Post-Translational Modifications of TRP Channels
Abstract
:1. Transient Receptor Potential Channels
2. The Various Types of Post-Translational Modifications
| Channel | Function * | Modification | Modified Site | Location of Modified Site | Modification Regulates | Reference |
|---|---|---|---|---|---|---|
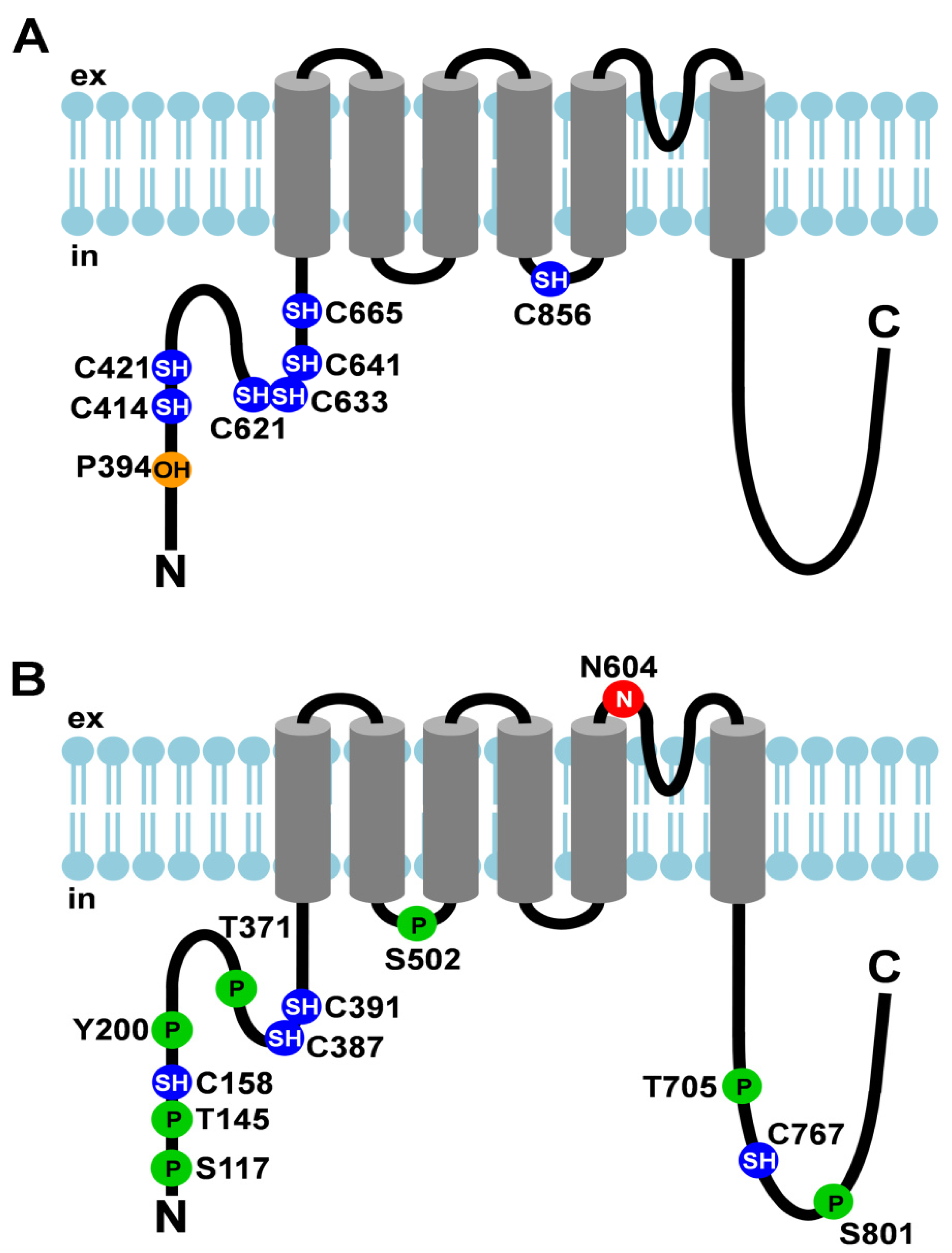
| TRPA1 (see Figure 1A) | thermo-sensation (noxious cold), chemo-sensation, nociception, O2 sensing | covalent modification by electrophiles | C621 C641 C665 | N-terminus | channel gating | [8] |
| covalent modification by electrophiles | C414 C421 C621 | [9] | ||||
| covalent modification by inflammatory mediators | C421 C621 | [10] | ||||
| hydroxylation | P394 | [11] | ||||
| oxidation | C633 | |||||
| C856 | 2nd intracellular loop | |||||
| TRPC3 | BDNF-signaling in the brain | N-linked glycosylation | N418 | 1st extracellular loop | channel activity (by surface expression?) | [12] |
| phosphorylation by PKC | T646 S712 | 2nd intracellular loop | channel gating | [13,14] | ||
| phosphorylation by PKG | T11 S263 | N-terminus | [15] | |||
| phosphorylation by Src kinase | [16] | |||||
| TRPC5 | brain development | S-nitrosylation | C553 C558 | adjacent to pore-forming loop | channel gating | [17] |
| phosphorylation by PKC | T970 | [18] | ||||
| TRPC6 | signaling in smooth muscle | N-linked glycosylation | N473 N561 | 1st and 2nd extracellular loop | channel activity (by surface expression?) | [12] |
| phosphorylation by PKC | channel inhibition | [19] | ||||
| phosphorylation by Src family kinase Fyn | T970 | C-terminus | carbachol-mediated desensitization | [20] | ||
| phosphorylation by CaMKII | channel activation | [21] | ||||
| TRPM4b | regulation of Ca2+ entry into the cell | N-linked glycosylation | N988 | adjacent to pore-forming loop | surface expression | [22] |
| TRPM7 | Mg2+ homeostasis and reabsorption in kidney and intestine, cell migration | autophorylation | several sites | C-terminus | substrate recognition | [23] |
| TRPM8 | thermo-sensation (cold), sperm motility, acrosome reaction | N-linked glycosylation | N934 | adjacent to pore-forming loop | response to cold and menthol | [24,25,26] |
| Polyester modification | several sites | N-terminus and S3-S4 linker | channel function | [27] | ||
| TRPV1 (see Figure 1B) | thermo-sensation (heat), nociception | N-linked glycosylation | N604 | adjacent to pore-forming loop | ligand binding or gating properties | [28,29] |
| cysteine modification | C158 | N-terminus | activation by cysteine-modifying compounds | [30] | ||
| cysteine modification | C158 C387 C391 | sensitization by oxidative stress | [31] | |||
| C767 | C-terminus | |||||
| phosphorylation by PKC | S502 | 1st intracellular loop | potentiation | [32] | ||
| S801 | C-terminus | |||||
| phosphorylation by PKA | S117 | N-terminus | prevention of desensitization | [33] | ||
| T145 T371 | sensitization | [34] | ||||
| S502 | 1st intracellular loop | |||||
| phosphorylation by c-Src | Y200 | N-terminus | surface expression | [35] | ||
