Src1 is a Protein of the Inner Nuclear Membrane Interacting with the Dictyostelium Lamin NE81
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vector Constructions and Expression of Recombinant Src1 for Immunizations
2.2. Microscopy
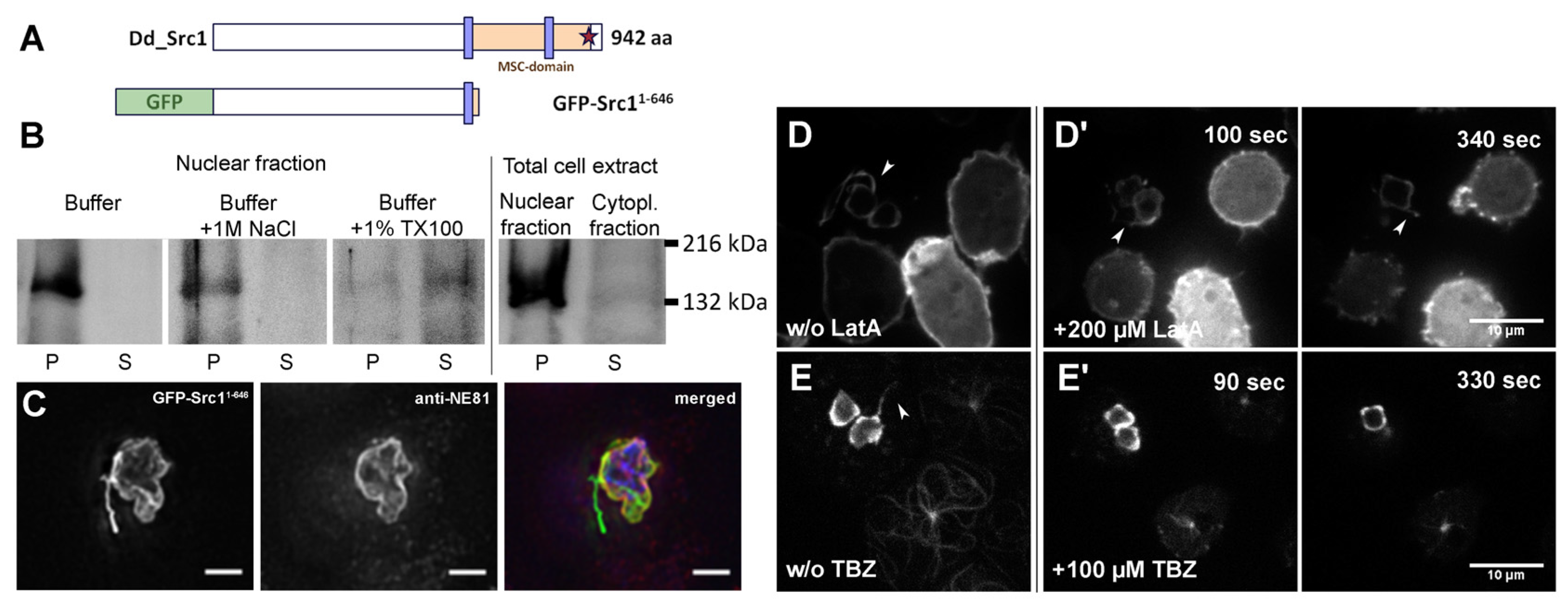
2.3. Membrane Protein Extractions, Electrophoresis, and Western Blotting
2.4. Antibodies and Streptavidin Conjugates
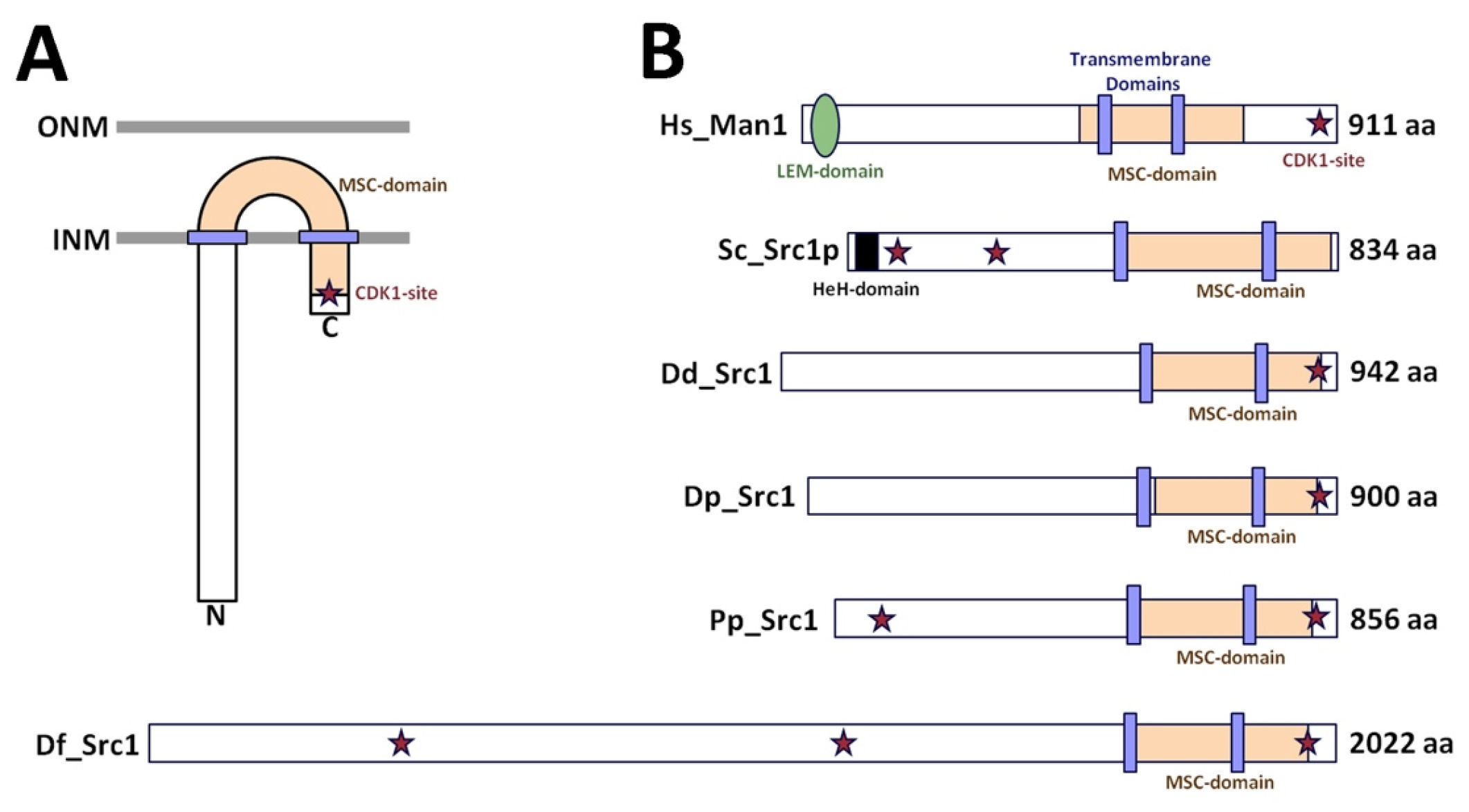
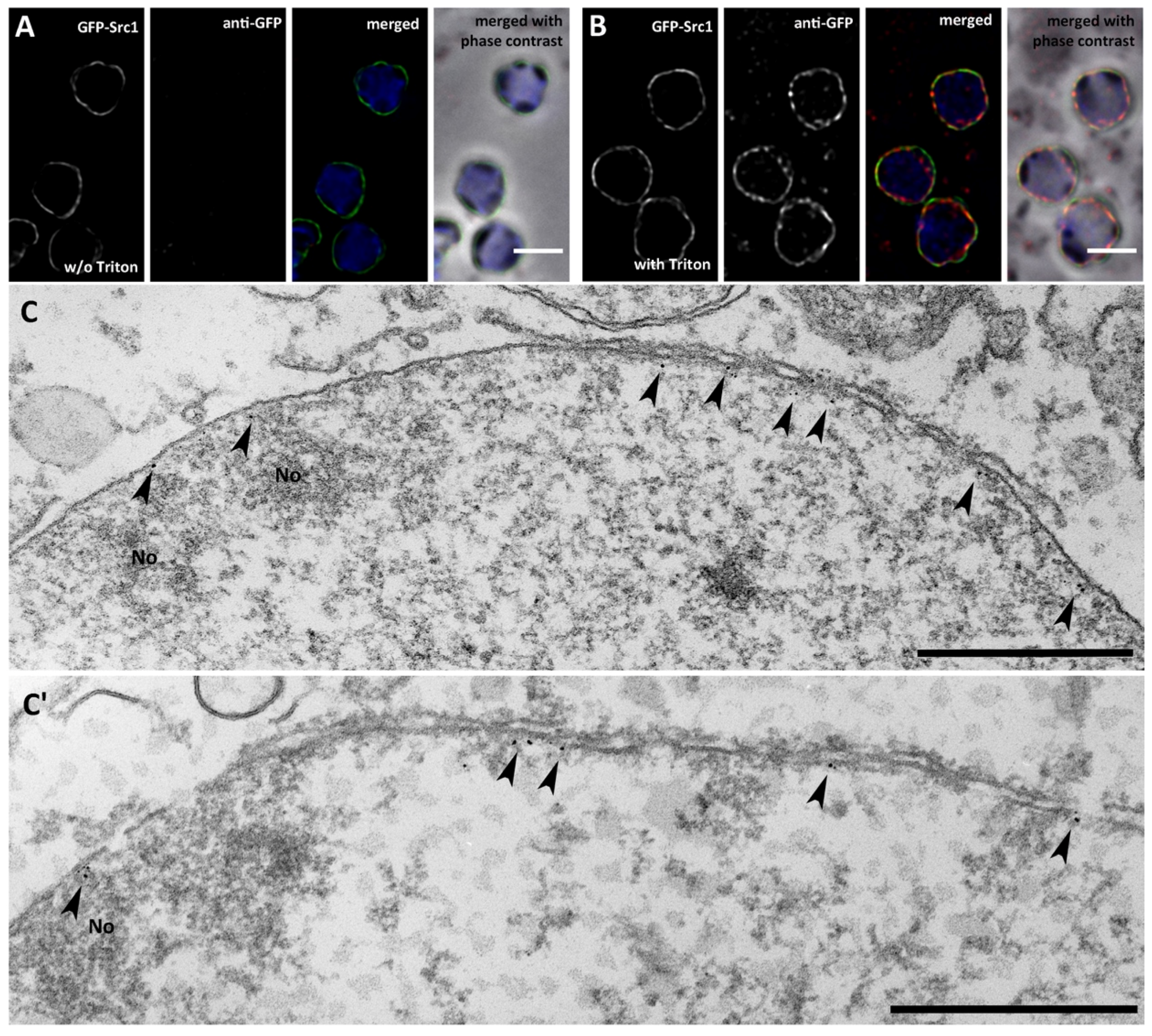
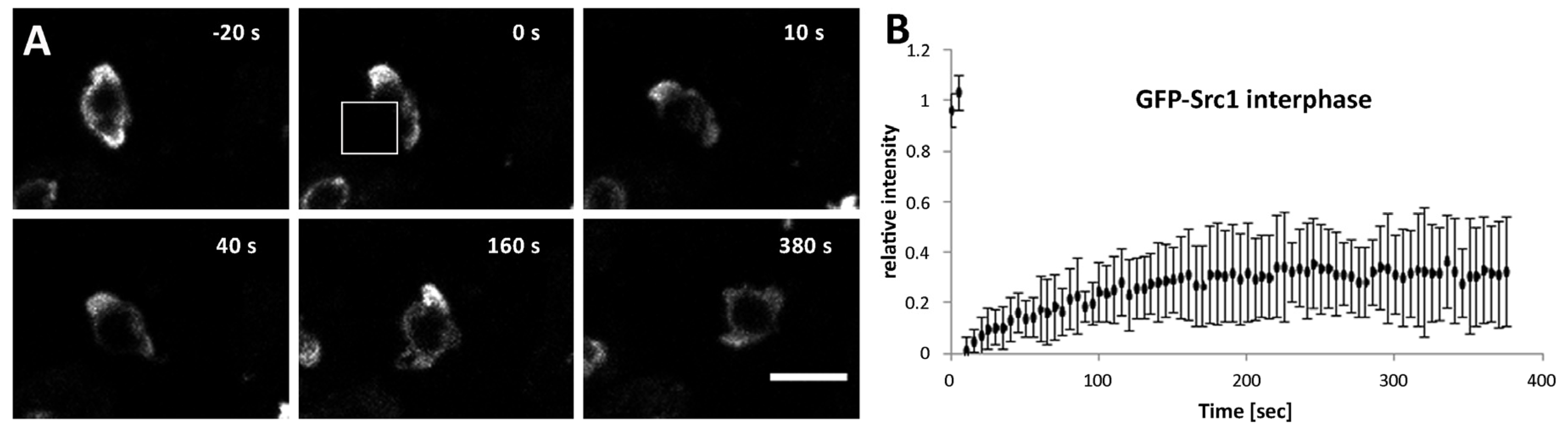
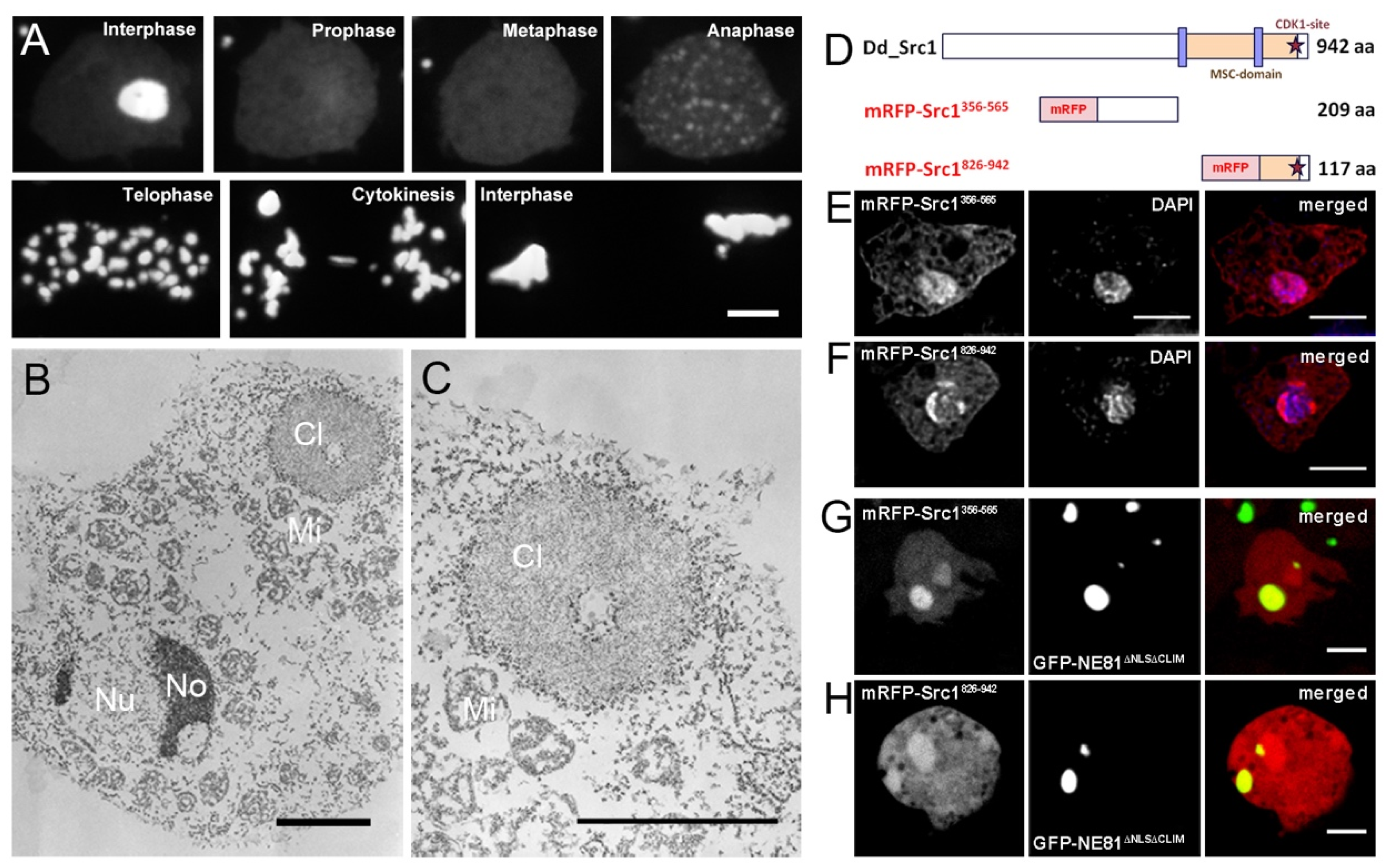
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BioID | Biotin identification |
| HeH | Helix-extension-helix |
| NE | Nuclear envelope |
| INM | Inner nuclear membrane |
| ONM | Outer nuclear membrane |
References
- Wilson, K.L.; Berk, J.M. The nuclear envelope at a glance. J. Cell Sci. 2010, 123, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, Y.; Medalia, O. Lamins: The structure and protein complexes. Curr. Opin. Cell Biol. 2015, 32, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, Y.; Margalit, A.; Goldman, R.D.; Shumaker, D.K.; Wilson, K.L. The nuclear lamina comes of age. Nat. Rev. Mol. Cell Biol. 2005, 6, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.N.; Wilson, K.L. Partners and post-translational modifications of nuclear lamins. Chromosoma 2013, 122, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Crisp, M.; Liu, Q.; Roux, K.; Rattner, J.B.; Shanahan, C.; Burke, B.; Stahl, P.D.; Hodzic, D. Coupling of the nucleus and cytoplasm: Role of the linc complex. J. Cell Biol. 2006, 172, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachner, A.; Foisner, R. Evolvement of lem proteins as chromatin tethers at the nuclear periphery. Biochem. Soc. Trans. 