Reproductive Strategies and Population Genetic Structure in Two Dryland River Floodplain Plants, Marsilea drummondii and Eleocharis acuta
Abstract
:1. Introduction
2. Materials and Methods
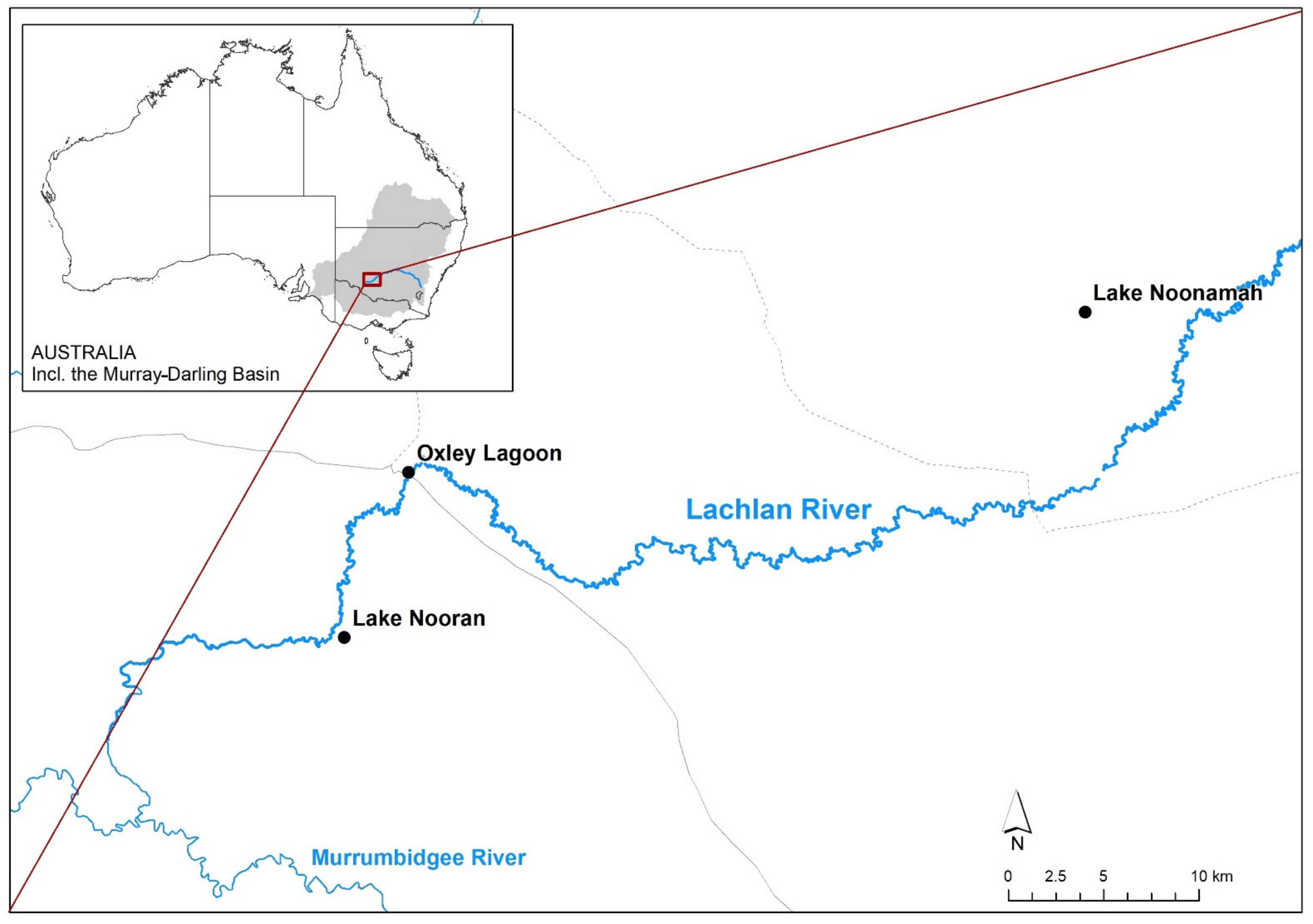
2.1. Study Area
2.2. Study Species
2.3. Study Design and Sampling Strategy
2.4. Genotyping
2.5. Data Analysis
3. Results
3.1. Clonality
3.2. Parent–Offspring Relationship through Self-Fertilisation
3.3. Parent–Offspring Relationship through Outcrossing
3.4. Other Sample Pairs with Few Locus Differences
3.5. Genetic Diversity
3.6. Population Genetic Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philbrick, C.T.; Les, D.H. Evolution of aquatic angiosperm reproductive systems. Bioscience 1996, 46, 813–826. [Google Scholar] [CrossRef]
- Li, W. Environmental opportunities and constraints in the reproduction and dispersal of aquatic plants. Aquat. Bot. 2014, 118, 62–70. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Ma, X.; Li, M.; Guo, X.; Yin, M.; Cai, Y.; Yu, X.; Du, N.; Wang, R. Impacts of the yellow River and Qingtongxia dams on genetic diversity of Phragmites australis in Ningxia Plain, China. Aquat. Bot. 2021, 169, 103341. [Google Scholar] [CrossRef]
- Dorken, M.E.; Eckert, C.G. Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J. Ecol. 2001, 89, 339–350. [Google Scholar] [CrossRef]
- Ivey, C.T.; Richards, J.H. Genetic diversity of everglades sawgrass, Cladium jamaicense (Cyperaceae). Int. J. Plant Sci. 2001, 162, 817–825. [Google Scholar] [CrossRef]
- Les, D.H. Breeding systems, population structure, and evolution in hydrophilous angiosperms. Ann. Mo. Bot. Gard. 1988, 75, 819–835. [Google Scholar] [CrossRef]
- Niklas, K.J.; Cobb, E.D. The evolutionary ecology (evo-eco) of plant asexual reproduction. Evol. Ecol. 2017, 31, 317–332. [Google Scholar] [CrossRef]
- Barrett, S.C. Sexual reproduction in Eichhornia crassipes (water hyacinth). II. Seed production in natural populations. J. Appl. Ecol. 1980, 17, 113–124. [Google Scholar] [CrossRef]
- McKee, J.; Richards, A. Variation in seed production and germinability in common reed (Phragmites australis) in Britain and France with respect to climate. New Phytol. 1996, 133, 233–243. [Google Scholar] [CrossRef]
