Emerging and Established Technologies to Increase Nitrogen Use Efficiency of Cereals
Abstract
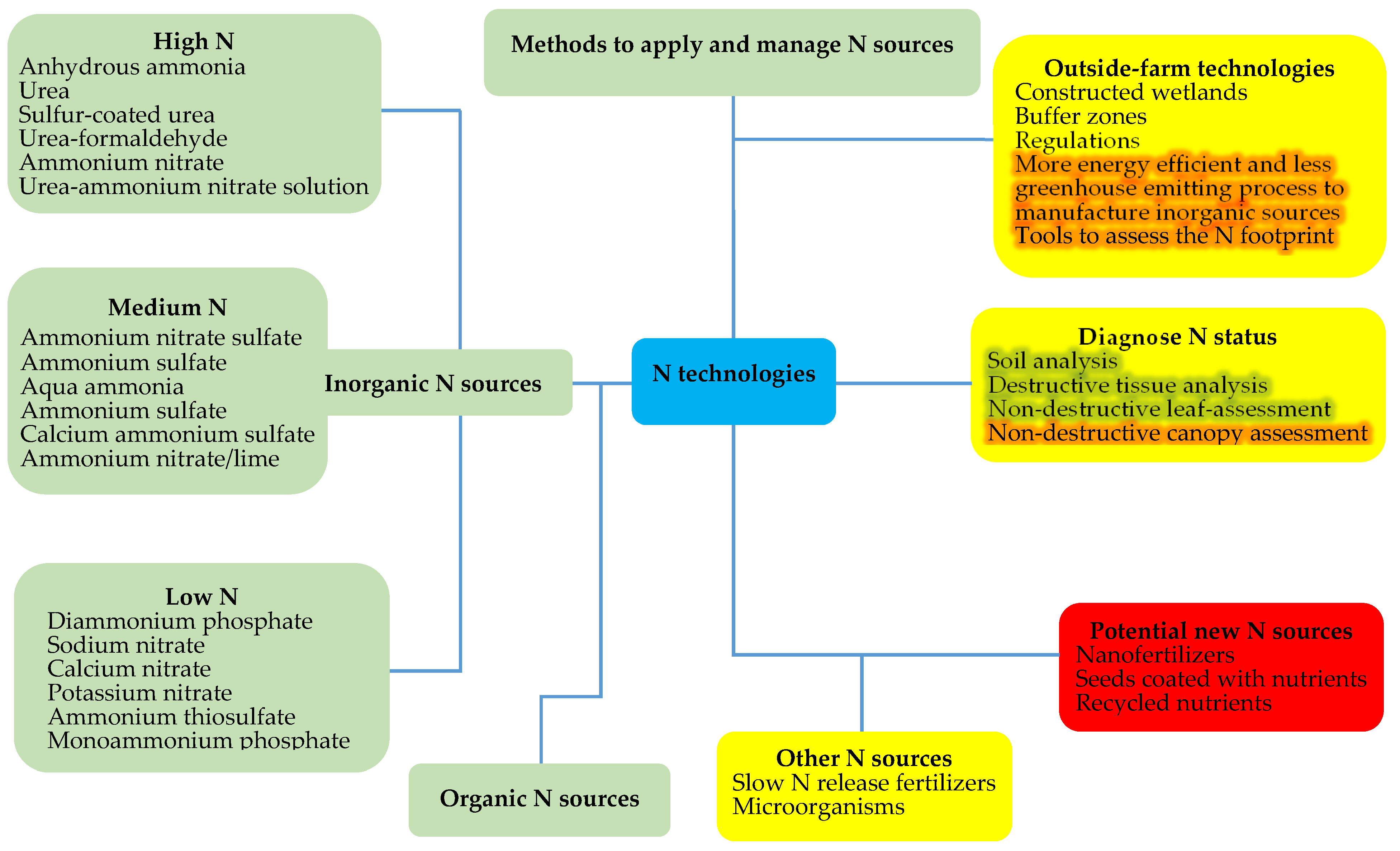
:1. Introduction
2. Literature Review
2.1. Classic Fertilizer Sources
2.2. Controlled and Slow N Release Fertilizers
2.3. Microorganisms Used for Crop N Nutrition
2.4. New Potential N Sources
2.5. Methods of Applying N Fertilizers
2.6. Technologies to Diagnose Crop N Status
2.7. Decision Support Systems
2.8. Outside-Farm Technologies
3. Conclusions
Conflicts of Interest
Abbreviations
| N | nitrogen |
| NI | nitrification inhibitor |
| PGPB | plant growth-promoting bacteria |
| NUE | nitrogen use efficiency |
References
- Godfrey, D.; Hawkesford, M.J.; Powers, S.J.; Millar, S.; Shewry, P.R. Effects of crop nutrition on wheat grain composition and end use quality. J. Agric. Food Chem. 2010, 58, 3012–3021. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Below, F.E.; Craftsbrandner, S.J.; Harper, J.E.; Hageman, R.H. Uptake, distribution, and remobilization of n-15-labeled urea applied to maize canopies. Agron. J. 1985, 77, 412–415. [Google Scholar] [CrossRef]
- Kaizzi, K.C.; Byalebeka, J.; Semalulu, O.; Alou, I.; Zimwanguyizza, W.; Nansamba, A.; Musinguzi, P.; Ebanyat, P.; Hyuha, T.; Wortmann, C.S. Sorghum response to fertilizer and nitrogen use efficiency in uganda. Agron. J. 2012, 104, 83–90. [Google Scholar] [CrossRef]
- Bange, M.P.; Hammer, G.L.; Rickert, K.G. Effect of specific leaf nitrogen on radiation use efficiency and growth of sunflower. Crop Sci. 1997, 37, 1201–1208. [Google Scholar] [CrossRef]
- Dreccer, M.F.; Schapendonk, A.; Slafer, G.A.; Rabbinge, R. Comparative response of wheat and oilseed rape to nitrogen supply: Absorption and utilisation efficiency of radiation and nitrogen during the reproductive stages determining yield. Plant Soil 2000, 220, 189–205. [Google Scholar] [CrossRef]
- Dreccer, M.F.; Schapendonk, A.; van Oijen, M.; Pot, C.S.; Rabbinge, R. Radiation and nitrogen use at the leaf and canopy level by wheat and oilseed rape during the critical period for grain number definition. Aust. J. Plant Physiol. 2000, 27, 899–910. [Google Scholar]
- Dreccer, M.F.; van Oijen, M.; Schapendonk, A.; Pot, C.S.; Rabbinge, R. Dynamics of vertical leaf nitrogen distribution in a vegetative wheat canopy. Impact on canopy photosynthesis. Ann. Bot. 2000, 86, 821–831. [Google Scholar] [CrossRef]
- Muchow, R.C. Effect of nitrogen supply on the comparative productivity of maize and sorghum in a semi-arid tropical environment I. Leaf growth and leaf nitrogen. Field Crops Res. 1988, 18, 1–16. [Google Scholar] [CrossRef]