2011, 39, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Blake, D.L.; Callebaut, I.; Skerjanc, I.S.; Holmer, L.; McBurney, M.W.; Paulin-Levasseur, M.; Worman, H.J. Man1, an inner nuclear membrane protein that shares the lem domain with lamina-associated polypeptide 2 and emerin. J. Biol. Chem. 2000, 275, 4840–4847. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, D.K.; Lee, K.K.; Tanhehco, Y.C.; Craigie, R.; Wilson, K.L. Lap2 binds to baf.DNA complexes: Requirement for the lem domain and modulation by variable regions. EMBO J. 2001, 20, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Huang, Y.; Ghirlando, R.; Wilson, K.L.; Craigie, R.; Clore, G.M. Solution structure of the constant region of nuclear envelope protein lap2 reveals two lem-domain structures: One binds baf and the other binds DNA. EMBO J. 2001, 20, 4399–4407. [Google Scholar] [CrossRef] [PubMed]
- Kind, J.; van Steensel, B. Genome-nuclear lamina interactions and gene regulation. Curr. Opin. Cell Biol. 2010, 22, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, S.; Igual, J.C.; Perez-Ortin, J.E. Src1: An intron-containing yeast gene involved in sister chromatid segregation. Yeast 2002, 19, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, K.; Seebacher, J.; Gygi, S.P.; Moazed, D. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature 2008, 456, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.; Gu, Y.; Oliferenko, S. Partitioning and remodeling of the schizosaccharomyces japonicus mitotic nucleus require chromosome tethers. Curr. Biol. 2013, 23, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Shindo, H.; Tase, A.; Kikuchi, Y.; Shimizu, M.; Yamazaki, T. Solution structures and DNA binding properties of the n-terminal sap domains of sumo e3 ligases from saccharomyces cerevisiae and oryza sativa. Proteins 2009, 75, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Caputo, S.; Couprie, J.; Duband-Goulet, I.; Konde, E.; Lin, F.; Braud, S.; Gondry, M.; Gilquin, B.; Worman, H.J.; Zinn-Justin, S. The carboxyl-terminal nucleoplasmic region of man1 exhibits a DNA binding winged helix domain. J. Biol. Chem. 2006, 281, 18208–18215. [Google Scholar] [CrossRef] [PubMed]
