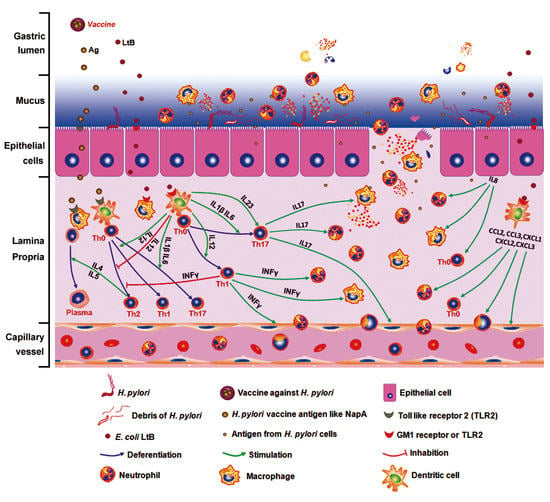
E. coli Enterotoxin LtB Enhances Vaccine-Induced Anti-H. pylori Protection by Promoting Leukocyte Migration into Gastric Mucus via Inflammatory Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria and Cultivation
2.2. L. lactis Production of NapA and LtB, Respectively
2.3. Oral Vaccination of Mice
2.4. Sampling Blood, Spleen and Intestinal Feces
2.5. ELISA Detection of IgG and SIgA Antibodies
2.6. Splenocyte Cultivation and Antigenic Stimulation
2.7. Assessment of Cytokines
2.8. H. pylori Challenges
2.9. Evaluation of H. pylori Colonization
2.10. Gastric Histological Examination
2.11. Statistical Analysis
3. Results
3.1. Cultivation of the Engineered L. lactis Strains
3.2. Immunization of Mice and Antibody Assays
3.3. Splenocyte Cultivation and Cytokine Assessment
3.4. H. pylori Challenge and Immune Protection
3.5. Gastric Histological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bloom, D.E.; Black, S.; Salisbury, D.; Rappuoli, R. Antimicrobial resistance and the role of vaccines. Proc. Natl. Acad. Sci. USA 2018, 115, 12868–12871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Brubaker, J.; Harro, C.; Weirzba, T.; Sack, D. Development of a novel multiplex electrochemiluminescent-based immunoassay to aid enterotoxigenic Escherichia coli vaccine development and evaluations. J. Immunol. Methods 2019, 470, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Walduck, A.K.; Raghavan, S. Immunity and Vaccine Development Against Helicobacter pylori. In Results and Problems in Cell Differentiation; Springer: New York, NY, USA, 2019; pp. 1–19. [Google Scholar]
- Stubljar, D.; Jukic, T.; Ihan, A. How far are we from vaccination against Helicobacter pylori infection? Expert Rev. Vaccines 2018, 17, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Ikewaki, J.; Nishizono, A.; Goto, T.; Fujioka, T.; Mifune, K. Therapeutic Oral Vaccination Induces Mucosal Immune Response Sufficient to Eliminate Long-TermHelicobacter pyloriInfection. Microbiol. Immunol. 2000, 44, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garhart, C.A.; Nedrud, J.G.; Heinzel, F.P.; Sigmund, N.E.; Czinn, S.J. Vaccine-Induced Protection against Helicobacter pylori in Mice Lacking Both Antibodies and Interleukin-4. Infect. Immun. 2003, 71, 3628–3633. [Google Scholar] [CrossRef] [PubMed]
- Gottwein, J.M.; Blanchard, T.G.; Targoni, O.S.; Eisenberg, J.C.; Zagorski, B.M.; Redline, R.W.; Nedrud, J.G.; Tary-Lehmann, M.; Lehmann, P.V.; Czinn, S.J. Protective Anti-Helicobacter Immunity Is Induced with Aluminum Hydroxide or Complete Freund’s Adjuvant by Systemic Immunization. J. Infect. Dis. 2001, 184, 308–314. [Google Scholar] [CrossRef]
- Ernst, P.B.; Pappo, J. T-cell-mediated mucosal immunity in the absence of antibody: Lessons from Helicobacter pylori infection. Acta Odontol. Scand. 2001, 59, 216–221. [Google Scholar] [CrossRef]
- Sun, H.; Yuan, H.; Tan, R.; Li, B.; Guo, G.; Zhang, J.; Jing, H.; Qin, Y.; Zhao, Z.; Zou, Q.; et al. Immunodominant antigens that induce Th1 and Th17 responses protect mice against Helicobacter pylori infection. Oncotarget 2018, 9, 12050–12063. [Google Scholar] [CrossRef]
- Ding, H.; Nedrud, J.G.; Blanchard, T.G.; Zagorski, B.M.; Li, G.; Shiu, J.; Xu, J.; Czinn, S.J. Th1-Mediated Immunity against Helicobacter pylori Can Compensate for Lack of Th17 Cells and Can Protect Mice in the Absence of Immunization. PLoS ONE 2013, 8, e69384. [Google Scholar] [CrossRef]