- Eckert, C.G.; Dorken, M.E.; Mitchell, S.A. Loss of sex in clonal populations of a flowering plant, Decodon verticillatus (Lythraceae). Evolution 1999, 53, 1079–1092. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
- Barrett, S.C. Influences of clonality on plant sexual reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 8859–8866. [Google Scholar] [CrossRef]
- Handel, S.N. The intrusion of clonal growth patterns on plant breeding systems. Am. Nat. 1985, 125, 367–384. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Eckert, C.G.; Dorken, M.E.; Barrett, S.C. Ecological and evolutionary consequences of sexual and clonal reproduction in aquatic plants. Aquat. Bot. 2016, 135, 46–61. [Google Scholar] [CrossRef]
- Wade, M.J. A critical review of the models of group selection. Q. Rev. Biol. 1978, 53, 101–114. [Google Scholar] [CrossRef]
- Boedeltje, G.; Bakker, J.P.; Ten Brinke, A.; Van Groenendael, J.M.; Soesbergen, M. Dispersal phenology of hydrochorous plants in relation to discharge, seed release time and buoyancy of seeds: The flood pulse concept supported. J. Ecol. 2004, 92, 786–796. [Google Scholar] [CrossRef]
- Van der Valk, A. Succession in wetlands: A Gleasonian approach. Ecology 1981, 62, 688–696. [Google Scholar] [CrossRef]
- Santamaría, L. Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecol. 2002, 23, 137–154. [Google Scholar] [CrossRef]
- Piquot, Y.; Saumitou-Laprade, P.; Petit, D.; Vernet, P.; Epplen, J. Genotypic diversity revealed by allozymes and oligonucleotide DNA fingerprinting in French populations of the aquatic macrophyte Sparganium erectum. Mol. Ecol. 1996, 5, 251–258. [Google Scholar] [CrossRef]
- Le Corre, V.; Machon, N.; Petit, R.J.; Kremer, A. Colonization with long-distance seed dispersal and genetic structure of maternally inherited genes in forest trees: A simulation study. Genet. Res. 1997, 69, 117–125. [Google Scholar] [CrossRef]
- Cain, M.L.; Milligan, B.G.; Strand, A.E. Long-distance seed dispersal in plant populations. Am. J. Bot. 2000, 87, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I. Metapopulation dynamics. Nature 1998, 396, 41–49. [Google Scholar] [CrossRef]
- Walker, K.F.; Sheldon, F.; Puckridge, J.T. A perspective on dryland river ecosystems. Regul. Rivers Res. Manag. 1995, 11, 85–104. [Google Scholar] [CrossRef]
