In Winter Wheat, No-Till Increases Mycorrhizal Colonization thus Reducing the Need for Nitrogen Fertilization
Abstract
:1. Introduction
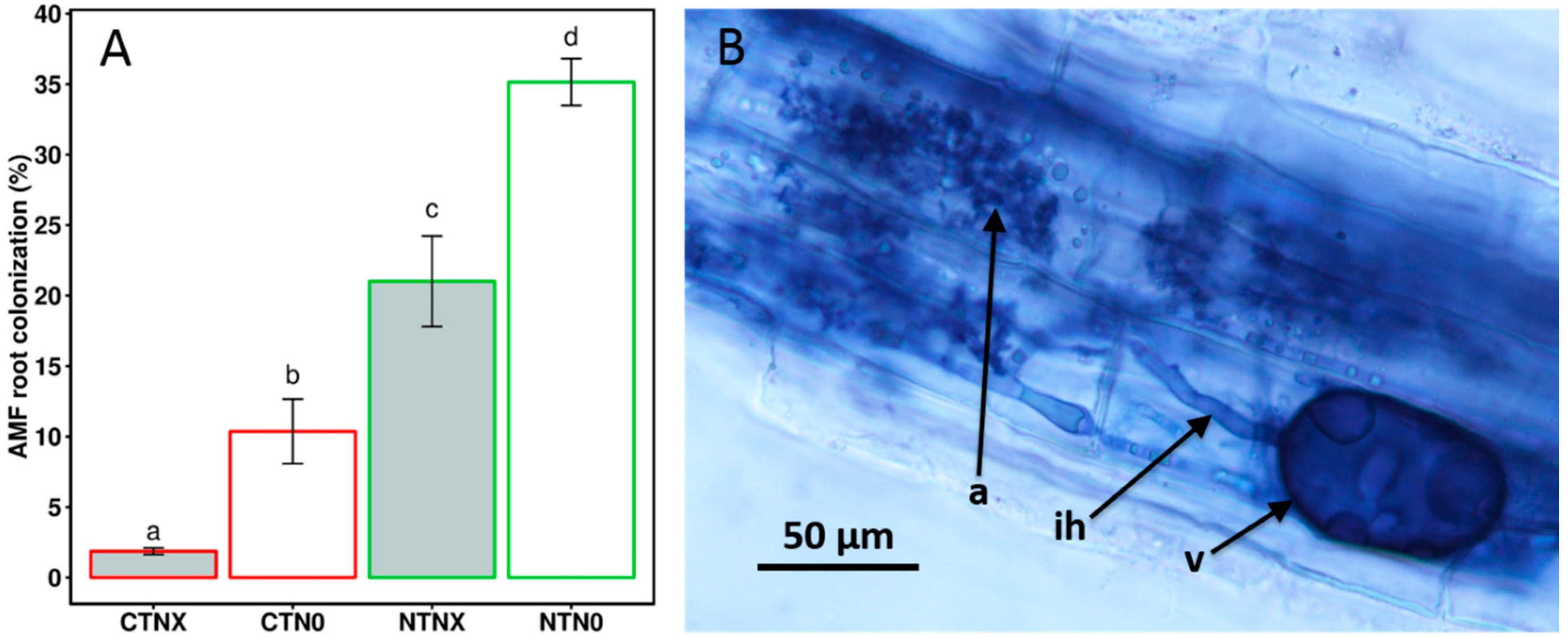
2. Results and Discussion
3. Materials and Methods
3.1. Site Description and Experimental Design
3.2. Sample Collection and Analyzes
3.3. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mardukhi, B.; Rejali, F.; Daei, G.; Ardakani, M.R.; Malakouti, M.J.; Miransari, M. Arbuscular mycorrhizas enhance nutrient uptake in different wheat genotypes at high salinity levels under field and greenhouse conditions. Comptes Rendu Biol. 2011, 334, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Bücking, H.; Kafle, A. Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Harrison, M.J. The arbuscular mycorrhizal symbiosis: An underground association. Trends Plant Sci. 1997, 2, 54–60. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Martinez, T.N.; Johnson, N.C. Agricultural management influences propagule densities and functioning of arbuscular mycorrhizas in low- and high-input agroecosystems in arid environments. Appl. Soil Ecol. 2010, 46, 300–306. [Google Scholar] [CrossRef]
- Corkidi, L.; Rowland, D.L.; Johnson, N.C.; Allen, E.B. Nitrogen fertilization alters the functioning of arbuscular mycorrhizas at two semiarid grasslands. Plant Soil 2002, 240, 299–310. [Google Scholar] [CrossRef]
- Egerton-Warburton, L.M.; Allen, E.B. Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol. Appl. 2012, 10, 484–496. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; De Carvalho, M. Effect of tillage and crop on arbuscular mycorrhiza colonization of winter wheat and triticale under Mediterranean conditions. Soil Use Manag. 2012, 28, 202–208. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Wu, Q.S. Effects of soil tillage and planting grass on arbuscular mycorrhizal fungal propagules and soil properties in citrus orchards in southeast China. Soil Tillage Res. 2016, 155, 54–61. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBryun, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Vierheilig, H.; Alt-Hug, M.; Engel-Streitwolf, R.; Mäder, P.; Wiemken, A. Studies on the attractional effect of root exudates on hyphal growth of an arbuscular mycorrhizal fungus in a soil compartment-membrane system. Plant Soil 1998, 203, 137–144. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kisugi, T.; Nomura, T.; Yoneyama, K. Nitrogen and phosphorus fertilization negatively affects strigolactone production and exudation in sorghum. Planta 2013, 238, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, E.; Öpik, M.; Bonari, E.; Ercoli, L. Responses of wheat to arbuscular mycorrhizal fungi: A meta-analysis of field studies from 1975 to 2013. Soil Biol. Biochem. 