, G.W. Comparison of the kinetics and extent of muscarinic M1-M5 receptor internalization, recycling and downregulation in Chinese hamster ovary cells. Eur. J. Pharmacol. 2011, 650, 534–543. [Google Scholar] [CrossRef]
- Roseberry, A.G.; Hosey, M.M. Trafficking of M(2) muscarinic acetylcholine receptors. J. Biol. Chem. 1999, 274, 33671–33676. [Google Scholar] [CrossRef]
- Feron, O.; Smith, T.W.; Michel, T.; Kelly, R.A. Dynamic targeting of the agonist-stimulated m2 muscarinic acetylcholine receptor to caveolae in cardiac myocytes. J. Biol. Chem. 1997, 272, 17744–17748. [Google Scholar] [CrossRef]
- Roseberry, A.G.; Hosey, M.M. Internalization of the M2 muscarinic acetylcholine receptor proceeds through an atypical pathway in HEK293 cells that is independent of clathrin and caveolae. J. Cell. Sci 2001, 114, 739–746. [Google Scholar]
- Delaney, K.A.; Murph, M.M.; Brown, L.M.; Radhakrishna, H. Transfer of M2 muscarinic acetylcholine receptors to clathrin-derived early endosomes following clathrin-independent endocytosis. J. Biol. Chem. 2002, 277, 33439–33446. [Google Scholar]
- Reiner, C.; Nathanson, N.M. The internalization of the M2 and M4 muscarinic acetylcholine receptors involves distinct subsets of small G-proteins. Life Sci. 2008, 82, 718–727. [Google Scholar] [CrossRef]
- Ferguson, S.M.; De Camilli, P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012, 13, 75–88. [Google Scholar]
- Macia, E.; Ehrlich, M.; Massol, R.; Boucrot, E.; Brunner, C.; Kirchhausen, T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 2006, 10, 839–850. [Google Scholar] [CrossRef]
- Mosser, V.A.; Jones, K.T.; Hoffman, K.M.; McCarty, N.A.; Jackson, D.A. Differential role of beta-arrestin ubiquitination in agonist-promoted down-regulation of M1 vs M2 muscarinic acetylcholine receptors. J. Mol. Signal. 2008, 3, 20. [Google Scholar] [CrossRef]
- Reiner, C.L.; McCullar, J.S.; Kow, R.L.; Le, J.H.; Goodlett, D.R.; Nathanson, N.M. RACK1 associates with muscarinic receptors and regulates M(2) receptor trafficking. PLoS One 2010, 5, e13517. [Google Scholar]
- Bolger, G.B.; Baillie, G.S.; Li, X.; Lynch, M.J.; Herzyk, P.; Mohamed, A.; Mitchell, L.H.; McCahill, A.; Hundsrucker, C.; Klussmann, E.; Adams, D.R.; Houslay, M.D. Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, beta-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5. Biochem. J. 2006, 398, 23–36. [Google Scholar] [CrossRef]
- Wennerberg, K.; Rossman, K.L.; Der, C.J. The Ras superfamily at a glance. J. Cell. Sci. 2005, 118, 843–846. [Google Scholar] [CrossRef]
- Donaldson, J.G. Multiple roles for Arf6: Sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003, 278, 41573–41576. [Google Scholar] [CrossRef]
- Donaldson, J.G.; Jackson, C.L. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2010, 12, 362–375. [Google Scholar] [CrossRef]
- Donaldson, J.G.; Honda, A. Localization and function of Arf family GTPases. Biochem. Soc. Trans. 2005, 33, 639–642. [Google Scholar] [CrossRef]
- Boman, A.L.; Taylor, T.C.; Melancon, P.; Wilson, K.L. A role for ADP-ribosylation factor in nuclear vesicle dynamics. Nature 1992, 358, 512–514. [Google Scholar]
- Dascher, C.; Balch, W.E. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 1994, 269, 1437–1448. [Google Scholar]
- Lenhard, J.M.; Kahn, R.A.; Stahl, P.D. Evidence for ADP-ribosylation factor (ARF) as a regulator ofin vitro endosome-endosome fusion . J. Biol. Chem. 1992, 267, 13047–13052. [Google Scholar]