- Ozinga, W.A.; Bekker, R.M.; Schaminee, J.H.; Van Groenendael, J.M. Dispersal potential in plant communities depends on environmental conditions. J. Ecol. 2004, 92, 767–777. [Google Scholar] [CrossRef]
- Pannell, J.R.; Charlesworth, B. Neutral genetic diversity in a metapopulation with recurrent local extinction and recolonization. Evolution 1999, 53, 664–676. [Google Scholar] [CrossRef]
- Reynes, L.; Thibaut, T.; Mauger, S.; Blanfuné, A.; Holon, F.; Cruaud, C.; Couloux, A.; Valero, M.; Aurelle, D. Genomic signatures of clonality in the deep water kelp Laminaria rodriguezii. Authorea Prepr. 2020, 30, 1806–1822. [Google Scholar]
- Schaible, R.; Bergmann, I.; Bögle, M.; Schoor, A.; Schubert, H. Genetic characterisation of sexually and parthenogenetically reproductive populations of Chara canescens (Charophyceae) using AFLP, rbc L, and SNP markers. Phycologia 2009, 48, 105–117. [Google Scholar] [CrossRef]
- MDBA. Assessment of Environmental Water Requirements for the Proposed Basin Plan. Series of 24 Reports, Australian Government, Murray-Darling Basin Authority. Available online: https://www.mdba.gov.au/publications/mdba-reports/assessing-environmental-water-requirements-basins-rivers (accessed on 6 May 2022).
- Higgisson, W.; Higgisson, B.; Powell, M.; Driver, P.; Dyer, F. Impacts of water resource development on hydrological connectivity of different floodplain habitats in a highly variable system. River Res. Appl. 2020, 36, 542–552. [Google Scholar] [CrossRef]
- Kingsford, R.T. Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecol. 2000, 25, 109–127. [Google Scholar] [CrossRef]
- Bureau of Meteorology. Climate Statistics for Australian Locations, Summary Statistics Hillston Airport. Available online: http://www.bom.gov.au/climate/data-services/station-data.shtml (accessed on 14 July 2020).
- Bureau of Meteorology. Monthly Mean Maximum Temperature, Hay Airport AWS. Available online: http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=122&p_display_type=dailyDataFile&p_startYear=&p_c=&p_stn_num=075019 (accessed on 21 June 2021).
- PlantNET. Marsilea drummondii A.Braun In ‘New South Wales Flora Online’ The NSW Plant Information Network System, Royal Botanic Gardens and Domain Trust, Sydney. 2021. Available online: https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Marsilea~drummondii (accessed on 13 February 2021).
