Response of Microbial Activities in Soil to Various Organic and Mineral Amendments as an Indicator of Soil Quality
Abstract
:1. Introduction
2. Materials and Methods
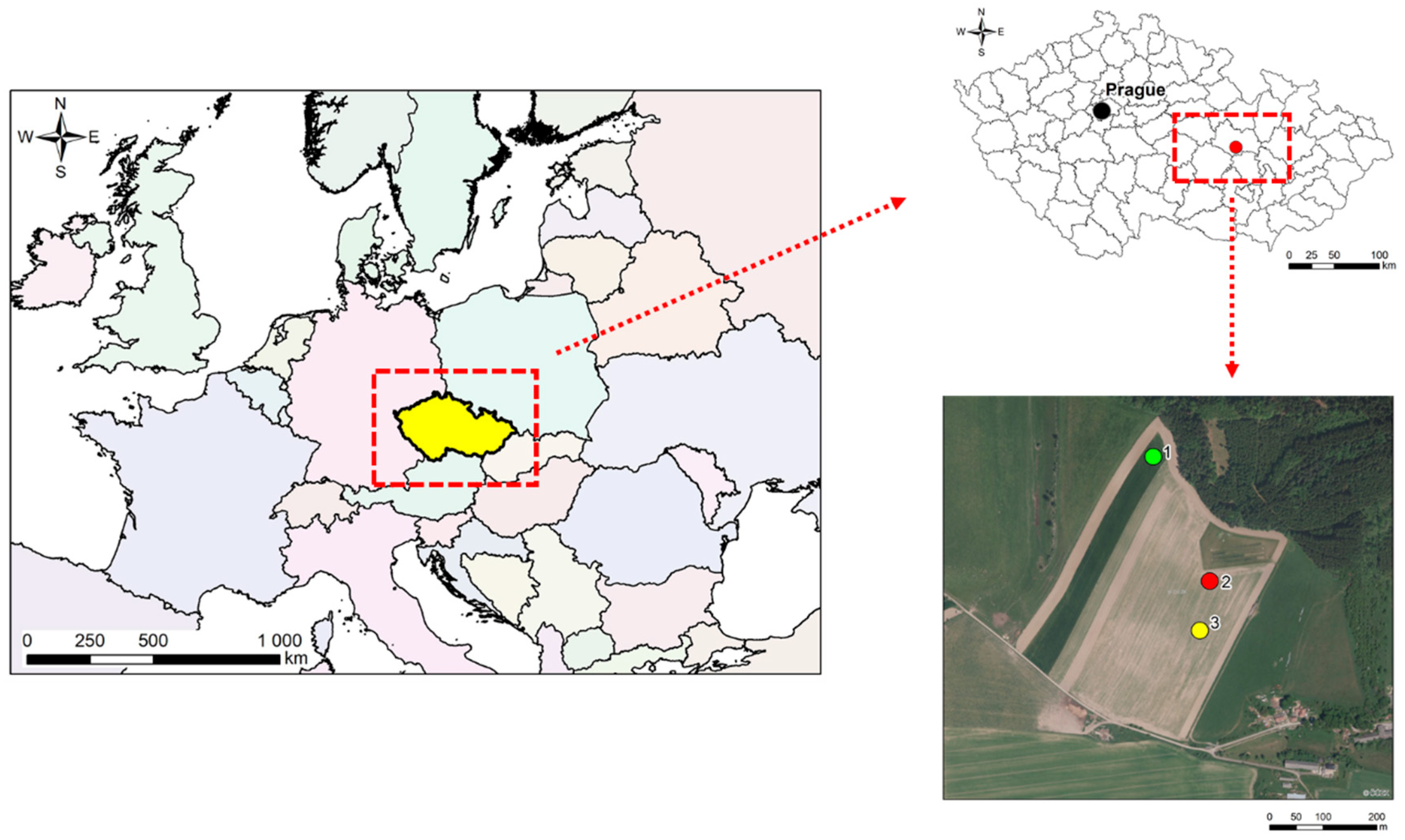
2.1. Experiment Design
2.2. Analysis of Arable Ssoil and Composts
2.3. Measurement of Basal and Substrate Induced Respiration
2.4. Determination of Enzymatic Activities and Content of Microbial Carbon
2.5. Statistical Analysis
3. Results and discussion
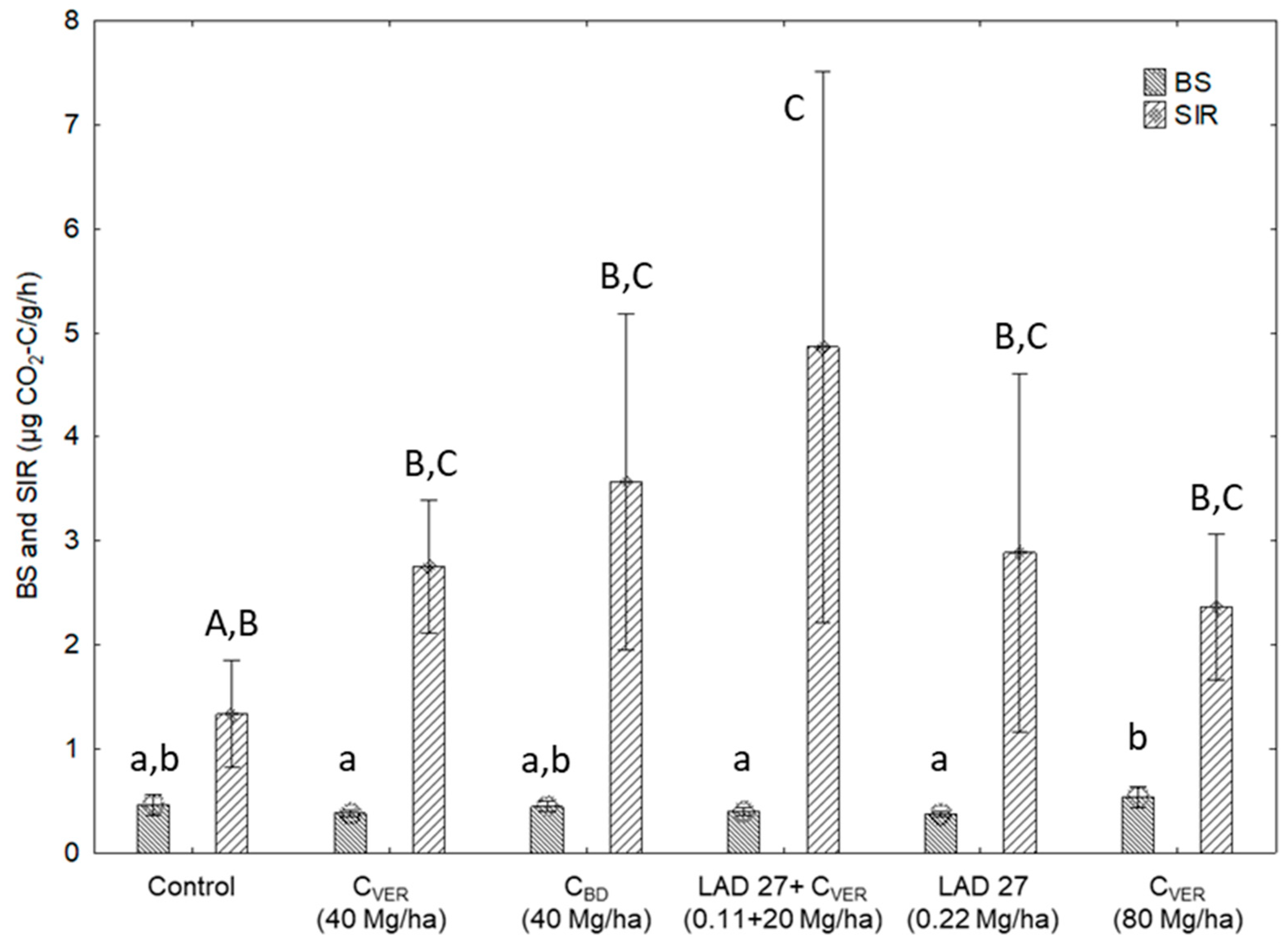
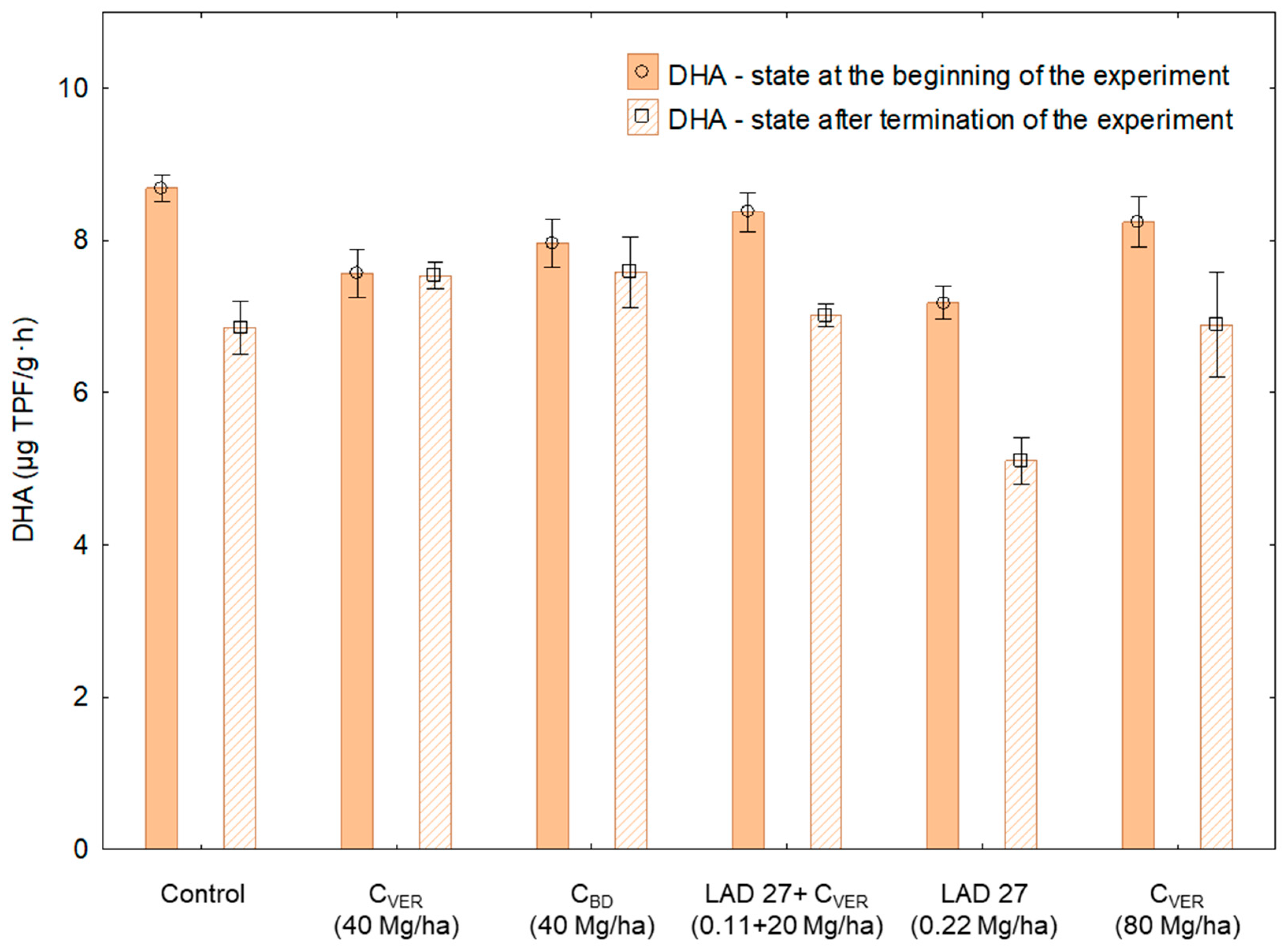
3.1. Respiration Activity
3.2. Content of Carbon in Microbial Biomass
3.3. Soil Enzyme Activities
3.4. Plant Biomass Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

| Sample | Plant Available Nutrient Content (mg/kg) | K:Mg | |||
|---|---|---|---|---|---|
| P | K | Ca | Mg | ||
| Arable soil | 146 | 370 | 3703 | 187 | 1.9 |
| Arable soil + CBD | 565 | 6422 | 11,235 | 1255 | 5.1 |
| Arable soil + CVER | 855 | 3475 | 6100 | 2407 | 3.9 |
| LAD 27 | 0 | 0 | 0 | 4 | - |
Appendix B
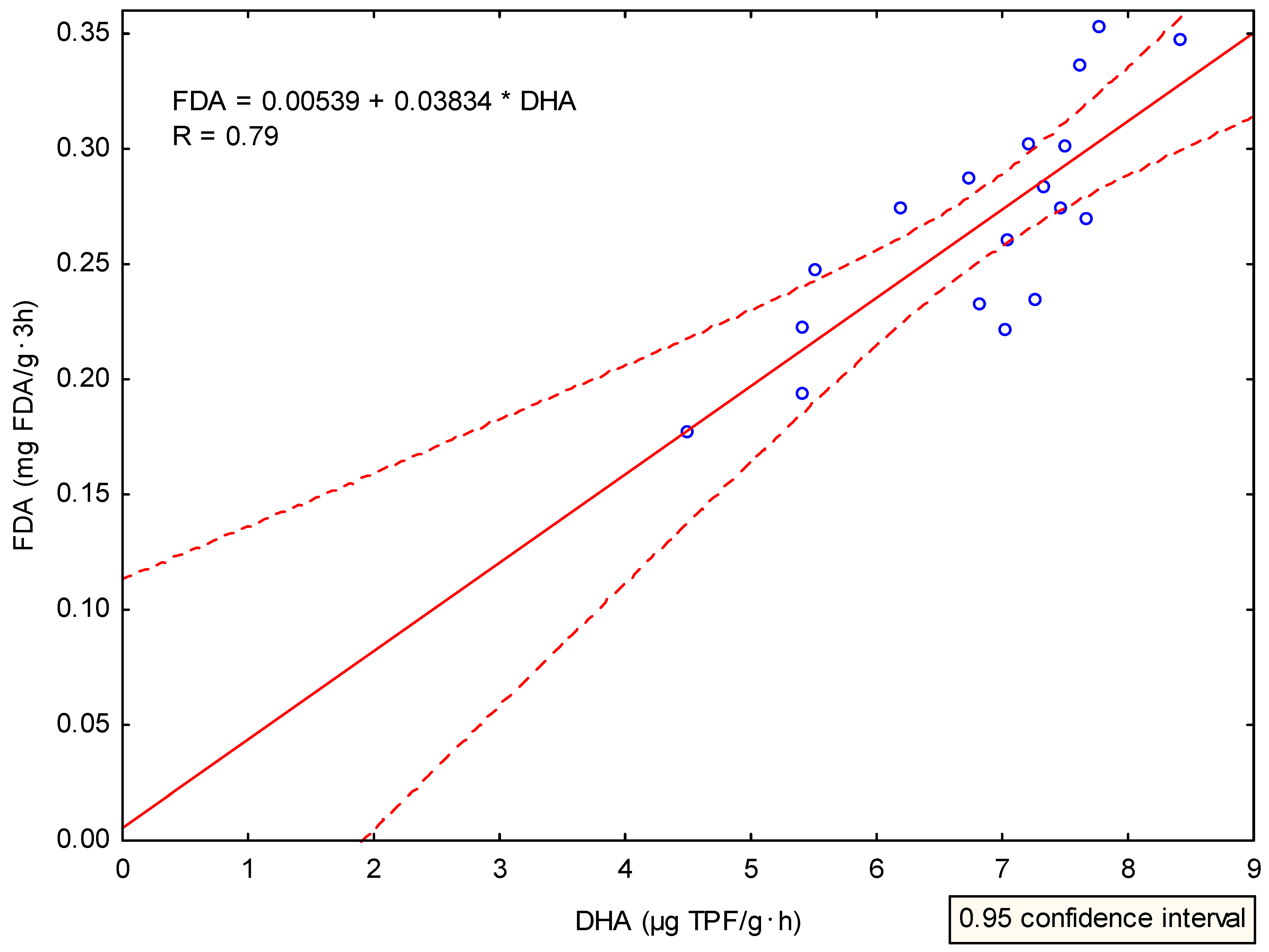

References
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Durigan, M.R.; Cherubin, M.R.; Camargo, P.B.; Ferreira, J.N.; Berenguer, E.; Gardner, T.A.; Barlow, J.; Dias, C.T.S.; Signor, D.; Oliveira, R.S., Jr.; et al. Soil organic matter responses to anthropogenic forest disturbance and land use change in the Eastern Brazilian Amazon. Sustainability 2017, 9, 379. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. B. Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A. The European Nitrogen Assessment: Sources, Effects and Policy Perspectives; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- White, R.E. Principles and Practice of Soil Science: The Soil as a Natural Resource; John Wiley & Sons Ltd.: Bognor Regis, UK, 2013; p. 384. [Google Scholar]