- Taylor, T.C.; Kahn, R.A.; Melancon, P. Two distinct members of the ADP-ribosylation factor family of GTP-binding proteins regulate cell-free intra-Golgi transport. Cell 1992, 70, 69–79. [Google Scholar]
- D'Souza-Schorey, C.; Li, G.; Colombo, M.I.; Stahl, P.D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science 1995, 267, 1175–1178. [Google Scholar] [CrossRef]
- Sabe, H. Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J. Biochem. 2003, 134, 485–489. [Google Scholar] [CrossRef]
- D'Souza-Schorey, C.; Boshans, R.L.; McDonough, M.; Stahl, P.D.; Van Aelst, L. A role for POR1, a Rac1-interacting protein, in ARF6-mediated cytoskeletal rearrangements. Embo. J. 1997, 16, 5445–5454. [Google Scholar] [CrossRef]
- Radhakrishna, H.; Klausner, R.D.; Donaldson, J.G. Aluminum fluoride stimulates surface protrusions in cells overexpressing the ARF6 GTPase. J. Cell. Biol. 1996, 134, 935–947. [Google Scholar] [CrossRef]
- Brown, F.D.; Rozelle, A.L.; Yin, H.L.; Balla, T.; Donaldson, J.G. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol 2001, 154, 1007–1017. [Google Scholar] [CrossRef]
- Franco, M.; Chardin, P.; Chabre, M.; Paris, S. Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg2+ levels. J. Biol. Chem. 1995, 270, 1337–1341. [Google Scholar]
- Zhang, Q.; Cox, D.; Tseng, C.C.; Donaldson, J.G.; Greenberg, S. A requirement for ARF6 in Fcgamma receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 1998, 273, 19977–19981. [Google Scholar]
- Caumont, A.S.; Galas, M.C.; Vitale, N.; Aunis, D.; Bader, M.F. Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J. Biol. Chem. 1998, 273, 1373–1379. [Google Scholar]
- D'Souza-Schorey, C. Disassembling adherens junctions: breaking up is hard to do. Trends Cell. Biol 2005, 15, 19–26. [Google Scholar] [CrossRef]
- Palacios, F.; D'Souza-Schorey, C. Modulation of Rac1 and ARF6 activation during epithelial cell scattering. J. Biol. Chem. 2003, 278, 17395–17400. [Google Scholar] [CrossRef]
- Palacios, F.; Schweitzer, J.K.; Boshans, R.L.; D'Souza-Schorey, C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 2002, 4, 929–936. [Google Scholar] [CrossRef]
- Martin, T.F. PI(4,5)P(2) regulation of surface membrane traffic. Curr. Opin. Cell Biol. 2001, 13, 493–499. [Google Scholar] [CrossRef]
- Wenk, M.R.; De Camilli, P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. USA 2004, 101, 8262–8269. [Google Scholar] [CrossRef]
- Powelka, A.M.; Sun, J.; Li, J.; Gao, M.; Shaw, L.M.; Sonnenberg, A.; Hsu, V.W. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 2004, 5, 20–36. [Google Scholar] [CrossRef]
- Radhakrishna, H.; Donaldson, J.G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell. Biol. 1997, 139, 49–61. [Google Scholar] [CrossRef]
- Franco, M.; Peters, P.J.; Boretto, J.; van Donselaar, E.; Neri, A.; D'Souza-Schorey, C.; Chavrier, P. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. Embo. J. 1999, 18, 1480–1491. [Google Scholar] [CrossRef]
- Houndolo, T.; Boulay, P.L.; Claing, A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J. Biol. Chem. 2005, 280, 5598–5604. [Google Scholar] [CrossRef]
- Lau, A.W.; Chou, M.M. The adaptor complex AP-2 regulates post-endocytic trafficking through the non-clathrin Arf6-dependent endocytic pathway. J. Cell. Sci. 2008, 121, 4008–4017. [Google Scholar] [CrossRef]
- Hein, L.; Ishii, K.; Coughlin, S.R.; Kobilka, B.K. Intracellular targeting and trafficking of thrombin receptors. A novel mechanism for resensitization of a G protein-coupled receptor. J. Biol. Chem. 1994, 269, 27719–27726. [Google Scholar]
- Pierce, K.L.; Luttrell, L.M.; Lefkowitz, R.J. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene 2001, 20, 1532–1539. [Google Scholar] [CrossRef]