- Jones, D.L. Marsileaceae. In Flora of Australia: Ferns, Gymnosperms and Allied Groups; CSIRO Publishing: Canberra, Australia, 1998; Volume 48, pp. 166–173. [Google Scholar]
- Nagalingum, N.S.; Schneider, H.; Pryer, K.M. Comparative morphology of reproductive structures in heterosporous water ferns and a reevaluation of the sporocarp. Int. J. Plant Sci. 2006, 167, 805–815. [Google Scholar] [CrossRef]
- Cunningham, G.; Mulham, W.; Milthorpe, P.; Leigh, J. Plants of Western New South Wales; CSIRO Publishing: Melbourne, Australia, 1981. [Google Scholar]
- Schneider, H.; Pryer, K.M. Structure and function of spores in the aquatic heterosporous fern family Marsileaceae. Int. J. Plant Sci. 2002, 163, 485–505. [Google Scholar] [CrossRef]
- Johnson, D.M. Systematics of the new world species of Marsilea (Marsileaceae). Syst. Bot. Monogr. 1986, 11, 1–87. [Google Scholar] [CrossRef]
- Lellinger, K. New records for longevity of Marsilea sporocarps. Am. Fern J. 1985, 75, 30–31. [Google Scholar]
- Malone, C.R.; Proctor, V.W. Dispersal of Marsilea mucronata by water birds. Am. Fern J. 1965, 55, 167–170. [Google Scholar] [CrossRef]
- PlantNET. Eleocharis acuta R.Br. In ‘New South Wales Flora Online’ The NSW Plant Information Network System, Royal Botanic Gardens and Domain Trust, Sydney. 2021. Available online: https://plantnet.rbgsyd.nsw.gov.au/cgi-bin/NSWfl.pl?page=nswfl&lvl=sp&name=Eleocharis~acuta (accessed on 6 January 2021).
- Higgisson, W.; Dyer, F. Seed germination and dispersal of Eleocharis acuta and Eleocharis sphacelata under experimental hydrological conditions. Aquat. Ecol. 2021, 55, 21–32. [Google Scholar] [CrossRef]
- Green, A.J.; Jenkins, K.; Bell, D.; Morris, P.; Kingsford, R. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshw. Biol. 2008, 53, 380–392. [Google Scholar] [CrossRef]
- Walters, S. Eleocharis R. Br. J. Ecol. 1949, 37, 192–206. [Google Scholar] [CrossRef]
- Demeda, C.L.B.; Seger, G.D.d.S.; Steiner, N.; Trevisan, R. Reproductive phenology and germination of Eleocharis laeviglumis R. Trevis. & Boldrini (Cyperaceae). Acta Bot. Bras. 2018, 32, 487–492. [Google Scholar]
- Cruz, V.M.; Kilian, A.; Dierig, D.A. Development of DArT marker platforms and genetic diversity assessment of the US collection of the new oilseed crop lesquerella and related species. PLoS ONE 2013, 8, e64062. [Google Scholar] [CrossRef]
- Kilian, A.; Wenzl, P.; Huttner, E.; Carling, J.; Xia, L.; Blois, H.; Caig, V.; Heller-Uszynska, K.; Jaccoud, D.; Hopper, C. Diversity arrays technology: A generic genome profiling technology on open platforms. Methods Mol. Biol. 2012, 888, 67–89. [Google Scholar]
- Altshuler, D.; Pollara, V.J.; Cowles, C.R.; Van Etten, W.J.; Baldwin, J.; Linton, L.; Lander, E.S. An SNP map of the human genome generated by reduced representation shotgun sequencing. Nature 2000, 407, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.A.; Etter, P.D.; Atwood, T.S.; Currey, M.C.; Shiver, A.L.; Lewis, Z.A.; Selker, E.U.; Cresko, W.A.; Johnson, E.A. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE 2008, 3, e3376. [Google Scholar] [CrossRef] [PubMed]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.; Unmack, P.J.; Berry, O.F.; Georges, A. dartr: An r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol. Ecol. Resour. 2018, 18, 691–699. [Google Scholar] [CrossRef]