- Kazemi, R.; Akhavian, A.; Amani, J.; Salimian, J.; Motamedi, M.J.; Mousavi, A.; Jafari, M.; Salmanian, A.H. Immunogenic properties of trivalent recombinant protein composed of B-subunits of LT, STX-2, and CT toxins. Microbes Infect. 2016, 18, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.C.; Chang, C.Y.; Tsai, P.S.; Chiou, H.Y.; Jeng, C.R.; Pang, V.F.; Chang, H.W. Efficacy of heat-labile enterotoxin B subunit-adjuvanted parenteral porcine epidemic diarrhea virus trimeric spike subunit vaccine in piglets. Appl. Microbiol. Biotechnol. 2018, 102, 7499–7507. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Recent Advances in Nontoxic Escherichia coli Heat-labile Toxin and Its Derivative Adjuvants. Expert Rev. Vaccines 2016, 15, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Kang, T.J. Expression of B subunit of E. coli heat-labile enterotoxin in the progenies of transgenic tobacco bred by crossing nuclear- and chloroplast-transgenic lines. Protein Expr. Purif. 2019, 155, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Liu, Q.; Xiao, K.P.; Li, P.; Liu, Q.; Zhao, X.X.; Kong, Q.K. Attenuated Salmonella typhimurium delivery of a novel DNA vaccine induces immune responses and provides protection against duck enteritis virus. Vet. Microbiol. 2016, 186, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bal, J.; Luong, N.N.; Park, J.; Song, K.D.; Jang, Y.S.; Kim, D.H. Comparative immunogenicity of preparations of yeast-derived dengue oral vaccine candidate. Microb. Cell Factories 2018, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordbacheh, E.; Nazarian, S.; Sadeghi, D.; Hajizadeh, A. An LTB-entrapped protein in PLGA nanoparticles preserves against enterotoxin of enterotoxigenic Escherichia coli. Iran. J. Basic Med. Sci. 2018, 21, 517–524. [Google Scholar]
- Yagnik, B.; Sharma, D.; Padh, H.; Desai, P. Oral immunization with LacVax® OmpA induces protective immune response against Shigella flexneri 2a ATCC 12022 in a murine model. Vaccine 2019, 37, 3097–3105. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Y.; Tong, P.; Li, C.; Yang, W.; Hu, J.; Ye, L.; Gu, W.; Shi, C.; Shan, B.; et al. Alleviation of enterotoxigenic Escherichia coli challenge by recombinant Lactobacillus plantarum expressing a FaeG-and DC-targeting peptide fusion protein. Benef. Microbes 2017, 8, 379–391. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Khavari-Nejad, R.A. Oral immunization with recombinant Lactococcus lactis NZ9000 expressing human papillomavirus type 16 E7 antigen and evaluation of its immune effects in female C57BL/6 mice. J. Med. Virol. 2019, 91, 296–307. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, R.; Duan, G.; Peng, X.; Wang, C.; Fan, Q.; Chen, S.; Xi, Y. An engineered food-grade Lactococcus lactis strain for production and delivery of heat-labile enterotoxin B subunit to mucosal sites. BMC Biotechnol. 2017, 17, 25. [Google Scholar] [CrossRef]
- Lee, M.H.; Roussel, Y.; Wilks, M.; Tabaqchali, S. Expression of Helicobacter pylori urease subunit B gene in Lactococcus lactis MG1363 and its use as a vaccine delivery system against H. pylori infection in mice. Vaccine 2001, 19, 3927–3935. [Google Scholar] [CrossRef]