- Boote, K.J.; Gallaher, R.N.; Robertson, W.K.; Hinson, K.; Hammond, L.C. Effect of foliar fertilization on photosynthesis, leaf nutrition, and yield of soybeans. Agron. J. 1978, 70, 787–791. [Google Scholar] [CrossRef]
- Harder, H.J.; Carlson, R.E.; Shaw, R.H. Leaf photosynthetic response to foliar fertilizer applied to corn plants during grain fill. Agron. J. 1982, 74, 759–761. [Google Scholar] [CrossRef]
- Killorn, R.; Zourarakis, D. Nitrogen fertilizer management effect on corn grain yield and nitrogen uptake. J. Prod. Agric. 1992, 5, 142–148. [Google Scholar] [CrossRef]
- Rajcan, I.; Tollenaar, M. Source: Sink ratio and leaf senescence in maize: I. Dry matter accumulation and partitioning during grain filling. Field Crops Res. 1999, 60, 245–253. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Dewit, C.T. Analysis of carbon and nitrogen limitations to soybean yield. Agron. J. 1976, 68, 319–324. [Google Scholar] [CrossRef]
- Fischer, R.A. Irrigated spring wheat and timing and amount of nitrogen fertilizer. II. Physiology of grain yield response. Field Crops Res. 1993, 33, 57–80. [Google Scholar] [CrossRef]
- Miralles, D.J.; Katz, S.D.; Colloca, A.; Slafer, G.A. Floret development in near isogenic wheat lines differing in plant height. Field Crops Res. 1998, 59, 21–30. [Google Scholar] [CrossRef]
- Uhart, S.A.; Andrade, F.H. Nitrogen and carbon accumulation and removilization during grain filling in maize under different source/sink ratios. Crop Sci. 1995, 35, 183–190. [Google Scholar] [CrossRef]
- Cooper, J.L.; Blakeney, A.B. The effect of two forms of nitrogen-fertilizer applied near anthesis on the grain quality of irrigated wheat. Aust. J. Exp. Agric. 1990, 30, 615–619. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T.; Bustamante, M.M.C.; Nardoto, G.B.; Mitre, S.K.; Pérez, T.; Ometto, J.P.H.B.; Ascarrunz, N.L.; Forti, M.C.; Longo, K.; Gavito, M.E.; et al. Latin America’s nitrogen challenge. Science 2013, 340, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, R.; Grigera, S. Analysis of soil fertility and management effects on yields of wheat and corn in the rolling pampa of Argentina. J. Agron. Crop Sci. 2005, 191, 321–329. [Google Scholar] [CrossRef]