- Harrison-Kirk, T.; Beare, M.H.; Meenken, E.D.; Condron, L.M. Soil organic matter and texture affect responses to dry/wet cycles: Changes in soil organic matter fractions and relationships with C and N mineralization. Soil Biol. Biochem. 2014, 74, 50–60. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R.; Coleman, K. Soil Organic Matter: Its importance in sustainable agriculture and carbon dioxide fluxes. Adv. Agron. 2009, 101, 1–57. [Google Scholar]
- Maková, J. The Influence of Fertilization on Selected Indicators of Soil Quality and Health; Slovak University of Agriculture: Nitra, Slovakia, 2015. [Google Scholar]
- Tan, K.M. Humic Matter in Soil and the Environment: Principles and Controversies, 2rd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Adamcová, D.; Vaverková, M. Does composting of biodegrable municipal solid waste on the landfill body make sense? J. Ecol. Eng. 2016, 17, 30–37. [Google Scholar] [CrossRef]
- Paul, E.A. Soil Microbiology, Ecology and Biochemistry, 4rd ed.; Academic Press: Waltham, MA, USA, 2015. [Google Scholar]
- Hussain, K.; Arif, M.; Anwar-ul-Haq, M.; Hussain, T.; Nasim, M. Compost for growing plants by applying em-biofertilizer. Pak. J. Agric. Sci. 2007, 44, 434–442. [Google Scholar]
- Tejada, M.; García-Martínez, A.M.; Parrado, J. Effects of a vermicompost composted with beet vinasse on soil properties, soil losses and soil restoration. Catena 2009, 77, 238–247. [Google Scholar] [CrossRef]
- Mendoza-Hernández, D.; Fornes, F.; Belda, R.M. Compost and vermicompost of horticultural waste as substrates for cutting rooting and growth of rosemary. Sci. Hortic. 2014, 178, 192–202. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, F.; Chang, Q.; Li, T.; Li, F. Effects of vermicomposts on tomato yield and quality and soil fertility in greenhouse under different soil water regimes. Agric. Water Manag. 2015, 160, 98–105. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- De la Rosa, D.; Sobral, R. Soil quality and methods for its assessment. In Land Use and Soil Resources, 1st ed.; Braimoh, A.K., Vlek, P.L.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 167–200. [Google Scholar]