- Gräf, R.; Batsios, P.; Meyer, I. Evolution of centrosomes and the nuclear lamina: Amoebozoan assets. Eur. J. Cell Biol. 2015, 94, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Batsios, P.; Peter, T.; Baumann, O.; Stick, R.; Meyer, I.; Gräf, R. A lamin in lower eukaryotes? Nucleus 2012, 3, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Batsios, P.; Baumann, O.; Luckert, E.; Schwarz, H.; Stick, R.; Meyer, I.; Gräf, R. Characterization of ne81, the first lamin-like nucleoskeleton protein in a unicellular organism. Mol. Biol. Cell 2012, 23, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Batsios, P.; Meyer, I.; Gräf, R. Proximity-dependent biotin identification (bioid) in dictyostelium amoebae. Methods Enzymol. 2016, 569, 23–43. [Google Scholar] [PubMed]
- Kollmar, M. Polyphyly of nuclear lamin genes indicates an early eukaryotic origin of the metazoan-type intermediate filament proteins. Sci. Rep. 2015, 5, 10652. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; Anantharaman, V.; Aravind, L.; Koonin, E.V. Comparative genomics, evolution and origins of the nuclear envelope and nuclear pore complex. Cell Cycle 2004, 3, 1612–1637. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.L.; Dawson, S.C. Evolution: Functional evolution of nuclear structure. J. Cell Biol. 2011, 195, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Schulz, I.; Baumann, O.; Samereier, M.; Zoglmeier, C.; Gräf, R. Dictyostelium sun1 is a dynamic membrane protein of both nuclear membranes and required for centrosomal association with clustered centromeres. Eur. J. Cell Biol. 2009, 88, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Haase, I.; Simmeth, E.; Gerisch, G.; Müller-Taubenberger, A. A brilliant monomeric red fluorescent protein to visualize cytoskeleton dynamics in dictyostelium. FEBS Lett. 2004, 577, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Veltman, D.M.; Akar, G.; Bosgraaf, L.; Van Haastert, P.J. A new set of small, extrachromosomal expression vectors for dictyostelium discoideum. Plasmid 2009, 61, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.K.O.; Devreotes, P.N.; Eliott, S.; Jermyn, K.; Kuspa, A.; Fechheimer, M.; Furukawa, R.; Parent, C.A.; Segall, J.; Shaulsky, G.; et al. Cell biological, molecular genetic, and biochemical methods used to examine dictyostelium. In Cell biology: A Laboratory Handbook, 2nd ed.; Celis, J.E., Ed.; Academic Press: San Diego, CA, USA, 1998; Volume 1, pp. 431–465. [Google Scholar]
- Batsios, P.; Baumann, O.; Gräf, R.; Meyer, I. Isolation of dictyostelium nuclei for light and electron microscopy. Methods Mol. Biol. 2013, 983, 283–294. [Google Scholar] [PubMed]
- Fukui, Y.; Yumura, S.; Yumura, T.K. Agar-overlay immunofluorescence: High resolution studies of cytoskeletal components and their changes during chemotaxis. Methods Cell Biol. 1987, 28, 347–356. [Google Scholar] [PubMed]
- Kuhnert, O.; Baumann, O.; Meyer, I.; Gräf, R. Functional characterization of cp148, a novel key component for centrosome integrity in dictyostelium. Cell. Mol. Life Sci. 2012, 69, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Samereier, M.; Meyer, I.; Koonce, M.P.; Gräf, R. Live cell-imaging techniques for analyses of microtubules in dictyostelium. Methods Cell Biol. 2010, 97, 341–357. [Google Scholar] [PubMed]
- Gräf, R.; Euteneuer, U.; Ueda, M.; Schliwa, M. Isolation of nucleation-competent centrosomes from dictyostelium discoideum. Eur. J. Cell Biol. 1998, 76, 167–175. [Google Scholar] [CrossRef]