- Spiess, E. Nitrogen, phosphorus and potassium balances and cycles of Swiss agriculture from 1975 to 2008. Nutr. Cycl. Agroecosyst. 2011, 91, 351–365. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Estimation of global NH3 volatilization loss from synthetic fertilizers and animal manure applied to arable lands and grasslands. Glob. Biogeochem. Cycl. 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Galloway, J.N.; Winiwarter, W.; Leip, A.; Leach, A.M.; Bleeker, A.; Erisman, J.W. Nitrogen footprints: Past, present and future. Environ. Res. Lett. 2014, 9, 115003. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Delogu, G.; Cattivelli, L.; Pecchioni, N.; De Falcis, D.; Maggiore, T.; Stanca, A.M. Uptake and agronomic efficiency of nitrogen in winter barley and winter wheat. Eur. J. Agron. 1998, 9, 11–20. [Google Scholar] [CrossRef]
- Rimski-Korsakov, H.; Rubio, G.; Lavado, R.S. Fate of the nitrogen from fertilizers in field-grown maize. Nutr. Cycl. Agroecosyst. 2012, 93, 253–263. [Google Scholar] [CrossRef]
- Rathke, G.W.; Behrens, T.; Diepenbrock, W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus l.): A review. Agric. Ecosyst. Environ. 2006, 117, 80–108. [Google Scholar] [CrossRef]
- Varvel, G.E.; Peterson, T.A. Nitrogen fertilizer recovery by grain sorghum in monoculture and rotation systems. Agron. J. 1991, 83, 617–622. [Google Scholar] [CrossRef]
- Varvel, G.E.; Peterson, T.A. Nitrogen fertilizer recovery by soybean in monoculture and rotation systems. Agron. J. 1992, 84, 215–218. [Google Scholar] [CrossRef]
- Scheiner, J.D.; Gutiérrez-Boem, F.H.; Lavado, R.S. Sunflower nitrogen requirement and 15n fertilizer recovery in western Pampas, Argentina. Eur. J. Agron. 2002, 17, 73–79. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Havlin, J. Impact of management systems on fertilizer nitrogen use efficiency. In Agriculture and the Nitrogen Cycle: Assessing the Impacts of Fertilizer Use on Food Production and the Environment; Mosier, A.R., Syers, J.K., Freney, J.R., Eds.; Island Press: Washington, DC, USA, 2004; pp. 167–178. [Google Scholar]
- Kaag, C.S.; Krishnamurthy, V.N. The fertilizer encyclopedia. Ref. User Serv. Quart. 2010, 50, 82–83. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; González-Torralba, J.; Arregui, L.M.; González-Murua, C.; González-Moro, M.B.; Estavillo, J.M. Ammonium as sole n source improves grain quality in wheat. J. Sci. Food Agric. 2013, 93, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Fuglie, K.O.; Heisey, P.W.; King, J.L.; Day-Rubenstein, K.A.; Schimmelpfennig, D.E.; Wang, S.L. Research Investments and Market Structure in the Food Processing, Agricultural Input, and Biofuel Industries Worldwide; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2011.