- Benintende, S.; Benintende, M.; Sterren, M.; Saluzzio, M.; Barbagelata, P. Biological variables as soil quality indicators: Effect of sampling time and ability to classify soils by their suitability. Ecol. Indic. 2015, 52, 147–152. [Google Scholar] [CrossRef]
- Stone, D.; Ritz, K.; Griffiths, B.G.; Orgiazzi, A.; Creamer, R.E. Selection of biological indicators appropriate for European soil monitoring. Appl. Soil Ecol. 2015, 97, 12–22. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martinez, V.; Debryun, J.; Schaeffer, S.M.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Dick, R.P. Soil enzyme activities as integrative indicators of soil health. In Biological Indicators of Soil Health, 1st ed.; Pankhurst, C.E., Doube, B.M., Gupta, V.S., Eds.; CAB International: Wallingford, UK, 1997; pp. 121–156. [Google Scholar]
- Sparling, G.P. Soil microbial biomass, activity and nutrient cycling as indicators of soil health. In Biological Indicators of Soil Health, 1st ed.; Pankhurst, C.E., Doube, B.M., Gupta, V.S., Eds.; CAB International: Wallingford, UK, 1997; pp. 97–119. [Google Scholar]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Dykyjova, D. Methods for Studying Grassland Ecosystem (Metody Studia Travinných Ekosystémů); Nakladatelství Československé Akademie Věd: Praha, Czech Republic, 1989. (In Czech) [Google Scholar]
- International Organization for Standardization. Soil Quality—Determination of pH; ISO 10 390:2005; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- Bremner, J.M. Nitrogen total. In Methods of Soil Analysis, Part 3: Chemical Methods, 1st ed.; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Filcheva, E.C.; Tsadilas, C. Influence of cliniptilolite and compost on soil properties. Commun. Soil Sci. Plant Anal. 2002, 33, 595–607. [Google Scholar] [CrossRef]
