1. Introduction
For over 50 years, California has been the US’s top agricultural state generating approximately
$54 billion a year in farm-gate value [
1]. California is one of the largest users of methyl bromide (MeBr) and other soil fumigants in the United States (US) [
2]. Due to its deleterious effects on stratospheric ozone, the import and manufacture of MeBr has largely been phased out under the provisions of the U.S. Clean Air Act and the Montreal Protocol [
3,
4]. California strawberry producers have continued using MeBr under a critical use exemption subject to annual review by the parties of the Montreal Protocol [
5]. In the US, 1,3-dichloropropene (1,3-D), chloropicrin, dimethyl disulfide (DMDS), and methyl isothiocyanate (MITC) have been proposed as the most likely chemical alternatives to MeBr [
3,
5,
6]. Of these, DMDS is not currently registered in California.
To reduce the use of methyl bromide fumigation and alleviate its detrimental effects to the soil, rotation with cover crops has been recommended [
7]. Cover crops grown in rotation with cash crops can alleviate the accumulation of weeds and pathogens in the soil, increase soil organic matter and improve soil physical, chemical, and biological properties [
8]. Therefore, strawberry fields in California are rotated with cover crops such as rye and barley, or other cash crops; however, in areas where land and water costs are high, cover crops are not economically feasible.
Several short-term studies on the recovery of soil microbial communities in fumigated fields concluded that soil microbial communities make a relatively rapid recovery (less than a year) after the application of fumigants [
9,
10,
11,
12]. However, there are limited studies on the effect of long-term, repeated application of fumigants on soil microbial communities. Reeve et al. [
13] proposed that long-term repeated application of fumigants can have significantly different and longer lasting impacts on soil microorganisms compared to single fumigation events.
It is important to study the effect of fumigants on soil microbial communities in regularly-fumigated fields, as soil microorganisms are crucial in sustaining long-term health of agricultural soil [
9]. Hence, understanding the impact of fumigants on beneficial soil microbial communities is essential to help further preserve the health and sustainability of these fumigated lands. Therefore, the main objective of this study was to determine the effect of long-term annual application of fumigants (15, 26, 33 and 39 years of annual fumigation) on soil microbial communities and compare them with non-fumigated sites.
4. Discussion
California is one of the largest users of MeBr and other soil fumigants in the US. To ensure better and more consistent crop yields and provide greater benefits to growers, soil fumigation has become an important agricultural practice. Therefore, producers of strawberries, tomatoes and other high value cash crops have been applying soil fumigants to control a wide array of target soil borne pests including nematodes, pathogens and weeds [
21]. For example, California alone used 3550 tons of MeBr in 2004 before it was banned [
22]. With the phase-out of MeBr because of its role in depleting stratospheric ozone, several alternatives have been used or in registration. However, the broad biocidal activity of fumigants and the continuous application can affect both target or harmful as well as non-target or beneficial soil organisms. Beneficial microorganisms are crucial in sustaining the health of agricultural soil by contributing to nutrient cycling, plant nutrient uptake, and agricultural productivity. This study helps to understand the effect of long-term repeated MeBr fumigation and early importance in studying the impact of alternative fumigants which will help to protect and create awareness among farmers on the importance of beneficial microorganisms.
Several short-term studies on the effect of soil microbial communities after fumigation have concluded that fumigation does not harm non-target soil microorganisms. Other studies have suggested there may be differences in deleterious and beneficial rhizosphere colonizers following fumigation in strawberry rhizosphere fumigated and non-fumigated soils [
23]. A study by Ibekwe et al. [
24] concluded that the lack of negative effects after fumigation may be due to the reduction in populations of plant pathogens and weeds, thus enhancing bacteria to rebound after the initial decline in population and use the available carbon sources for growth. Moreover, Ibekwe et al. [
25] confirmed that MeBr has the greatest impact on soil microbial communities and that 1,3-D has the least impact. Therefore, whether it is a short-term or a long-term study, the residual effects of different fumigants on beneficial microorganisms are still unclear. Almost all of the studies conducted to investigate the effect of fumigants on soil microbial communities have focused on the short-term effect. In the present study, we have focused on the field sites where MeBr was applied annually for 15 years or every other year for 26, 33 and 39 years.