- Ni, K.; Koster, J.R.; Seidel, A.; Pacholski, A. Field measurement of ammonia emissions after nitrogen fertilization-a comparison between micrometeorological and chamber methods. Eur. J. Agron. 2015, 71, 115–122. [Google Scholar] [CrossRef]
- Bedmar, E.J.; Robles, E.F.; Delgado, M.J. The complete denitrification pathway of the symbiotic, nitrogen-fixing bacterium bradyrhizobium japonicum. Biochem. Soc. Trans. 2005, 33, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Shaviv, A. Advances in controlled release fertilizers. Adv. Agron. 2000, 71, 1–49. [Google Scholar]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Chapter 8 recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv. Agron. 2009, 102, 267–322. [Google Scholar]
- Du, C.; Tang, D.; Zhou, J.; Wang, H.; Shaviv, A. Prediction of nitrate release from polymer-coated fertilizers using an artificial neural network model. Biosyst. Eng. 2008, 99, 478–486. [Google Scholar] [CrossRef]
- Upadhyay, L.S.B. Urease inhibitors: A review. Indian J. Biotechnol. 2012, 11, 381–388. [Google Scholar]
- Shoji, S.; Delgado, J.; Mosier, A.; Miura, Y. Use of controlled release fertilizers and nitrification inhibitors to increase nitrogen use efficiency and to conserve air and water quality. Commun. Soil Sci. Plant Anal. 2001, 32, 1051–1070. [Google Scholar] [CrossRef]
- Delgado, J.A.; Mosier, A.R. Mitigation alternatives to decrease nitrous oxides emissions and urea-nitrogen loss and their effect on methane flux. J. Environ. Qual. 1996, 25, 1105–1111. [Google Scholar] [CrossRef]
- McCarty, G.W.; Bremner, J.M. Persistence of effects of nitrification inhibitors added to soils. Commun. Soil Sci. Plant Anal. 1990, 21, 639–648. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management 2004.
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef]
- Collino, D.J.; Salvagiotti, F.; Perticari, A.; Piccinetti, C.; Ovando, G.; Urquiaga, S.; Racca, R.W. Biological nitrogen fixation in soybean in Argentina: Relationships with crop, soil, and meteorological factors. Plant Soil 2015, 392, 239–252. [Google Scholar] [CrossRef]
- Hunter, W.J. Increased nodulation of soybean by a strain of Bradyrhizobium japonicum with altered tryptophan metabolism. Lett. Appl. Microbiol. 1994, 18, 340–342. [Google Scholar] [CrossRef]
- De Leij, F.A.A.M.; Lynch, J.M. Functional diversity of the rhizosphere. In Plant Growth-Promoting Rhizobacteria. Present, Status and Future Prospects; Ogoshi, A., Kobayashi, K., Homma, Y., Kodama, F., Kondo, N., Akino, S., Eds.; Nakanishi Printing: Sapporo, Japan, 1997; pp. 38–43. [Google Scholar]
- Pang, P.C.; Paul, E.A. Effect of vesicular-arbuscular-mycorrhiza on 14C and 15N distribution in nodulated faba beans. Can. J. Soil Sci. 1980, 60, 241–250. [Google Scholar] [CrossRef]
- Harris, D.; Pacovsky, R.S.; Paul, E.A. Carbon economy of soybean-rhizobium-glomus associations. New Phytol. 1985, 101, 427–440. [Google Scholar] [CrossRef]
- García de Salamone, I.E.; Funes, J.M.; Di Salvo, L.P.; Escobar-Ortega, J.S.; D’Auria, F.; Ferrando, L.; Fernandez-Scavino, A. Inoculation of paddy rice with azospirillum brasilense and pseudomonas fluorescens: Impact of plant genotypes on rhizosphere microbial communities and field crop production. Appl. Soil Ecol. 2012, 61, 196–204. [Google Scholar] [CrossRef]
- Pereg, L.; De-Bashan, L.E.; Bashan, Y. Assessment of affinity and specificity of Azospirillum for plants. Plant Soil 2016, 399, 389–414. [Google Scholar] [CrossRef]
- El-Sirafy, Z.M.; Woodard, H.J.; El-Norjar, E.M. Contribution of biofertilizers and fertilizer nitrogen to nutrient uptake and yield of Egyptian winter wheat. J. Plant Nutr. 2006, 29, 587–599. [Google Scholar] [CrossRef]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E. Seed and root bacterization. Annu. Rev. Phytopathol. 1974, 12, 181–197. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Pohlmann, E.L.; Roberts, G.P. Identification of critical residues in glnb for its activation of Nifa activity in the photosynthetic bacterium Rhodospirillum rubrum. Proc. Natl. Acad. Sci. USA 2004, 101, 2782–2787. [Google Scholar] [CrossRef] [PubMed]