- Tong, H.; Rockman, H.A.; Koch, W.J.; Steenbergen, C.; Murphy, E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ. Res. 2004, 94, 1133–1141. [Google Scholar] [CrossRef]
- Premont, R.T.; Claing, A.; Vitale, N.; Freeman, J.L.; Pitcher, J.A.; Patton, W.A.; Moss, J.; Vaughan, M.; Lefkowitz, R.J. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA 1998, 95, 14082–14087. [Google Scholar] [CrossRef]
- Claing, A.; Chen, W.; Miller, W.E.; Vitale, N.; Moss, J.; Premont, R.T.; Lefkowitz, R.J. beta-Arrestin-mediated ADP-ribosylation factor 6 activation and beta 2-adrenergic receptor endocytosis. J. Biol. Chem. 2001, 276, 42509–42513. [Google Scholar]
- Claing, A.; Laporte, S.A.; Caron, M.G.; Lefkowitz, R.J. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 2002, 66, 61–79. [Google Scholar] [CrossRef]
- Madziva, M.T.; Birnbaumer, M. A role for ADP-ribosylation factor 6 in the processing of G-protein-coupled receptors. J. Biol. Chem. 2006, 281, 12178–12186. [Google Scholar] [CrossRef]
- Weigert, R.; Yeung, A.C.; Li, J.; Donaldson, J.G. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol. Biol. Cell. 2004, 15, 3758–3770. [Google Scholar] [CrossRef]
- Wallukat, G.; Nissen, E.; Morwinski, R.; Muller, J. Autoantibodies against the beta- and muscarinic receptors in cardiomyopathy. Herz 2000, 25, 261–266. [Google Scholar] [CrossRef]
- Nussinovitch, U.; Shoenfeld, Y. The diagnostic and clinical significance of anti-muscarinic receptor autoantibodies. Clin. Rev. Allergy Immunol. 2012, 42, 298–308. [Google Scholar] [CrossRef]
- Beltrame, S.P.; Auger, S.R.; Bilder, C.R.; Waldner, C.I.; Goin, J.C. Modulation of M(2) muscarinic receptor-receptor interaction by immunoglobulin G antibodies from Chagas' disease patients. Clin. Exp. Immunol. 2011, 164, 170–179. [Google Scholar] [CrossRef]
- Leiros, C.P.; Sterin-Borda, L.; Borda, E.S.; Goin, J.C.; Hosey, M.M. Desensitization and sequestration of human m2 muscarinic acetylcholine receptors by autoantibodies from patients with Chagas' disease. J. Biol. Chem. 1997, 272, 12989–12993. [Google Scholar]
- Fox, R.I.; Stern, M.; Michelson, P. Update in Sjogren syndrome. Curr. Opin. Rheumatol. 2000, 12, 391–398. [Google Scholar] [CrossRef]
- He, J.; Qiang, L.; Ding, Y.; Wei, P.; Li, Y.N.; Hua, H.; Li, Z.G. The role of muscarinic acetylcholine receptor type 3 polypeptide (M3RP205–220) antibody in the saliva of patients with primary Sjogren's syndrome. Clin. Exp. Rheumatol. 2012, 30, 322–326. [Google Scholar]
- Jin, M.; Hwang, S.M.; Davies, A.J.; Shin, Y.; Bae, J.S.; Lee, J.H.; Lee, E.B.; Song, Y.W.; Park, K. Autoantibodies in primary Sjogren's syndrome patients induce internalization of muscarinic type 3 receptors. Biochimica et biophysica acta 2012, 1822, 161–167. [Google Scholar]
- Bjur, D.; Danielson, P.; Alfredson, H.; Forsgren, S. Presence of a non-neuronal cholinergic system and occurrence of up- and down-regulation in expression of M2 muscarinic acetylcholine receptors: new aspects of importance regarding Achilles tendon tendinosis (tendinopathy). Cell Tissue Res. 2008, 331, 385–400. [Google Scholar] [CrossRef]
- Fisher, J.T.; Vincent, S.G.; Gomeza, J.; Yamada, M.; Wess, J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004, 18, 711–713. [Google Scholar]
- Jimenez, E.; Montiel, M. Activation of MAP kinase by muscarinic cholinergic receptors induces cell proliferation and protein synthesis in human breast cancer cells. J. Cell. Physiol. 2005, 204, 678–686. [Google Scholar] [CrossRef]