| phosphorylation by CaMKII | S502 | 1st intracellular loop | channel activity | [36] | ||
| T705 | C-terminus | |||||
| TRPV2 | thermo-sensation (noxious heat), nociception | N-linked glycosylation | N570 (alignment) | adjacent to pore-forming loop | [37,38] | |
| TRPV4 | tonicity sensing | N-linked glycosylation | N651 | adjacent to pore-forming loop | channel activity (through surface expression?) | [39] |
| phosphorylation by Src | Y253 | N-terminus | channel activity | [39,40] | ||
| TRPV5 | Ca2+ reabsorption in kidney | N-linked glycosylation | N358 | 1st extracellular loop | surface expression | [41] |
| TRPV6 | Ca2+ reabsorption in intestine | N-linked glycosylation | N357 | 1st extracellular loop | surface expression | |
| dTRP (see Figure 2A) | generation of the photoreceptor potential | phosphorylation | S15 | N-terminus | [42,43] | |
| S717 S721 | C-terminus | |||||
| dTRPL (see Figure 2B) | generation of the photoreceptor potential | phosphorylation | S20 | N-terminus | [44] | |
| S730 S927 | C-terminus | channel stability |
3. N-Linked Glycosylation of TRP Channels
4. Covalent Modification of TRP Cysteine Residues
4.1. Covalent Modification of TRPC5
4.2. Covalent Modification of TRPA1
4.3. Covalent Modification of TRPV1
5. Polyester Modification of TRPM8
6. Phosphorylation of Mammalian TRP Channels
6.1. Phosphorylation of TRPC Channels
6.2. Phosphorylation of TRPV1
6.3. Phosphorylation of TRPV4
6.4. Phosphorylation of TRPM7
7. Phosphorylation of Drosophila TRP and TRPL
7.1. Drosophila Phototransduction
7.2. Phosphorylation of TRP
7.3. Phosphorylation of TRPL

8. Concluding Remarks

Acknowledgments
Conflicts of Interest
References
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef]
- Minke, B.; Wu, C.; Pak, W.L. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 1975, 258, 84–87. [Google Scholar] [CrossRef]
- Montell, C.; Rubin, G.M. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Hardie, R.C.; Minke, B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 1992, 8, 643–651. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef]
- Hinman, A.; Chuang, H.-H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamamoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008, 2, 287–298. [Google Scholar] [CrossRef]
- Takahashi, N.; Kuwaki, T.; Kiyonaka, S.; Numata, T.; Kozai, D.; Mizuno, Y.; Yamamoto, S.; Naito, S.; Knevels, E.; Carmeliet, P.; et al. TRPA1 underlies a sensing mechanism for O2. Nat. Chem. Biol. 2011, 7, 701–711. [Google Scholar] [CrossRef]
- Dietrich, A.; Mederos y Schnitzler, M.; Emmel, J.; Kalwa, H.; Hofmann, T.; Gudermann, T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J. Biol. Chem. 2003, 278, 47842–47852. [Google Scholar]
- Trebak, M.; Hempel, N.; Wedel, B.J.; Smyth, J.T.; Bird, G.S.J.; Putney, J.W., Jr. Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol. Pharmacol. 2005, 67, 558–563. [Google Scholar]
- Becker, E.B.; Oliver, P.L.; Glitsch, M.D.; Banks, G.T.; Achilli, F.; Hardy, A.; Nolan, P.M.; Fisher, E.M.; Davies, K.E. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc. Natl. Acad. Sci. USA 2009, 106, 6706–6711. [Google Scholar]