- Wehland, J.; Willingham, M.C.; Sandoval, I.V. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. Ii. Effects on cell movement, organization of microtubules, and intermediate filaments, and arrangement of golgi elements. J. Cell Biol. 1983, 97, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Faix, J.; Weber, I.; Mintert, U.; Kohler, J.; Lottspeich, F.; Marriott, G. Recruitment of cortexillin into the cleavage furrow is controlled by rac1 and iqgap-related proteins. EMBO J. 2001, 20, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Fey, P.; Pandit, Y.; Dodson, R.; Kibbe, W.A.; Chisholm, R.L. Dictybase 2013: Integrating multiple dictyostelid species. Nucleic Acids Res. 2013, 41, D676–D683. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.R.; Roos, U.P.; Neighbors, B.; McDonald, K.L. Architecture of the microtubule component of mitotic spindles from dictyostelium discoideum. J. Cell Sci. 1985, 75, 93–129. [Google Scholar] [PubMed]
- O'Day, D.H.; Budniak, A. Nucleocytoplasmic protein translocation during mitosis in the social amoebozoan dictyostelium discoideum. Biol. Rev. Camb. Philos. Soc. 2015, 90, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Benichou, J.C.; Quiviger, B.; Ryter, A. Cytochemical study of the nucleolus of the slime mold dictyostelium discoideum. J. Ultrastruct. Res. 1983, 84, 60–66. [Google Scholar] [CrossRef]
- Catalano, A.; O'Day, D.H. Evidence for nucleolar subcompartments in dictyostelium. Biochem. Biophys. Res. Commun. 2015, 456, 901–907. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H.; Catalano, A. The nucleolus of dictyostelium and other lower eukaryotes. In Proteins of the nucleolus; O’Day, D.H., Catalano, A., Eds.; Springer: New York, 2013. [Google Scholar]