- Fu, H.W. Helicobacter pylori neutrophil-activating protein: From molecular pathogenesis to clinical applications. World J. Gastroenterol. 2014, 20, 5294–5301. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, R.; Duan, G.; Wang, C.; Sun, N.; Zhang, L.; Chen, S.; Fan, Q.; Xi, Y. Production and delivery of Helicobacter pylori NapA in Lactococcus lactis and its protective efficacy and immune modulatory activity. Sci. Rep. 2018, 8, 6435. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sinha, S.; Saxena, A. Helicobacter pylori neutrophil-activating protein: A potential Treg modulator suppressing allergic asthma? Front. Microbiol. 2015, 6, 493. [Google Scholar] [CrossRef]
- Zhang, X.J.; Feng, S.Y.; Li, Z.T.; Feng, Y.M. Expression of Helicobacter pylori hspA Gene in Lactococcus lactis NICE System and Experimental Study on Its Immunoreactivity. Gastroenterol. Res. Pract. 2015, 2015, 750932. [Google Scholar] [CrossRef]
- Ma, Y.J.; Duan, G.C.; Zhang, R.G.; Fan, Q.T.; Zhang, W.D. Mutation of iceA in Helicobacter pylori compromised IL-8 induction from human gastric epithelial cells. J. Basic Microbiol. 2010, 50, S83–S88. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, R.; Duan, G.; Shi, J. Food-Grade Expression of Helicobacter pylori UreB Subunit in Lactococcus lactis and its Immunoreactivity. Curr. Microbiol. 2011, 62, 1726. [Google Scholar] [CrossRef]
- Kang, Q.Z.; Duan, G.C.; Fan, Q.T.; Xi, Y.L. Fusion expression of Helicobacter pylori neutrophil-activating protein in E. coli. World J. Gastroenterol. 2005, 11, 454–456. [Google Scholar] [CrossRef]
- Guo, L.; Yang, H.; Tang, F.; Yin, R.; Liu, H.; Gong, X.; Wei, J.; Zhang, Y.; Xu, G.; Liu, K. Oral Immunization with a Multivalent Epitope-Based Vaccine, Based on NAP, Urease, HSP60, and HpaA, Provides Therapeutic Effect on H. pylori Infection in Mongolian gerbils. Front. Microbiol. 2017, 7, 349. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Zhou, H.; Wu, M. Quantitative Proteomic Analysis of Escherichia coli Heat-Labile Toxin B Subunit (LTB) with Enterovirus 71 (EV71) Subunit VP1. Int. J. Mol. Sci. 2016, 17, 1419. [Google Scholar] [CrossRef]
- Ji, J.; Griffiths, K.L.; Milburn, P.J.; Hirst, T.R.; O’Neill, H.C. The B subunit of Escherichia coli heat-labile toxin alters the development and antigen-presenting capacity of dendritic cells. J. Cell. Mol. Med. 2015, 19, 2019–2031. [Google Scholar] [CrossRef]
- Thiam, F.; Charpilienne, A.; Poncet, D.; Kohli, E.; Basset, C. B subunits of cholera toxin and thermolabile enterotoxin of Escherichia coli have similar adjuvant effect as whole molecules on rotavirus 2/6-VLP specific antibody responses and induce a Th17-like response after intrarectal immunization. Microb. Pathog. 2015, 89, 27–34. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yang, F.; Wu, W.; Sun, H.; Xie, Q.; Si, W.; Zou, Q.; Yang, Z. Immunization with Heat Shock Protein A and γ-Glutamyl Transpeptidase Induces Reduction on the Helicobacter pylori Colonization in Mice. PLoS ONE 2015, 10, e0130391. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, G.; Wang, Q.; Wang, Z.; Yang, W.; Gu, W.; Shi, C.; Wang, J.; Huang, H.; Wang, C. Molecular mechanisms underlying protection against H9N2 influenza virus challenge in mice by recombinant Lactobacillus plantarum with surface displayed HA2-LTB. J. Biotechnol. 2017, 259, 6–14. [Google Scholar] [CrossRef]
- Lee, C.H.; Hajishengallis, G.; Connell, T.D. Dendritic cell-mediated mechanisms triggered by LT-IIa-B5, a mucosal adjuvant derived from a type II heat-labile enterotoxin of Escherichia coli. J. Microbiol. Biotechnol. 2017, 27, 709–717. [Google Scholar] [CrossRef]