- Goudet, J. Hierfstat, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes 2005, 5, 184–186. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Kamvar, Z.; Tabima, J.; Grünwald, N. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Boedeltje, G.; Ozinga, W.A.; Prinzing, A. The trade-off between vegetative and generative reproduction among angiosperms influences regional hydrochorous propagule pressure. Glob. Ecol. Biogeogr. 2008, 17, 50–58. [Google Scholar] [CrossRef]
- Sarneel, J. The dispersal capacity of vegetative propagules of riparian fen species. Hydrobiologia 2013, 710, 219–225. [Google Scholar] [CrossRef]
- Capon, S.J.; Brock, M.A. Flooding, soil seed bank dynamics and vegetation resilience of a hydrologically variable desert floodplain. Freshw. Biol. 2006, 51, 206–223. [Google Scholar] [CrossRef]
- Vitalis, R.; Riba, M.; Colas, B.; Grillas, P.; Olivieri, I. Multilocus genetic structure at contrasted spatial scales of the endangered water fern Marsilea strigosa Willd.(Marsileaceae, Pteridophyta). Am. J. Bot. 2002, 89, 1142–1155. [Google Scholar] [CrossRef]
- Whitehead, M.R.; Lanfear, R.; Mitchell, R.J.; Karron, J.D. Plant mating systems often vary widely among populations. Front. Ecol. Evol. 2018, 6, 38. [Google Scholar] [CrossRef]
- Costa, A.; Machado, I. Flowering dynamics and pollination system of the sedge Rhynchospora ciliata (Vahl) Kükenth (Cyperaceae): Does ambophily enhance its reproductive success? Plant Biol. 2012, 14, 881–887. [Google Scholar] [CrossRef]
- Pollux, B.; Luteijn, A.; Van Groenendael, J.; Ouborg, N. Gene flow and genetic structure of the aquatic macrophyte Sparganium emersum in a linear unidirectional river. Freshw. Biol. 2009, 54, 64–76. [Google Scholar] [CrossRef]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef]
- McGinness, H. Waterbird Breeding and Movements: Knowledge for Water Managers. Available online: https://research.csiro.au/ewkrwaterbirds/research-results/ (accessed on 5 May 2021).



| (1) Genetically Identical Pairs through Asexual Reproduction | (2) Pairs in a Parent–Offspring Rel. through Self-Fertilisation | (3) Pairs in a Parent–Offspring Relationship through Outcrossing | |||
|---|---|---|---|---|---|
| Possible Loci | Can Be: | Possible Loci | Can Be: | Possible Loci | Can Be: |
| AA | AA | AA | AA | AA | AB, AA |
| AB | AB | AB | AA, AB, BB | AB | AA, AB, BB |
| BB | BB | BB | BB | BB | AB, BB |
| (n) Genotypes | |||||
|---|---|---|---|---|---|
| Wetland | Patch | MD | EA | ||
| Wetland | Patch | Wetland | Patch | ||
| Lake Nooran | 1 | 21 (26) | 7 (13) | 26 (26) | 13 (13) |
| 2 | 13 (13) | 13 (13) | |||
| Noonamah Wetland | 1 | 37 (39) | 12 (13) | 38 (39) | 13 (13) |
| 2 | 13 (13) | 12 (13) | |||
| 3 | 13 (13) | 13 (13) | |||