- Brachner, A.; Reipert, S.; Foisner, R.; Gotzmann, J. Lem2 is a novel man1-related inner nuclear membrane protein associated with a-type lamins. J. Cell Sci. 2005, 118, 5797–5810. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Birendra, K.C.; Zhu, W.; Motamedchaboki, K.; Doye, V.; Roux, K.J. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc. Natl. Acad. Sci. USA 2014, 111, E2453–E2461. [Google Scholar] [CrossRef] [PubMed]
- Roux, K.J.; Kim, D.I.; Raida, M.; Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012, 196, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.A.; Sengupta, K.; Goldman, R.D. Regulation of nuclear lamin polymerization by importin alpha. J. Biol. Chem. 2008, 283, 8462–8468. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, T.; Diez, S.; Anderson, K.; Heuser, J.; Clarke, M.; Müller-Taubenberger, A.; Köhler, J.; Gerisch, G. Dynamic actin patterns and arp2/3 assembly at the substrate-attached surface of motile cells. Curr. Biol. 2004, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rehberg, M.; Gräf, R. Dictyostelium eb1 is a genuine centrosomal component required for proper spindle formation. Mol. Biol. Cell 2002, 13, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Rehberg, M.; Kleylein-Sohn, J.; Faix, J.; Ho, T.H.; Schulz, I.; Gräf, R. Dictyostelium lis1 is a centrosomal protein required for microtubule/cell cortex interactions, nucleus/centrosome linkage, and actin dynamics. Mol. Biol. Cell 2005, 16, 2759–2771. [Google Scholar] [CrossRef] [PubMed]
- Sameshima, M. The orientation of nucleus, nucleus-associated body and protruding nucleolus in aggregating dictyostelium discoideum. Exp. Cell Res. 1985, 156, 341–350. [Google Scholar] [CrossRef]
- Sameshima, M.; Fujimoto, H.; Imai, Y.; Tsukita, S.; Hashimoto, Y. Relation of nucleolar structure and position to the cytoplasmic microtubule system in dictyostelium. Cell Motil. Cytoskeleton 1991, 18, 293–303. [Google Scholar] [CrossRef]