- Kwan, H.Y.; Huang, Y.; Yao, X. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc. Natl. Acad. Sci. USA 2004, 101, 2625–2630. [Google Scholar] [CrossRef]
- Vazquez, G.; Wedel, B.J.; Kawasaki, B.T.; Bird, G.S.; Putney, J.W., Jr. Obligatory role of Src kinase in the signaling mechanism for TRPC3 cation channels. J. Biol. Chem. 2004, 279, 40521–40528. [Google Scholar]
- Yoshida, T.; Inoue, R.; Morii, T.; Takahashi, N.; Yamamoto, S.; Hara, Y.; Tominaga, M.; Shimizu, S.; Sato, Y.; Mori, Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006, 2, 596–607. [Google Scholar] [CrossRef]
- Zhu, M.H.; Chae, M.; Kim, H.J.; Lee, Y.M.; Kim, M.J.; Jin, N.G.; Yang, D.K.; So, I.; Kim, K.W. Desensitization of canonical transient receptor potential channel 5 by protein kinase C. Am. J. Physiol., Cell Physiol. 2005, 289, C591–C600. [Google Scholar] [CrossRef]
- Zhang, L.; Saffen, D. Muscarinic acetylcholine receptor regulation of TRP6 Ca2+ channel isoforms. Molecular structures and functional characterization. J. Biol. Chem. 2001, 276, 13331–13339. [Google Scholar] [CrossRef]
- Hisatsune, C.; Kuroda, Y.; Nakamura, K.; Inoue, T.; Nakamura, T.; Michikawa, T.; Mizutani, A.; Mikoshiba, K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J. Biol. Chem. 2004, 279, 18887–18894. [Google Scholar] [CrossRef]
- Shi, J.; Mori, E.; Mori, Y.; Mori, M.; Li, J.; Ito, Y.; Inoue, R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J. Physiol. (Lond.) 2004, 561, 415–432. [Google Scholar] [CrossRef]
- Woo, S.K.; Kwon, M.S.; Ivanov, A.; Geng, Z.; Gerzanich, V.; Simard, J.M. Complex N-glycosylation stabilizes surface expression of transient receptor potential melastatin 4b. J. Biol. Chem. 2013, 288, 36409–36417. [Google Scholar] [CrossRef]
- Clark, K.; Middelbeek, J.; Morrice, N.A.; Figdor, C.G.; Lasonder, E.; van Leeuwen, F.N. Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS One 2008, 3, e1876. [Google Scholar]
- Pertusa, M.; Madrid, R.; Morenilla-Palao, C.; Belmonte, C.; Viana, F. N-glycosylation of TRPM8 ion channels modulates temperature sensitivity of cold thermoreceptor neurons. J. Biol. Chem. 2012, 287, 18218–18229. [Google Scholar] [CrossRef]
- Dragoni, I.; Guida, E.; McIntyre, P. The cold and menthol receptor TRPM8 contains a functionally important double cysteine motif. J. Biol. Chem. 2006, 281, 37353–37360. [Google Scholar] [CrossRef]
- Erler, I.; Al-Ansary, D.M.M.; Wissenbach, U.; Wagner, T.F.J.; Flockerzi, V.; Niemeyer, B.A. Trafficking and assembly of the cold-sensitive TRPM8 channel. J. Biol. Chem. 2006, 281, 38396–38404. [Google Scholar]
- Cao, C.; Yudin, Y.; Bikard, Y.; Chen, W.; Liu, T.; Li, H.; Jendrossek, D.; Cohen, A.; Pavlov, E.; Rohacs, T.; et al. Polyester modification of the mammalian TRPM8 channel protein: Implications for structure and function. Cell Rep. 2013, 4, 302–315. [Google Scholar] [CrossRef]
- Jahnel, R.; Dreger, M.; Gillen, C.; Bender, O.; Kurreck, J.; Hucho, F. Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur. J. Biochem. 2001, 268, 5489–5496. [Google Scholar] [CrossRef]