- Hurley, B.P.; Pirzai, W.; Eaton, A.D.; Harper, M.; Roper, J.; Zimmermann, C.; Ladics, G.S.; Layton, R.J.; Delaney, B. An experimental platform using human intestinal epithelial cell lines to differentiate between hazardous and non-hazardous proteins. Food Chem. Toxicol. 2016, 92, 75–87. [Google Scholar] [CrossRef]
- Williams, S.M.; Chen, Y.T.; Andermann, T.M.; Carter, J.E.; McGee, D.J.; Ottemann, K.M. Helicobacter pylori Chemotaxis Modulates Inflammation and Bacterium-Gastric Epithelium Interactions in Infected Mice. Infect. Immun. 2007, 75, 3747–3757. [Google Scholar] [CrossRef]
- Patry, R.T.; Stahl, M.; Perez-Munoz, M.E.; Nothaft, H.; Wenzel, C.Q.; Sacher, J.C.; Coros, C.; Walter, J.; Vallance, B.A.; Szymanski, C.M. Bacterial AB5 toxins inhibit the growth of gut bacteria by targeting ganglioside-like glycoconjugates. Nat. Commun. 2019, 10, 1390. [Google Scholar] [CrossRef]
- Liu, W.; Tan, Z.; Liu, H.; Zeng, Z.; Luo, S.; Yang, H.; Zheng, L.; Xi, T.; Xing, Y. Nongenetically modified Lactococcus lactis-adjuvanted vaccination enhanced innate immunity against Helicobacter pylori. Helicobacter 2017, 22, e12426. [Google Scholar] [CrossRef]
- Bagheri, N.; Razavi, A.; Pourgheysari, B.; Azadegan-Dehkordi, F.; Rahimian, G.; Pirayesh, A.; Shafigh, M.; Rafieian-Kopaei, M.; Fereidani, R.; Tahmasbi, K.; et al. Up-regulated Th17 cell function is associated with increased peptic ulcer disease in Helicobacter pylori-infection. Infect. Genet. Evol. 2018, 60, 117–125. [Google Scholar] [CrossRef]
- Harbour, S.N.; Mitchell, H.M.; Sutton, P. Host Nonresponsiveness Does not Interfere With Vaccine-Mediated Protection Against Gastric Helicobacter Infection. Helicobacter 2015, 20, 217–222. [Google Scholar] [CrossRef]
- Couch, R.B. Nasal Vaccination, Escherichia coli Enterotoxin, and Bell’s Palsy. N. Engl. J. Med. 2004, 350, 860–861. [Google Scholar] [CrossRef]
- Li, Y.M.; Jing, Z.P. Lessons from the Chinese defective vaccine case. Lancet Infect. Dis. 2019, 19, 245. [Google Scholar] [Green Version]
- Zeng, M.; Mao, X.H.; Li, J.X.; Tong, W.D.; Wang, B.; Zhang, Y.J.; Guo, G.; Zhao, Z.J.; Li, L.; Wu, D.L.; et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Zhang, R.; Wang, C.; Yu, F.; Yu, M.; Chen, S.; Fan, Q.; Xi, Y.; Duan, G. E. coli Enterotoxin LtB Enhances Vaccine-Induced Anti-H. pylori Protection by Promoting Leukocyte Migration into Gastric Mucus via Inflammatory Lesions. Cells 2019, 8, 982. https://doi.org/10.3390/cells8090982
Peng X, Zhang R, Wang C, Yu F, Yu M, Chen S, Fan Q, Xi Y, Duan G. E. coli Enterotoxin LtB Enhances Vaccine-Induced Anti-H. pylori Protection by Promoting Leukocyte Migration into Gastric Mucus via Inflammatory Lesions. Cells. 2019; 8(9):982. https://doi.org/10.3390/cells8090982
Chicago/Turabian StylePeng, Xiaoyan, Rongguang Zhang, Chen Wang, Feiyan Yu, Mingyang Yu, Shuaiyin Chen, Qingtang Fan, Yuanlin Xi, and Guangcai Duan. 2019. "E. coli Enterotoxin LtB Enhances Vaccine-Induced Anti-H. pylori Protection by Promoting Leukocyte Migration into Gastric Mucus via Inflammatory Lesions" Cells 8, no. 9: 982. https://doi.org/10.3390/cells8090982
APA StylePeng, X., Zhang, R., Wang, C., Yu, F., Yu, M., Chen, S., Fan, Q., Xi, Y., & Duan, G. (2019). E. coli Enterotoxin LtB Enhances Vaccine-Induced Anti-H. pylori Protection by Promoting Leukocyte Migration into Gastric Mucus via Inflammatory Lesions. Cells, 8(9), 982. https://doi.org/10.3390/cells8090982