- Parnell, E.A.; Calleja-Macias, I.E.; Kalantari, M.; Grando, S.A.; Bernard, H.U. Muscarinic cholinergic signaling in cervical cancer cells affects cell motility via ERK1/2 signaling. Life Sci. 2012, 91, 1093–1098. [Google Scholar] [CrossRef]
- Fiszman, G.L.; Middonno, M.C.; de la Torre, E.; Farina, M.; Espanol, A.J.; Sales, M.E. Activation of muscarinic cholinergic receptors induces MCF-7 cells proliferation and angiogenesis by stimulating nitric oxide synthase activity. Cancer Biol. Ther. 2007, 6, 1106–1113. [Google Scholar] [CrossRef]
- Frucht, H.; Jensen, R.T.; Dexter, D.; Yang, W.L.; Xiao, Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin. Cancer Res. 1999, 5, 2532–2539. [Google Scholar]
- Cheng, K.; Zimniak, P.; Raufman, J.P. Transactivation of the epidermal growth factor receptor mediates cholinergic agonist-induced proliferation of H508 human colon cancer cells. Cancer Res. 2003, 63, 6744–6750. [Google Scholar]
- Xie, G.; Cheng, K.; Shant, J.; Raufman, J.P. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am. J. Physiol. Gastrointest Liver Physiol. 2009, 296, G755–G763. [Google Scholar] [CrossRef]
- Ferretti, M.; Fabbiano, C.; Di Bari, M.; Ponti, D.; Calogero, A.; Tata, A.M. M2 muscarinic receptors inhibit cell proliferation in human glioblastoma cell lines. Life Sci. 2012, 91, 1134–1137. [Google Scholar] [CrossRef]
- Fucile, S.; Napolitano, M.; Mattei, E. Cholinergic stimulation of human microcytoma cell line H69. Biochem. Biophys Res. Commun. 1997, 230, 501–504. [Google Scholar] [CrossRef]
- Williams, C.L.; Lennon, V.A. Activation of muscarinic acetylcholine receptors inhibits cell cycle progression of small cell lung carcinoma. Cell Regul. 1991, 2, 373–381. [Google Scholar]
- Zhao, Y.; Wang, X.; Wang, T.; Hu, X.; Hui, X.; Yan, M.; Gao, Q.; Chen, T.; Li, J.; Yao, M.; Wan, D.; Gu, J.; Fan, J.; He, X. Acetylcholinesterase, a key prognostic predictor for hepatocellular carcinoma, suppresses cell growth and induces chemosensitization. Hepatology 2011, 53, 493–503. [Google Scholar] [CrossRef]
- Wess, J.; Duttaroy, A.; Zhang, W.; Gomeza, J.; Cui, Y.; Miyakawa, T.; Bymaster, F.P.; McKinzie, L.; Felder, C.C.; Lamping, K.G.; Faraci, F.M.; Deng, C.; Yamada, M. M1-M5 muscarinic receptor knockout mice as novel tools to study the physiological roles of the muscarinic cholinergic system. Recept. Channels 2003, 9, 279–290. [Google Scholar] [CrossRef]
- Ito, Y.; Oyunzul, L.; Seki, M.; Fujino Oki, T.; Matsui, M.; Yamada, S. Quantitative analysis of the loss of muscarinic receptors in various peripheral tissues in M1-M5 receptor single knockout mice. Br. J. Pharmacol. 2009, 156, 1147–1153. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ockenga, W.; Kühne, S.; Bocksberger, S.; Banning, A.; Tikkanen, R. Non-Neuronal Functions of the M2 Muscarinic Acetylcholine Receptor. Genes 2013, 4, 171-197. https://doi.org/10.3390/genes4020171
Ockenga W, Kühne S, Bocksberger S, Banning A, Tikkanen R. Non-Neuronal Functions of the M2 Muscarinic Acetylcholine Receptor. Genes. 2013; 4(2):171-197. https://doi.org/10.3390/genes4020171
Chicago/Turabian StyleOckenga, Wymke, Sina Kühne, Simone Bocksberger, Antje Banning, and Ritva Tikkanen. 2013. "Non-Neuronal Functions of the M2 Muscarinic Acetylcholine Receptor" Genes 4, no. 2: 171-197. https://doi.org/10.3390/genes4020171
APA StyleOckenga, W., Kühne, S., Bocksberger, S., Banning, A., & Tikkanen, R. (2013). Non-Neuronal Functions of the M2 Muscarinic Acetylcholine Receptor. Genes, 4(2), 171-197. https://doi.org/10.3390/genes4020171