- Wirkner, K.; Hognestad, H.; Jahnel, R.; Hucho, F.; Illes, P. Characterization of rat transient receptor potential vanilloid 1 receptors lacking the N-glycosylation site N604. Neuroreport 2005, 16, 997–1001. [Google Scholar] [CrossRef]
- Salazar, H.; Llorente, I.; Jara-Oseguera, A.; García-Villegas, R.; Munari, M.; Gordon, S.E.; Islas, L.D.; Rosenbaum, T. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat. Neurosci. 2008, 11, 255–261. [Google Scholar]
- Chuang, H.-H.; Lin, S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc. Natl. Acad. Sci. USA 2009, 106, 20097–20102. [Google Scholar] [CrossRef]
- Numazaki, M.; Tominaga, T.; Toyooka, H.; Tominaga, M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase C epsilon and identification of two target serine residues. J. Biol. Chem. 2002, 277, 13375–13378. [Google Scholar]
- Bhave, G.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau, R.W., 4th. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 2002, 35, 721–731. [Google Scholar] [CrossRef]
- Rathee, P.K.; Distler, C.; Obreja, O.; Neuhuber, W.; Wang, G.K.; Wang, S.Y.; Nau, C.; Kress, M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J. Neurosci. 2002, 22, 4740–4745. [Google Scholar]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef]
- Jung, J.; Shin, J.S.; Lee, S.Y.; Hwang, S.W.; Koo, J.; Cho, H.; Oh, U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 2004, 279, 7048–7054. [Google Scholar]
- Jahnel, R.; Bender, O.; Munter, L.M.; Dreger, M.; Gillen, C.; Hucho, F. Dual expression of mouse and rat VRL-1 in the dorsal root ganglion derived cell line F-11 and biochemical analysis of VRL-1 after heterologous expression. Eur. J. Biochem. 2003, 270, 4264–4271. [Google Scholar] [CrossRef]
- Cohen, D.M. Regulation of TRP channels by N-linked glycosylation. Semin. Cell Dev. Biol. 2006, 17, 630–637. [Google Scholar] [CrossRef]
- Xu, H.; Fu, Y.; Tian, W.; Cohen, D.M. Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am. J. Physiol. Renal. Physiol. 2006, 290, 1103–1109. [Google Scholar]
- Xu, H.; Zhao, H.; Tian, W.; Yoshida, K.; Roullet, J.-B.; Cohen, D.M. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J. Biol. Chem. 2003, 278, 11520–11527. [Google Scholar]
- Chang, Q.; Hoefs, S.; van der Kemp, A.W.; Topala, C.N.; Bindels, R.J.; Hoenderop, J.G. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 2005, 310, 490–493. [Google Scholar] [CrossRef]
- Voolstra, O.; Beck, K.; Oberegelsbacher, C.; Pfannstiel, J.; Huber, A. Light-dependent phosphorylation of the Drosophila transient receptor potential (TRP) ion channel. J. Biol. Chem. 2010, 285, 14275–14284. [Google Scholar]
- Voolstra, O.; Bartels, J.-P.; Oberegelsbacher, C.; Pfannstiel, J.; Huber, A. Phosphorylation of the Drosophila transient receptor potential ion channel is regulated by the phototransduction cascade and involves several protein kinases and phosphatases. PLoS One 2013, 8, e73787. [Google Scholar]