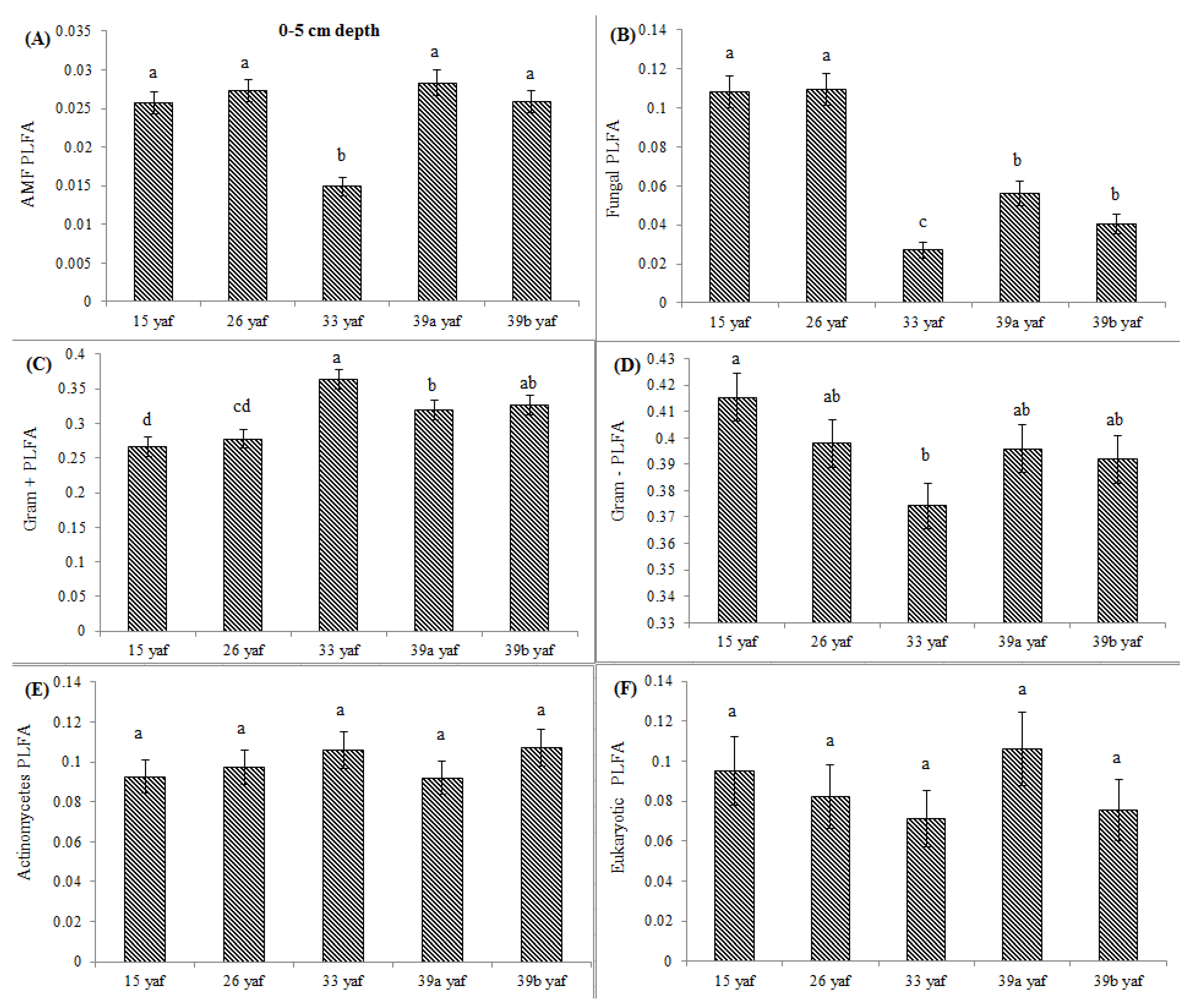
Soil microbial biomass (total PLFA) was affected by long-term repeated fumigation in the 33 yaf site. Soil microbial biomass in the non-fumigated 33 year old site was higher than in the 33 yaf site (
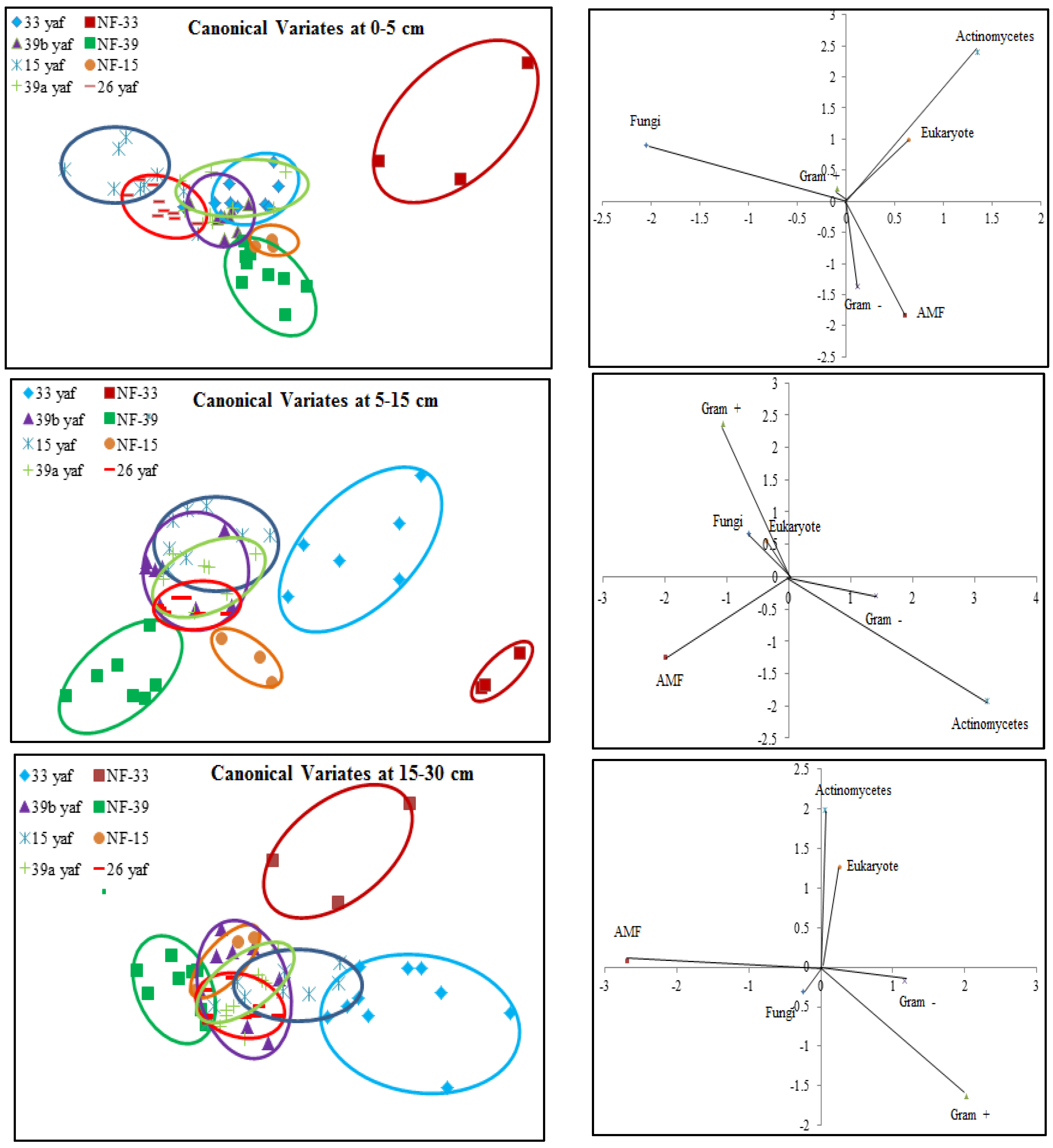
Figure 1); however, the different years (15, 33 and 39) did not affect soil microbial biomass. Nevertheless, canonical multivariate analysis showed that microbial communities in fumigated and non-fumigated sites are completely different and similarities were also observed between 15 and 26 yaf clay loam fields and 33, 39a, and 39b yaf sandy loam fields at the soil surface. Also, the shift in microbial community occurred in the 15 yaf continuously fumigated site at 5–15 cm depth, as evidenced by elevated levels of the stress rations S/M and Cy/Pre. These stress markers reflect the degree to which microorganisms are affected by temperature, nutrient, pesticide application and tillage [
17,
26]. However, other fumigated and non-fumigated sites in 0–5 and 15–30 cm depths did not show differences in S/M and Cy/Pre ratios.