- Bundy, L.G.; Meisinger, J.J. Nitrogen availability indices. In Methods of Soil Analysis, Part 2: Biochemical and Microbiological Properties, 1st ed.; Weaver, R.W., Ed.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 981–984. [Google Scholar]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A Review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensor 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Šimek, M.; Virtanen, S.; Krištůfek, V.; Simojoki, A.; Yli-Halla, M. Evidence of rich microbial communities in the subsoil of a boreal acid sulphate soil conducive to greenhouse gas emissions. Agric. Ecosyst. Environ. 2011, 140, 113–122. [Google Scholar] [CrossRef]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimalization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenylphosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.C. An extraction method for measuring soil microbial biomass. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bloem, J.; Schouten, A.J.; Sørensen, S.J.; Rutgers, M.; Van der Werf, A.; Breure, A.M. Microbiological methods for assessing soil quality. In Monitoring and Evaluating Soil Quality; Bloem, J., Benedetti, A., Hopkins, D.W., Eds.; CAB International: Wallingford, UK, 2006; pp. 23–49. [Google Scholar]
- Creamer, R.E.; Schulte, R.P.O.; Stone, D.; Gal, A.; Krogh, P.H.; Lo Pap, G.; Murray, P.J.; Péres, G.; Foerster, B.; Rutgers, M.; et al. Measuring basal soil respiration across Europe: Do incubation temperature and incubation period matter? Ecol. Indic. 2014, 36, 409–418. [Google Scholar] [CrossRef]
- Tian, W.; Wang, L.; Li, Y.; Zhuang, K.; Li, G.; Zhang, J.; Xiao, X.; Yungan, X. Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agric. Ecosyst. Environ. 2015, 213, 219–227. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Bound, A.S.; Doyle, R.; Bowamn, J.P. Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl. Soil Ecol. 2016, 98, 243–253. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen use by soil microorganisms—A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Aira, M.; Domíngues, J. Substrate-induced respiration as a measure of microbial biomass in vermicomposting studies. Bioresour. Technol. 2010, 101, 7173–7176. [Google Scholar] [CrossRef] [PubMed]