- Cerny, A.C.; Oberacker, T.; Pfannstiel, J.; Weigold, S.; Will, C.; Huber, A. Mutation of light-dependent phosphorylation sites of the Drosophila transient receptor potential-like (TRPL) ion channel affects its subcellular localization and stability. J. Biol. Chem. 2013, 288, 15600–15613. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef]
- Helenius, A.; Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004, 73, 1019–1049. [Google Scholar] [CrossRef]
- Hoenderop, J.G.J.; van Leeuwen, J.P.T.M.; van der Eerden, B.C.J.; Kersten, F.F.J.; van der Kemp, A.W.C.M.; Mérillat, A.-M.; Waarsing, J.H.; Rossier, B.C.; Vallon, V.; Hummler, E.; et al. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest 2003, 112, 1906–1914. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Imura, A.; Iwano, A.; Tohyama, O.; Tsuji, Y.; Nozaki, K.; Hashimoto, N.; Fujimori, T.; Nabeshima, Y.-I. Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004, 565, 143–147. [Google Scholar] [CrossRef]
- Tohyama, O.; Imura, A.; Iwano, A.; Freund, J.-N.; Henrissat, B.; Fujimori, T.; Nabeshima, Y.-I. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J. Biol. Chem. 2004, 279, 9777–9784. [Google Scholar]
- Boros, S.; Xi, Q.; Dimke, H.; van der Kemp, A.W.; Tudpor, K.; Verkaart, S.; Lee, K.P.; Bindels, R.J.; Hoenderop, J.G. Tissue transglutaminase inhibits the TRPV5-dependent calcium transport in an N-glycosylation-dependent manner. Cell. Mol. Life Sci. 2012, 69, 981–992. [Google Scholar] [CrossRef]
- Vannier, B.; Zhu, X.; Brown, D.; Birnbaumer, L. The membrane topology of human transient receptor potential 3 as inferred from glycosylation-scanning mutagenesis and epitope immunocytochemistry. J. Biol. Chem. 1998, 273, 8675–8679. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef]
- Liedtke, W.; Choe, Y.; Marti-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP Channel that Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Chuang, H.-H.; Neuhausser, W.M.; Julius, D. The Super-Cooling Agent Icilin Reveals a Mechanism of Coincidence Detection by a Temperature-Sensitive TRP Channel. Neuron 2004, 43, 859–869. [Google Scholar] [CrossRef]
- Bödding, M.; Wissenbach, U.; Flockerzi, V. Characterisation of TRPM8 as a pharmacophore receptor. Cell Calcium. 2007, 42, 618–628. [Google Scholar] [CrossRef]
- Franco, M.I.; Turin, L.; Mershin, A.; Skoulakis, E.M.C. Molecular vibration-sensing component in Drosophila melanogaster olfaction. Proc. Natl. Acad. Sci. USA 2011, 108, 3797–3802. [Google Scholar] [CrossRef]
- Gane, S.; Georganakis, D.; Maniati, K.; Vamvakias, M.; Ragoussis, N.; Skoulakis, E.M.C.; Turin, L. Molecular vibration-sensing component in human olfaction. PLoS One 2013, 8, e55780. [Google Scholar]
- Brookes, J.C.; Horsfield, A.P.; Stoneham, A.M. The swipe card model of odorant recognition. Sensors (Basel) 2012, 12, 15709–15749. [Google Scholar] [CrossRef]
- Yao, X.; Garland, C.J. Recent developments in vascular endothelial cell transient receptor potential channels. Circ. Res. 2005, 97, 853–863. [Google Scholar] [CrossRef]
- Chang, A.S.; Chang, S.M.; Garcia, R.L.; Schilling, W.P. Concomitant and hormonally regulated expression of trp genes in bovine aortic endothelial cells. FEBS Lett. 1997, 415, 335–340. [Google Scholar] [CrossRef]