2015, 84, 210–217. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Chen, B.; Rillig, M.C. Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biol. Biochem. 2012, 46, 53–62. [Google Scholar] [CrossRef]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2015, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Pohlert, T. PMCMR: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Version 4.1. 2016. Available online: http://cran.r-project.org/ (accessed on 22 January 2016).
| H (p) | CTNX | CTN0 | NTNX | NTN0 | |
|---|---|---|---|---|---|
| AG biomass (g·plant−1) | 10.61 (0.014) | 7.02 ± 0.43 b | 4.88 ± 0.27 a | 6.07 ± 0.30 ab | 7.13 ± 0.65 b |
| Plant N concentration (mg·g−1) | NS | 10.35 ± 0.45 | 9.56 ± 0.40 | 10.89 ± 0.66 | 9.68 ± 0.55 |
| Plant N uptake (mg·plant−1) | 9.25 (0.03) | 72.37 ± 4.95 b | 46.98 ± 4.06 a | 66.83 ± 7.03 ab | 68.02 ± 5.37 ab |
| Soil N (g·kg−1) | 12.49 (0.006) | 1.46 ± 0.01 b | 1.45 ± 0.01 b | 1.40 ± 0.03 b | 1.31 ± 0.02 a |
| Soil C (g·kg−1) | NS | 13.15 ± 0.17 | 12.93 ± 0.10 | 14.10 ± 0.33 | 13.17 ± 0.35 |
| Soil C:N ratio | 17.64 (0.0005) | 9.01 ± 0.08 a | 8.93 ± 0.10 a | 10.08 ± 0.14 b | 10.04 ± 0.21 b |
| Soil compaction (MPa) | NS | 0.13 ± 0.01 | 0.11 ± 0.03 | 0.23 ± 0.05 | 0.20 ± 0.03 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verzeaux, J.; Roger, D.; Lacoux, J.; Nivelle, E.; Adam, C.; Habbib, H.; Hirel, B.; Dubois, F.; Tetu, T. In Winter Wheat, No-Till Increases Mycorrhizal Colonization thus Reducing the Need for Nitrogen Fertilization. Agronomy 2016, 6, 38. https://doi.org/10.3390/agronomy6020038
Verzeaux J, Roger D, Lacoux J, Nivelle E, Adam C, Habbib H, Hirel B, Dubois F, Tetu T. In Winter Wheat, No-Till Increases Mycorrhizal Colonization thus Reducing the Need for Nitrogen Fertilization. Agronomy. 2016; 6(2):38. https://doi.org/10.3390/agronomy6020038
Chicago/Turabian StyleVerzeaux, Julien, David Roger, Jérôme Lacoux, Elodie Nivelle, Clément Adam, Hazzar Habbib, Bertrand Hirel, Frédéric Dubois, and Thierry Tetu. 2016. "In Winter Wheat, No-Till Increases Mycorrhizal Colonization thus Reducing the Need for Nitrogen Fertilization" Agronomy 6, no. 2: 38. https://doi.org/10.3390/agronomy6020038
APA StyleVerzeaux, J., Roger, D., Lacoux, J., Nivelle, E., Adam, C., Habbib, H., Hirel, B., Dubois, F., & Tetu, T. (2016). In Winter Wheat, No-Till Increases Mycorrhizal Colonization thus Reducing the Need for Nitrogen Fertilization. Agronomy, 6(2), 38. https://doi.org/10.3390/agronomy6020038