- Holz, M.; Zarebanadkouki, M.; Kaestner, A.; Kuzyakov, Y.; Carminati, A. Rhizodeposition under drought is controlled by root growth rate and rhizosphere water content. Plant Soil 2018, 423, 429–442. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Severi, J.; Butterly, C.R.; Baldock, J.A. Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol. 2016, 211, 864–873. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, W.; Dai, X.; Schaeffer, S.; Yang, F.; Radosevich, M.; Xu, L.; Liu, X.; Sun, X. Responses of absolute and specific soil enzyme activities to long term additions of organic and mineral fertilizer. Sci. Total Environ. 2015, 536, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, F.; Wei, Y.; Chen, X.; Fu, X. Changes of bacterial and fungal community compositions during vermicomposting of vegetable wastes by Eisenia foetida. Bioresour. Technol. 2013, 150, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Nikaeen, M.; Nafez, A.H.; Bina, B.; Nabavi, B.F.; Hassanzadeh, A. Respiration and enzymatic activities as indicators of stabilization of sewage sludge composting. Waste Manag. 2015, 39, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sadet-Bourgeteau, S.; Houot, S.; Dequiedt, S.; Nowak, V.; Tardy, V.; Terrat, S.; Montenach, D.; Mercier, V.; Karimi, B.; Prévost-Bouréa, N.C.; et al. Lasting effect of repeated application of organic waste products on microbial communities in arable soils. Appl. Soil Ecol. 2018, 125, 278–287. [Google Scholar] [CrossRef]
- Komilis, D.; Kontou, I.; Ntougias, S. A modified static respiration assay and its relationship with an enzymatic test to assess compost stability and maturity. Bioresour. Technol. 2011, 102, 5863–5872. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mina, B.L.; Gopinath, K.A.; Kundu, S.; Gupta, H.S. Relative changes in phosphatase activities as influenced by source and application rate of organic composts in field crops. Bioresour. Technol. 2008, 99, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Długosz, A.; Wilczewski, E. Soil phosphatase activity and phosphorus content as influenced by catch crops cultivated as green manure. Pol. J. Environ. Stud. 2014, 23, 157–165. [Google Scholar]