- Dhaka, A.; Viswanath, V.; Patapoutian, A. Trp ion channels and temperature sensation. Annu. Rev. Neurosci. 2006, 29, 135–161. [Google Scholar] [CrossRef]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE. 2005, 2005, re3. [Google Scholar]
- Clapham, D.E. TRP channels as cellular sensors. Nature. 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Jordt, S.-E.; Bautista, D.M.; Chuang, H.-H.; McKemy, D.D.; Zygmunt, P.M.; Hogestatt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.-E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Hogestatt, E.D.; Julius, D.; Jordt, S.-E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient Receptor Potential Cation Channels in Disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef]
- Wang, L.; Cvetkov, T.L.; Chance, M.R.; Moiseenkova-Bell, V.Y. Identification of in vivo disulfide conformation of TRPA1 ion channel. J. Biol. Chem. 2012, 287, 6169–6176. [Google Scholar] [CrossRef]
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Reusch, R.N. Poly-beta-hydroxybutyrate/calcium polyphosphate complexes in eukaryotic membranes. Proc. Soc. Exp. Biol. Med. 1989, 191, 377–381. [Google Scholar] [CrossRef]
- Reusch, R.N. Streptomyces lividans potassium channel contains poly-(R)-3-hydroxybutyrate and inorganic polyphosphate. Biochemistry 1999, 38, 15666–15672. [Google Scholar] [CrossRef]
- Seebach, D.; Brunner, A.; Bürger, H.M.; Schneider, J.; Reusch, R.N. Isolation and 1H-NMR spectroscopic identification of poly(3-hydroxybutanoate) from prokaryotic and eukaryotic organisms. Determination of the absolute configuration (R) of the monomeric unit 3-hydroxybutanoic acid from Escherichia coli and spinach. Eur. J. Biochem. 1994, 224, 317–328. [Google Scholar] [CrossRef]
- Okada, T.; Inoue, R.; Yamazaki, K.; Maeda, A.; Kurosaki, T.; Yamakuni, T.; Tanaka, I.; Shimizu, S.; Ikenaka, K.; Imoto, K.; et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 1999, 274, 27359–27370. [Google Scholar] [CrossRef]
- Trebak, M.; St J Bird, G.; McKay, R.R.; Birnbaumer, L.; Putney, J.W. Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J. Biol. Chem. 2003, 278, 16244–16252. [Google Scholar]
- Venkatachalam, K.; Zheng, F.; Gill, D.L. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J. Biol. Chem. 2003, 278, 29031–29040. [Google Scholar] [CrossRef]
- Cesare, P.; McNaughton, P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc. Natl. Acad. Sci. USA 1996, 93, 15435–15439. [Google Scholar] [CrossRef]
- Cesare, P.; Dekker, L.V.; Sardini, A.; Parker, P.J.; McNaughton, P.A. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 1999, 23, 617–624. [Google Scholar] [CrossRef]
- Lopshire, J.C.; Nicol, G.D. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J. Neurosci. 1998, 18, 6081–6092. [Google Scholar]
- Docherty, R.J.; Yeats, J.C.; Bevan, S.; Boddeke, H.W. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996, 431, 828–837. [Google Scholar] [CrossRef]