- Barneix, A.J.; Saubidet, M.I.; Fatta, N.; Kade, M. Effect of rhizobacteria on growth and grain protein in wheat. Agron. Sustain. Dev. 2005, 25, 505–511. [Google Scholar] [CrossRef]
- Meyer, J.R.; Linderman, R.G. Response of subterranean clover to dual inoculation with vesicular-arbuscular mycorrhizal fungi and a plant growth-promoting rhizobacteria pseudomonas putida. Soil Biol. Biochem. 1986, 18, 185–190. [Google Scholar] [CrossRef]
- Groppa, M.D.; Zawoznik, M.S.; Tomaro, M.L. Effect of co-incubation with Bradyrhizobium japonicum and Azospirillum brasilense on soybean plants. Eur. J. Soil Biol. 1998, 34, 75–80. [Google Scholar] [CrossRef]
- Grimes, H.D.; Mount, M.S. Influence of pseudomonas putida on nodulation of phaseolus vulgaris. Soil Biol. Biochem. 1984, 16, 27–30. [Google Scholar] [CrossRef]
- Leggett, M.; Newlands, N.K.; Greenshields, D.; West, L.; Inman, S.; Koivunen, M.E. Maize yield response to a phosphorus-solubilizing microbial inoculant in field trials. J. Agric. Sci. 2015, 153, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freiberg, E. Microclimatic parameters influencing nitrogen fixation in the phyllosphere in a Costa Rican premontane rain forest. Oecologia 1998, 117, 9–18. [Google Scholar] [CrossRef]
- Papen, H.; Geßler, A.; Zumbusch, E.; Rennenberg, H. Chemolithoautotrophic nitrifiers in the phyllosphere of a spruce ecosystem receiving high atmospheric nitrogen input. Curr. Microbiol. 2002, 44, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Nkoa, R.; Coulombe, J.; Desjardins, Y.; Tremblay, N. Towards optimization of growth via nutrient supply phasing: Nitrogen supply phasing increases broccoli (Brassica oleracea var. italica) growth and yield. J. Exp. Bot. 2001, 52, 821–827. [Google Scholar] [PubMed]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 1–21. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Manikandan, A.; Thirunavukkarasu, M.; Rahale, C.S. Nano-fertilizers for balanced crop nutrition. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer International Publishing: Heidelberg, Germany, 2015; pp. 69–80. [Google Scholar]
- Egley, G.H. Ethylene, nitrate and nitrite interactions in the promotion of dark germination of common purslane seeds. Ann. Bot. 1984, 53, 833–840. [Google Scholar]
- Nijënstein, H. Nutrient Seed Coating for Grasses. Available online: http://ceb.dlf.com/upload/etsc2008_iseed-nijenstein-_final.pdf (accessed on 15 April 2016).
- IPNI. Nutrient Stewardship. Available online: http://www.nutrientstewardship.com (accessed on 15 April 2016).
- Gu, B.; Zhu, Y.; Chang, J.; Peng, C.; Liu, D.; Min, Y.; Luo, W.; Howarth, R.W.; Ge, Y. The role of technology and policy in mitigating regional nitrogen pollution. Environ. Res. Lett. 2011, 6, 014011. [Google Scholar] [CrossRef]
- Maddonni, G.A.; Ruiz, R.A.; Villarino, P.; Garcia Salomone, I. Fertilización en los cultivos para grano. In Producción de Granos, Bases Funcionales Para su Manejo; Satorre, E.H., Benech Arnold, R., de la Fuente, E.B., Miralles, D.J., Otegui, M.E., Savin, R., Eds.; Facultad de Agronomía: Buenos Aires, Argentina, 2003. [Google Scholar]
- Peterson, G.A.; Fryre, W.W. Fertilizer nitrogen management. In Nitrogen Management and Ground Water Protection; Follett, R.F., Ed.; Elseiver: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Boswell, F.C.; Meisinger, J.J.; Case, N.L. Poduction, marketing, and use of nitrogen fertilizers. In Fertilizer Technology and Use; Engelstad, O.P., Ed.; Soil Science Society of America: Madison, WI, USA, 1985; pp. 229–292. [Google Scholar]