- Lemanowicz, J.; Bartkowiak, A.; Breza-Boruta, B. Changes in phosphorus content, phosphatase activity and some physicochemical and microbiological parameters of soil within the range of impact of illegal dumping sites in Bydgoszcz (Poland). Environ. Earth Sci. 2016, 75, 510. [Google Scholar] [CrossRef]
- Jakubus, M. Estimation of phosphorus bioavailability from composted organic wastes. Chem. Speciat. Bioavailab. 2016, 28, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Bahl, G.S. Phosphorus availability to maize as influenced by organic manures and fertilizer P associated phosphatase activity in soils. Bioresour. Technol. 2008, 99, 5773–5777. [Google Scholar] [CrossRef]
- Horta, C.; Roboredo, M.; Carneiro, J.P.; Duarte, A.C.; Torrent, J.; Sharpley, A. Organic amendments as a source of phosphorus: Agronomic and environmental impact of different animal manures applied to an acid soil. Arch. Agron. Soil Sci. 2018, 64, 257–271. [Google Scholar] [CrossRef]
- Bedada, W.; Lemenih, M.; Karltun, E. Soil nutrient build-up, input interaction effects and plot level N and P balances under long-term addition of compost and NP fertilizer. Agric. Ecosyst. Environ. 2016, 218, 220–231. [Google Scholar] [CrossRef]
- Atiyeha, R.M.; Sublera, S.; Edwards, C.A.; Bachman, G.; Metzger, J.D.; Shustera, W. Effects of vermicomposts and composts on plant growth in horticultural container media and soil. Pedobiologia 2000, 44, 579–590. [Google Scholar] [CrossRef] [Green Version]
- Moreno, B.; Vivas, A.; Nogales, R.; Macci, C.; Masciandaro, G.; Benitez, E. Restoring biochemical activity and bacterial diversity in a trichloroethylene-contaminated soil: The reclamation effect of vermicomposted olive wastes. Environ. Sci. Pollut. Res. 2009, 16, 253–264. [Google Scholar] [CrossRef]