- Jin, X.; Morsy, N.; Winston, J.; Pasricha, P.J.; Garrett, K.; Akbarali, H.I. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am. J. Physiol. Cell Physiol. 2004, 287, 558–563. [Google Scholar] [CrossRef]
- Tilly, B.C.; van den Berghe, N; Tertoolen, L.G.; Edixhoven, M.J.; de Jonge, H.R. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J. Biol. Chem. 1993, 268, 19919–19922. [Google Scholar]
- Sadoshima, J.; Qiu, Z.; Morgan, J.P.; Izumo, S. Tyrosine kinase activation is an immediate and essential step in hypotonic cell swelling-induced ERK activation and c-fos gene expression in cardiac myocytes. EMBO J. 1996, 15, 5535–5546. [Google Scholar]
- Zhang, Z.; Cohen, D.M. Hypotonicity increases transcription, expression, and action of Egr-1 in murine renal medullary mIMCD3 cells. Am. J. Physiol. 1997, 273, F837–F842. [Google Scholar]
- Zhang, Z.; Yang, X.Y.; Cohen, D.M. Hypotonicity activates transcription through ERK-dependent and -independent pathways in renal cells. Am. J. Physiol. 1998, 275, 1104–1112. [Google Scholar]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef]
- Langeslag, M.; Clark, K.; Moolenaar, W.H.; van Leeuwen, F.N.; Jalink, K. Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J. Biol. Chem. 2007, 282, 232–239. [Google Scholar]
- Clark, K.; Langeslag, M.; van Leeuwen, B.; Ran, L.; Ryazanov, A.G.; Figdor, C.G.; Moolenaar, W.H.; Jalink, K.; van Leeuwen, F.N. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006, 25, 290–301. [Google Scholar] [CrossRef]
- De la Roche, M.A.; Smith, J.L.; Betapudi, V.; Egelhoff, T.T.; Côté, G.P. Signaling pathways regulating Dictyostelium myosin II. J. Muscle Res. Cell. Motil. 2002, 23, 703–718. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell 2002, 110, 139–142. [Google Scholar] [CrossRef]
- Burridge, K.; Wennerberg, K. Rho and Rac take center stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Tsunoda, S.; Sierralta, J.; Sun, Y.; Bodner, R.; Suzuki, E.; Becker, A.; Socolich, M.; Zuker, C.S. A multivalent PDZ-domain protein assembles signalling complexes in a G- protein-coupled cascade. Nature 1997, 388, 243–249. [Google Scholar] [CrossRef]
- Huber, A. Scaffolding proteins organize multimolecular protein complexes for sensory signal transduction. Eur. J. Neurosci. 2001, 14, 769–776. [Google Scholar] [CrossRef]
- Adamski, F.M.; Zhu, M.Y.; Bahiraei, F.; Shieh, B.H. Interaction of eye protein kinase C and INAD in Drosophila. Localization of binding domains and electrophysiological characterization of a loss of association in transgenic flies. J. Biol. Chem. 1998, 273, 17713–17719. [Google Scholar]
- Chevesich, J.; Kreuz, A.J.; Montell, C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron 1997, 18, 95–105. [Google Scholar] [CrossRef]
- Huber, A.; Sander, P.; Gobert, A.; Bahner, M.; Hermann, R.; Paulsen, R. The transient receptor potential protein (Trp), a putative store- operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 1996, 15, 7036–7045. [Google Scholar]
- Kimple, M.E.; Siderovski, D.P.; Sondek, J. Functional relevance of the disulfide-linked complex of the N-terminal PDZ domain of InaD with NorpA. EMBO J. 2001, 20, 4414–4422. [Google Scholar] [CrossRef]