Reeve et al. [
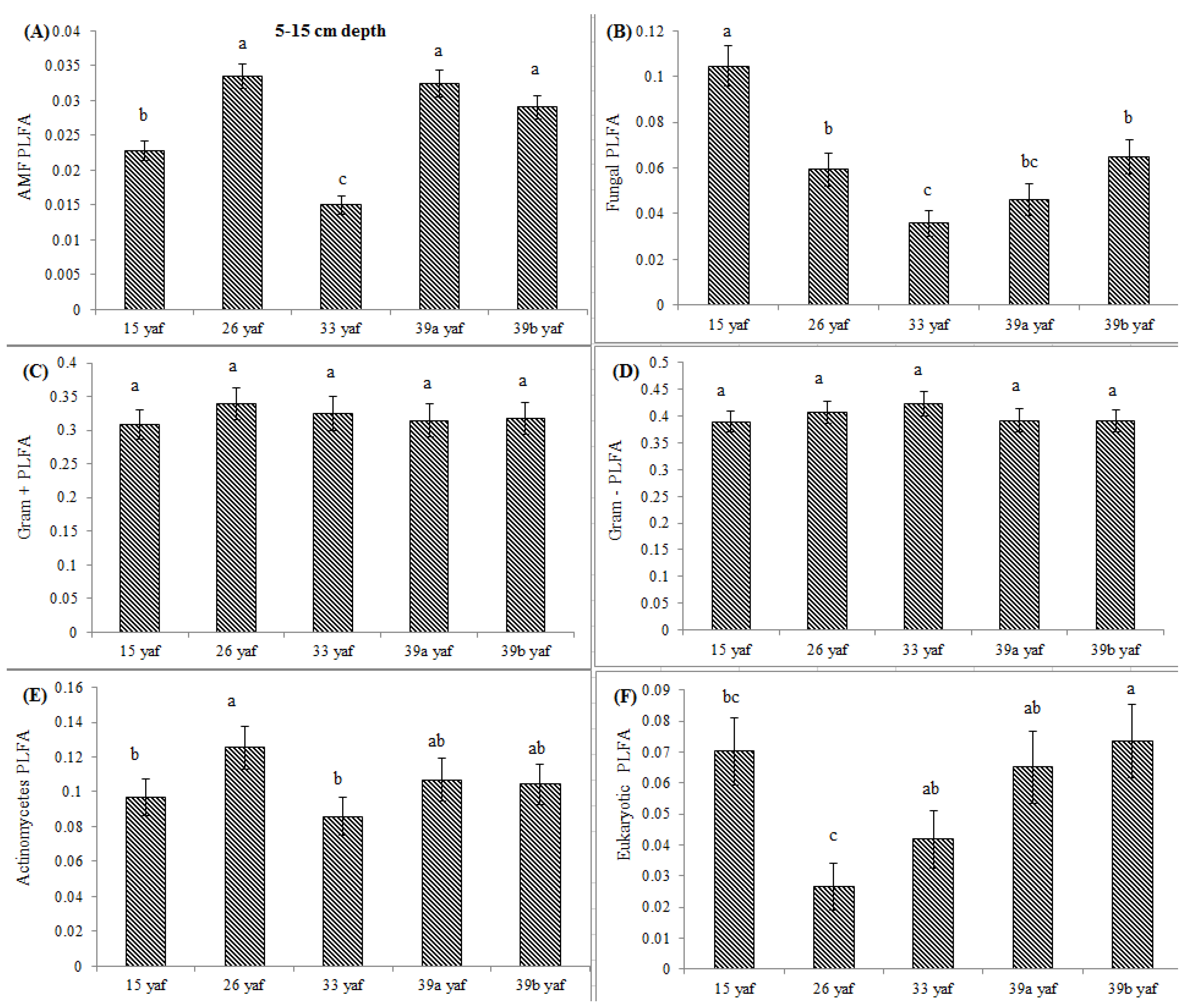
13] proposed that the long-term repeated use of fumigants may have more significant and lasting effects on soil microbial populations than have been measured in a single fumigation events study. To test the long-term effect on microbial community structure, we collected soil samples from different years of continuous fumigation. Our study shows that the proportion of fungal biomarkers was significantly higher in 15 and 26 yaf as compared to sites with 33 and 39 years of annual fumigation. However, AMF proportion was higher in 39a and 39b yaf sites compared to 33 yaf site. Lower fungal biomass is typical of intensively cultivated agricultural soils and it has been attributed to different factors such as physical disturbance, and altered amount and complexity of nutrient inputs and decrease in soil organic matter as compared to undisturbed soil [
27]. Gram + bacterial proportion was lower in 15 and 26 yaf sites than older fumigated sites, i.e., 33, 39a and 39b yaf sites. A study by Ibekwe et al. [
25] and Zelles et al. [
28] also found that Gram + bacteria were less injured by fumigation and concluded that this is due to protection by the cell wall structure of the bacteria by formation of spores and ability to adapt to fumigant vapor more quickly. Other studies on the effect of fumigation on soil microbial composition determined that application of high doses of metam sodium (MS) fumigant increased actinomycetes and Gram + bacterial population following fumigation; moreover, fungal and arbuscular mycorrhizal fungal populations were sensitive to MS [
29].
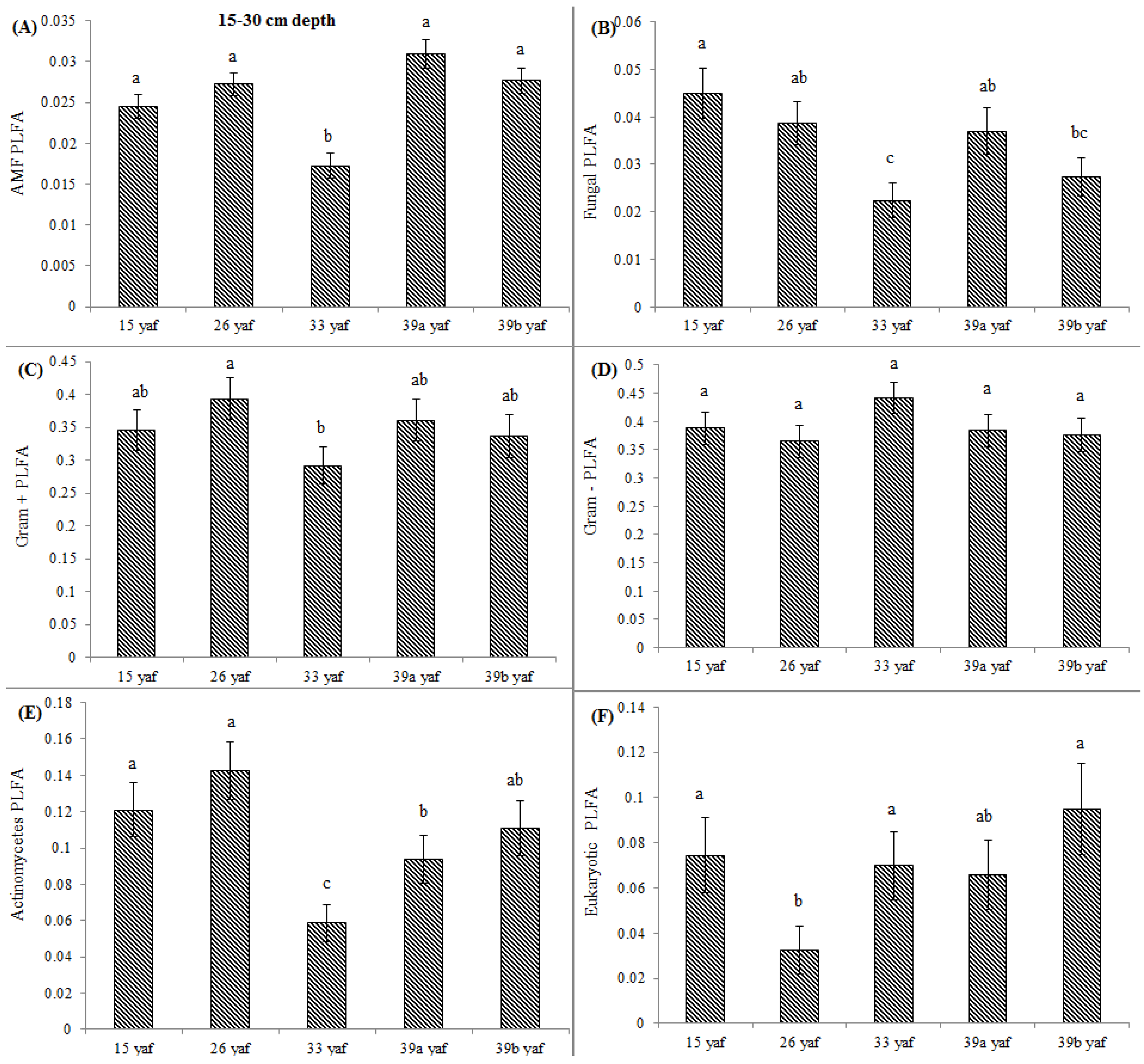
The soil textural effect (sandy loam and clay loam) of long-term annual fumigation on soil microbial communities was also studied. Ibekwe et al. [