- Diaz, L.F.; Bertoldi, M.; Bidlingmaier, W. Compost Science and Technology; Elsevier: Boston, NL, USA, 2007; p. 380. [Google Scholar]
- Mehlich, A. Mehlich III soil test extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Zbíral, J.; Honsa, I.; Malý, S.; Váňa, M. Unified Techniques—Analysis of Soils I, 4th ed.; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2016; pp. 68–74. [Google Scholar]

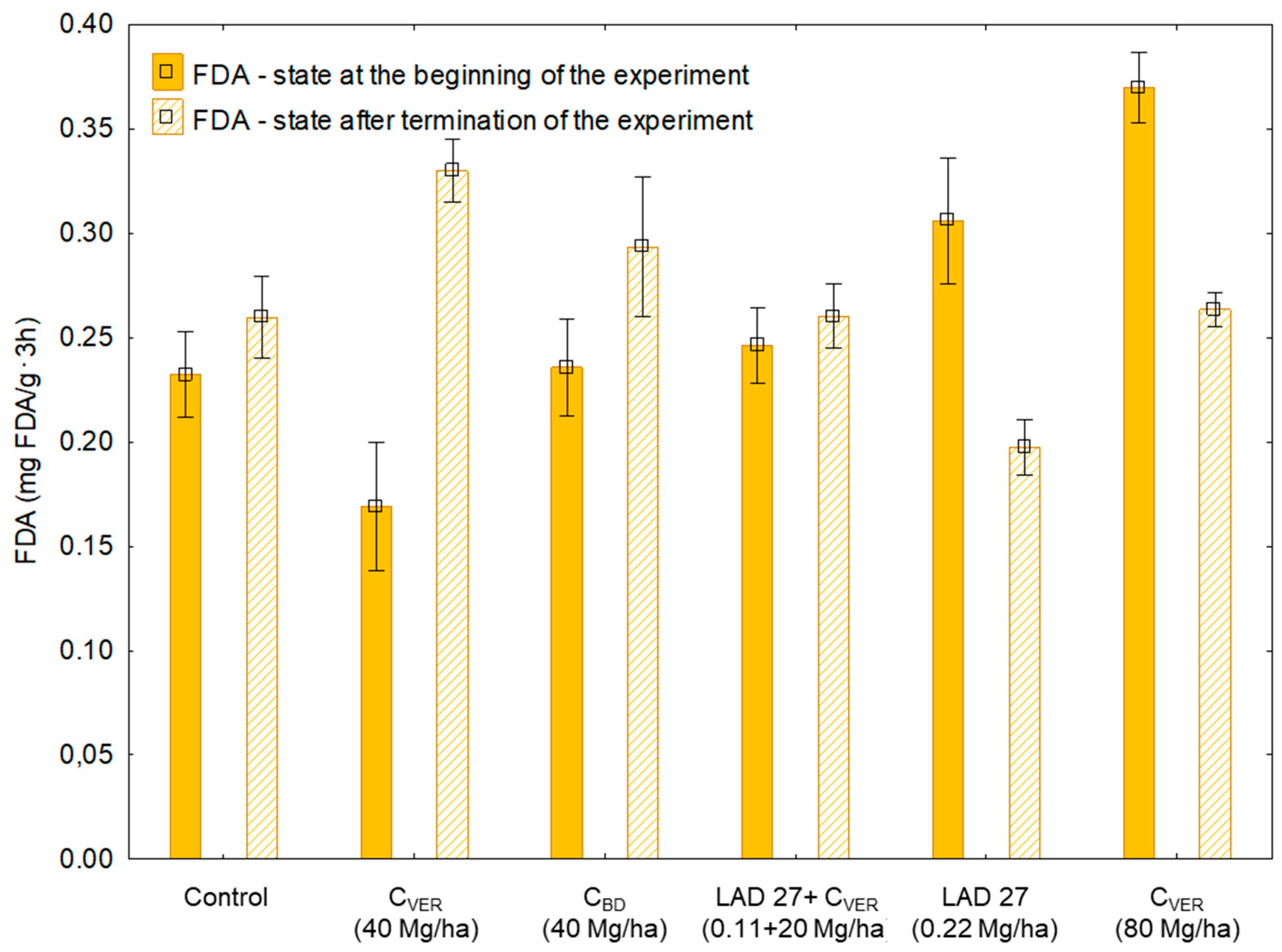
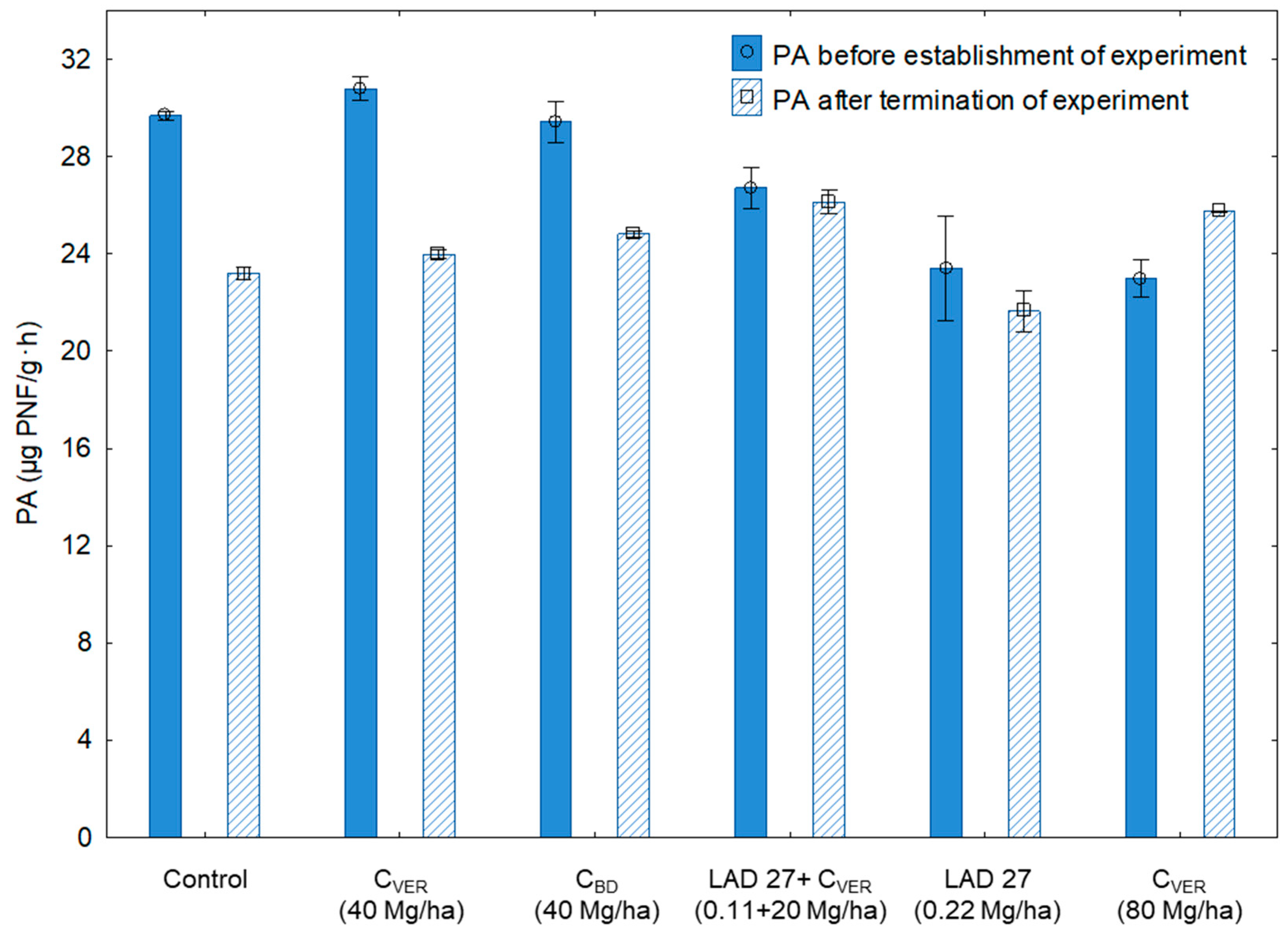
| Treatment No. | Characteristics | Dose of Fertilizers (Mg/ha) |
|---|---|---|
| 1 | Control without fertilizers | 0 |
| 2 | CVER | 40 |
| 3 | CBD | 40 |
| 4 | Combination of LAD 27 and CVER | 0.11 + 20 |
| 5 | LAD 27 | 0.22 |
| 6 | Double dose of CVER | 80 |
| Sample | pH (CaCl2) | Cox [mg·kg−1] | Ntot [mg·kg−1] | Ninorg [mg·kg−1] | C/N |
|---|---|---|---|---|---|
| Soil | 5.62 | 10.30 | 1.41 | 16.10 | 7.3 |
| CBD | 8.06 | 158.31 | 15.00 | 33.67 | 10.6 |
| CVER | 7.91 | 132.56 | 14.86 | 37.95 | 8.9 |
| LAD | - | - | 270 | 270 | - |
| Treatment No. | Dose of Fertilizer | HSD (p < 0.05) | ||
|---|---|---|---|---|
| Sampling 1 | Sampling 2 | S1 vs S2 | ||
| 1 | 0 – control | b | b | * |
| 2 | CVER 40 Mg/ha | a | b | * |
| 3 | CBD 40 Mg/ha | b | b | -* |
| 4 | LAD + CVER (0.11 + 20 Mg/ha) | b | a | - |
| 5 | LAD 0.22 Mg/ha | a | a | * |
| 6 | CVER 80 Mg/ha | b | c | * |
| Treatment No. | Dose of Fertilizer | HSD (p < 0.05) | ||
|---|---|---|---|---|
| Sampling 1 | Sampling 2 | S1 vs S2 | ||
| 1 | 0 – control | b | b | * |
| 2 | CVER 40 Mg/ha | a | c | - |
| 3 | CBD 40 Mg/ha | a,b | b,c | - |
| 4 | LAD + CVER (0.11 + 20 Mg/ha) | a,b | b | * |
| 5 | LAD 0.22 Mg/ha | a | a | * |
| 6 | CVER 80 Mg/ha | a,b | b,c | |
| Treatment No. | Dose of Fertilizer | HSD (p < 0.05) | ||
|---|---|---|---|---|
| Sampling 1 | Sampling 2 | S1 vs S2 | ||
| 1 | 0 – control | a,b | b | - |
| 2 | CVER 40 Mg/ha | a | c | * |
| 3 | CBD 40 Mg/ha | a,b | b,c | - |
| 4 | LAD + CVER (0.11 + 20 Mg/ha) | a,b | b | - |