| Oxley Lagoon | 1 | 26 (26) | 13 (13) | 26 (26) | 13 (13) |
| 2 | 13 (13) | 13 (13) | |||
| All—Lachlan | 83 (91) | 90 (91) | |||
| Ho | He | FIS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wetland | Patch | MD | EA | MD | EA | MD | EA | ||||
| Wetland | Patch | Wetland | Patch | Wetland | Patch | Wetland | Patch | ||||
| Lake Nooran | 1 | 0.117 | 0.035 | 0.244 | 0.248 | 0.105 | 0.021 | 0.190 | 0.172 | −0.669 | −0.403 |
| 2 | 0.160 | 0.241 | 0.093 | 0.173 | −0.693 | −0.354 | |||||
| Lake Noonamah | 1 | 0.035 | 0.045 | 0.060 | 0.056 | 0.050 | 0.059 | 0.056 | 0.051 | 0.264 | −0.076 |
| 2 | 0.040 | 0.063 | 0.050 | 0.056 | 0.229 | −0.090 | |||||
| 3 | 0.019 | 0.061 | 0.013 | 0.051 | −0.362 | −0.157 | |||||
| Oxley Lagoon | 1 | 0.205 | 0.278 | 0.239 | 0.244 | 0.203 | 0.189 | 0.193 | 0.188 | −0.439 | −0.265 |
| 2 | 0.128 | 0.233 | 0.125 | 0.183 | 0.018 | −0.236 | |||||
| All—Lachlan | 0.105 | 0.165 | 0.144 | 0.220 | |||||||
| Source of Variation | df | Sum of Squares | Mean Sum of Squares | Estimated (%) Variation | p Value |
|---|---|---|---|---|---|
| M.drummondii | |||||
| Between wetlands | 2 | 7092 | 3546 | 31% | 0.01 * |
| Between samples within wetlands | 81 | 12,227 | 150 | 5% | 0.12 |
| Within samples | 84 | 10,943 | 130 | 64% | 0.01 * |
| Total | 167 | 30,262 | 181 | 100% | |
| E.acuta | |||||
| Between wetlands | 2 | 64,774 | 32,387 | 32% | 0.01 * |
| Between samples within wetlands | 87 | 99,304 | 1141 | 1% | 0.34 |
| Within samples | 90 | 98,384 | 1093 | 67% | 0.01 * |
| Total | 179 | 262,462 | 1466 | 100% | |
| Lake Nooran 1 | Lake Nooran 2 | Noonamah 1 | Noonamah 2 | Noonamah 3 | Oxley 1 | Oxley 2 | |
|---|---|---|---|---|---|---|---|
| Lake Nooran 1 | --- | 0.075 | 0.307 | 0.301 | 0.309 | 0.058 | 0.055 |
| Lake Nooran 2 | 0.334 | --- | 0.311 | 0.305 | 0.313 | 0.062 | 0.057 |
| Noonamah 1 | 0.339 | 0.277 | --- | 0.033 | 0.046 | 0.279 | 0.275 |
| Noonamah 2 | 0.165 | 0.167 | 0.066 | --- | 0.040 | 0.274 | 0.270 |
| Noonamah 3 | 0.395 | 0.308 | 0.155 | 0.026 | --- | 0.281 | 0.276 |
| Oxley 1 | 0.318 | 0.266 | 0.277 | 0.201 | 0.303 | --- | 0.039 |
| Oxley 2 | 0.173 | 0.031 | 0.158 | 0.116 | 0.170 | 0.199 | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higgisson, W.; Broadhurst, L.; Shams, F.; Gruber, B.; Dyer, F. Reproductive Strategies and Population Genetic Structure in Two Dryland River Floodplain Plants, Marsilea drummondii and Eleocharis acuta. Genes 2022, 13, 1506. https://doi.org/10.3390/genes13091506
Higgisson W, Broadhurst L, Shams F, Gruber B, Dyer F. Reproductive Strategies and Population Genetic Structure in Two Dryland River Floodplain Plants, Marsilea drummondii and Eleocharis acuta. Genes. 2022; 13(9):1506. https://doi.org/10.3390/genes13091506
Chicago/Turabian StyleHiggisson, William, Linda Broadhurst, Foyez Shams, Bernd Gruber, and Fiona Dyer. 2022. "Reproductive Strategies and Population Genetic Structure in Two Dryland River Floodplain Plants, Marsilea drummondii and Eleocharis acuta" Genes 13, no. 9: 1506. https://doi.org/10.3390/genes13091506