30] suggested clayey soils might protect microorganisms from the effects of fumigation, and clay loam soil in 15 and 26 yaf sites might have protected fungal communities somewhat from the effects of fumigation as compared to the sandy loam at the 33, 39a and 39b yaf sites. However, the proportional level of AMF PLFA was significantly lower in fumigated 33 and 39 yaf sandy loam soils and 15 and 26 yaf clay loam soils compared to their non-fumigated counterparts. Similarly, a study on effects of soil type and farm management on soil ecological functional genes and microbial activities conducted by Reeve et al. [
13] concluded that over the long term under field conditions some genes were more affected by management on silty clay loam soil compared to sandy loam.
Cover crops can improve soil quality [
31] by reducing chemical inputs such as methyl bromide and other fumigations in strawberry production. Continuous strawberry production on the same site can decrease strawberry yield by causing proliferation of weeds and accumulation of pathogens in the soil [
32]. Limited information is available on the effect on microbial community in strawberry after rotation with cover crops in comparison with continuous strawberry production. However, continuous strawberry production at the 15 yaf site was associated with significantly higher proportions of fungal biomarkers as compared to rotation sites. Similar to our results, a study by Zhang et al. [
33] on short-term effects of crop rotation on microbial communities found that soils under corn-soybean rotation had lower fungal biomass compared to soils under monoculture corn. In the present study, cover crop such as barley in 39 yaf site and cash crop such as peas or beans at the 33 yaf site possibly increased Gram + bacterial proportion. Long-term repeated fumigation without any crop rotation at the 15 yaf site was associated with a lower proportion of Gram + bacteria and actinomycetes.