- Wiki.dictybase.org. Available online: http://wiki.dictybase.org/dictywiki/index.php/DDB_G0293138, (accessed on 17 March 2016).
- Catalano, A.; O'Day, D.H. Nucleolar localization and identification of nuclear/nucleolar localization signals of the calmodulin-binding protein nucleomorphin during growth and mitosis in dictyostelium. Histochem. Cell Biol. 2011, 135, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Grund, S.E.; Fischer, T.; Cabal, G.G.; Antunez, O.; Perez-Ortin, J.E.; Hurt, E. The inner nuclear membrane protein src1 associates with subtelomeric genes and alters their regulated gene expression. J. Cell Biol. 2008, 182, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Steglich, B.; Filion, G.J.; van Steensel, B.; Ekwall, K. The inner nuclear membrane proteins man1 and ima1 link to two different types of chromatin at the nuclear periphery in s. Pombe. Nucleus 2012, 3, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, Y.; Saito, A.; Sazer, S. Fission yeast lem2 and man1 perform fundamental functions of the animal cell nuclear lamina. Nucleus 2012, 3, 60–76. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batsios, P.; Ren, X.; Baumann, O.; Larochelle, D.A.; Gräf, R. Src1 is a Protein of the Inner Nuclear Membrane Interacting with the Dictyostelium Lamin NE81. Cells 2016, 5, 13. https://doi.org/10.3390/cells5010013
Batsios P, Ren X, Baumann O, Larochelle DA, Gräf R. Src1 is a Protein of the Inner Nuclear Membrane Interacting with the Dictyostelium Lamin NE81. Cells. 2016; 5(1):13. https://doi.org/10.3390/cells5010013
Chicago/Turabian StyleBatsios, Petros, Xiang Ren, Otto Baumann, Denis A. Larochelle, and Ralph Gräf. 2016. "Src1 is a Protein of the Inner Nuclear Membrane Interacting with the Dictyostelium Lamin NE81" Cells 5, no. 1: 13. https://doi.org/10.3390/cells5010013