- Vogt, K.; Kirschfeld, K. Chemical identity of the chromophores of fly visual pigment. Naturwissenschaften 1984, 71, 211–213. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.-S.; Hughes, S.A.; Postma, M.; Schwiening, C.J.; Hardie, R.C. Activation of TRP Channels by Protons and Phosphoinositide Depletion in Drosophila Photoreceptors. Curr. Biol. 2010, 20, 189–197. [Google Scholar] [CrossRef]
- Hardie, R.C.; Franze, K. Photomechanical responses in Drosophila photoreceptors. Science 2012, 338, 260–263. [Google Scholar] [CrossRef]
- Huber, A.; Sander, P.; Paulsen, R. Phosphorylation of the InaD gene product, a photoreceptor membrane protein required for recovery of visual excitation. J. Biol. Chem. 1996, 271, 11710–11717. [Google Scholar] [CrossRef]
- Huber, A.; Sander, P.; Bahner, M.; Paulsen, R. The TRP Ca2+ channel assembled in a signaling complex by the PDZ domain protein INAD is phosphorylated through the interaction with protein kinase C (ePKC). FEBS Lett. 1998, 425, 317–322. [Google Scholar] [CrossRef]
- Liu, M.; Parker, L.L.; Wadzinski, B.E.; Shieh, B.H. Reversible phosphorylation of the signal transduction complex in Drosophila photoreceptors. J. Biol. Chem. 2000, 275, 12194–12199. [Google Scholar] [CrossRef]
- Popescu, D.C.; Ham, A.J.; Shieh, B.H. Scaffolding protein INAD regulates deactivation of vision by promoting phosphorylation of transient receptor potential by eye protein kinase C in Drosophila. J. Neurosci. 2006, 26, 8570–8577. [Google Scholar] [CrossRef]
- Biggs, W.H., III; Zavitz, K.H.; Dickson, B.; van der, S.A.; Brunner, D.; Hafen, E.; Zipursky, S.L. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 1994, 13, 1628–1635. [Google Scholar]
- Brunner, D.; Oellers, N.; Szabad, J.; Biggs, W.H., III; Zipursky, S.L.; Hafen, E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 1994, 76, 875–888. [Google Scholar]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005, 1, 15–25. [Google Scholar] [CrossRef]
- Cao, J.; Li, Y.; Xia, W.; Reddig, K.; Hu, W.; Xie, W.; Li, H.S.; Han, J. A Drosophila metallophosphoesterase mediates deglycosylation of rhodopsin. EMBO J. 2011, 30, 3701–3713. [Google Scholar] [CrossRef]
- Bähner, M.; Frechter, S.; Da Silva, N.; Minke, B.; Paulsen, R.; Huber, A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron 2002, 34, 83–93. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Voolstra, O.; Huber, A. Post-Translational Modifications of TRP Channels. Cells 2014, 3, 258-287. https://doi.org/10.3390/cells3020258
Voolstra O, Huber A. Post-Translational Modifications of TRP Channels. Cells. 2014; 3(2):258-287. https://doi.org/10.3390/cells3020258
Chicago/Turabian StyleVoolstra, Olaf, and Armin Huber. 2014. "Post-Translational Modifications of TRP Channels" Cells 3, no. 2: 258-287. https://doi.org/10.3390/cells3020258
APA StyleVoolstra, O., & Huber, A. (2014). Post-Translational Modifications of TRP Channels. Cells, 3(2), 258-287. https://doi.org/10.3390/cells3020258