In the current study, to present comparative data from fumigated and non-fumigated sites, we collected soil samples from differently aged fumigated sites as well as from never-fumigated sites, and organic sites that have not been fumigated for at least 10 years. Arbuscular mycorrhizal fungi play a crucial role in nutrient acquisition and soil fertility. Our results show that proportions of AMF were significantly lower at the 15, 33 and 39 yaf sites compared to their non-fumigated counterparts. Other studies have also found MeBr to be clearly detrimental to AMF [
34], which causes stunting or poor production in several cropping systems [
35]. Also, [
36] showed that AMF spore abundance and species diversity was significantly higher in the organic than in the conventional systems.
Microbial biomass is often correlated with soil organic C [
37,
38] and may be more highly correlated with active C than total C [
39]. In the present study, the non-fumigated site compared to the 33 yaf site had significantly greater total C as well as greater microbial biomass (total PLFA) at the surface soil. Total carbon (organic carbon) can have a beneficial impact on soil quality, enhancing soil structure and fertility, and increasing water infiltration and storage [
40]. However, no notable difference in DOC between fumigated and non-fumigated sites was found. Also, TN was significantly higher at the 15 yaf site compared to its representative non-fumigated site. Levels of macronutrients were significantly lower in 15 yaf surface soil compared to its non-fumigated counterpart, similar to the 39 years old yaf surface soil in comparison to organic surface soil, excluding Ca being notably higher in NF-33 soil.