| 5 | LAD 0.22 Mg/ha | b,c | a | * |
| 6 | CVER 80 Mg/ha | c | b | * |
| Treatment No. | Dose of Fertilizer | HSD (p < 0.05) | ||
|---|---|---|---|---|
| Sampling 1 | Sampling 2 | S1 vs S2 | ||
| 1 | 0 – control | c | b,c | * |
| 2 | CVER 40 Mg/ha | c | c | * |
| 3 | CBD 40 Mg/ha | b,c | c | * |
| 4 | LAD + CVER (0.11 + 20 Mg/ha) | b | d | - |
| 5 | LAD 0.22 Mg/ha | a | a | - |
| 6 | CVER 80 Mg/ha | a | d | * |
| Treatment No. | Dose of Fertilizer | P | ||
|---|---|---|---|---|
| mg/kg | ±SD | HSD (p < 0.05) | ||
| 1 | 0 – control | 144 | 3.47 | a |
| 2 | CVER 40 Mg/ha | 169 | 4.66 | b,c |
| 3 | CBD 40 Mg/ha | 160 | 1.87 | b |
| 4 | LAD + CVER (0.11 + 20 Mg/ha) | 158 | 2.69 | a,b |
| 5 | LAD 0.22 Mg/ha | 153 | 3.85 | a,b |
| 6 | CVER 80 Mg/ha | 179 | 1.87 | c |
| Treatment No. | Dose of Fertilizer | Plant Biomass | ||
|---|---|---|---|---|
| Mg/ha | ±SD | HSD (p < 0.05) | ||
| 1 | 0 – control | 7.98 | 0.45 | c |
| 2 | CVER 40 Mg/ha | 10.01 | 0.72 | a,b |
| 3 | CBD 40 Mg/ha | 8.82 | 0.77 | b |
| 4 | LAD + CVER (0.11 + 20 Mg/ha) | 8.09 | 0.31 | c |
| 5 | LAD 0.22 Mg/ha | 8.44 | 0.61 | b |
| 6 | CVER 80 Mg/ha | 9.54 | 0.98 | b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbl, J.; Maková, J.; Javoreková, S.; Medo, J.; Kintl, A.; Lošák, T.; Lukas, V. Response of Microbial Activities in Soil to Various Organic and Mineral Amendments as an Indicator of Soil Quality. Agronomy 2019, 9, 485. https://doi.org/10.3390/agronomy9090485
Elbl J, Maková J, Javoreková S, Medo J, Kintl A, Lošák T, Lukas V. Response of Microbial Activities in Soil to Various Organic and Mineral Amendments as an Indicator of Soil Quality. Agronomy. 2019; 9(9):485. https://doi.org/10.3390/agronomy9090485
Chicago/Turabian StyleElbl, Jakub, Jana Maková, Soňa Javoreková, Juraj Medo, Antonín Kintl, Tomáš Lošák, and Vojtěch Lukas. 2019. "Response of Microbial Activities in Soil to Various Organic and Mineral Amendments as an Indicator of Soil Quality" Agronomy 9, no. 9: 485. https://doi.org/10.3390/agronomy9090485