- Altman, D.W.; McCuistion, W.L.; Kronstad, W.E. Grain protein percentage, kernel hardness, and grain yield of winter wheat with foliar applied urea. Agron. J. 1983, 75, 87–91. [Google Scholar] [CrossRef]
- Deckard, E.L.; Lambert, R.J.; Hageman, R.H. Nitrate reductase-activity in corn leaves as related to yields of grain and grain protein. Crop Sci. 1973, 13, 343–350. [Google Scholar] [CrossRef]
- Maddux, L.D.; Barnes, P.L. Effects of time and rate of applied nitrogen and nitrapyrin on irrigated corn. J. Fertil. Issues 1985, 2, 124–129. [Google Scholar]
- Walters, D.T.; Malzer, G.L. Nitrogen management and nitrification inhibitor effects on n-15 urea.1. Yield and fertilizer use efficiency. Soil Sci. Soc. Am. J. 1990, 54, 115–122. [Google Scholar] [CrossRef]
- Wang, J.; Mao, H.; Zhao, H.; Huang, D.; Wang, Z. Different increases in maize and wheat grain zinc concentrations caused by soil and foliar applications of zinc in loess plateau, China. Field Crops Res. 2012, 135, 89–96. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem- and phloem-based transport of cuo nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef] [PubMed]
- Bingham, I.J.; Blackwood, J.M.; Stevenson, E.A. Relationship between tissue sugar content, phloem import and lateral root initiation in wheat. Physiol. Plant. 1998, 103, 107–113. [Google Scholar] [CrossRef]
- Yasuor, H.; Ben-Gal, A.; Yermiyahu, U.; Beit-Yannai, E.; Cohen, S. Nitrogen management of greenhouse pepper production: Agronomic, nutritional, and environmental implications. HortScience 2013, 48, 1241–1249. [Google Scholar]
- Milani, N.; McLaughlin, M.J.; Stacey, S.P.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.G.; Cornelis, G. Dissolution kinetics of macronutrient fertilizers coated with manufactured zinc oxide nanoparticles. J. Agric. Food Chem. 2012, 60, 3991–3998. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.A.; Follett, R.F.; Shaffer, M. Simulation of nitrate-nitrogen dynamics for cropping systems with different rooting depths. Soil Sci. Soc. Am. J. 2000, 64, 1050–1054. [Google Scholar] [CrossRef]
- Boon-Long, P.; Egli, D.B.; Leggett, J.E. Leaf N and photosynthesis during reproductive growth in soybeans. Crop Sci. 1983, 23, 617–620. [Google Scholar] [CrossRef]
- Malhi, S.S.; Grant, C.A.; Johnston, A.M.; Gill, K.S. Nitrogen fertilization management for no-till cereal production in the Canadian Great Plains: A review. Soil Tillage Res. 2001, 60, 101–122. [Google Scholar] [CrossRef]
- Zimmer, S.; Messmer, M.; Haase, T.; Piepho, H.-P.; Mindermann, A.; Schulz, H.; Habekuß, A.; Ordon, F.; Wilbois, K.-P.; Heß, J. Effects of soybean variety and bradyrhizobium strains on yield, protein content and biological nitrogen fixation under cool growing conditions in germany. Eur. J. Agron. 2016, 72, 38–46. [Google Scholar] [CrossRef]
- Olfs, H.W.; Blankenau, K.; Brentrup, F.; Jasper, J.; Link, A.; Lammel, J. Soil- and plant-based nitrogen-fertilizer recommendations in arable farming. J. Plant Nutr. Soil Sci. 2005, 168, 414–431. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Pena-Fleitas, M.T.; Gallardo, M.; Thompson, R.B.; Farneselli, M.; Padilla, F.M. Assessing crop n status of fertigated vegetable crops using plant and soil monitoring techniques. Ann. Appl. Biol. 2015, 167, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, N.; Fallon, E.; Ziadi, N. Sensing of crop nitrogen status: Opportunities, tools, limitations, and supporting information requirements. HortTechnology 2011, 21, 274–281. [Google Scholar]
- Perry, E.M.; Davenport, J.R. Spectral and spatial differences in response of vegetation indices to nitrogen treatments on apple. Comput. Electron. Agric. 2007, 59, 56–65. [Google Scholar] [CrossRef]
- Lee, D.; Nguyen, V.; Littlefield, S. Comparison of methods for determination of nitrogen levels in soil, plant and body tissues, and water. Commun. Soil Sci. Plant Anal. 1996, 27, 783–793. [Google Scholar] [CrossRef]
- Feng, W.; Guo, B.-B.; Wang, Z.-J.; He, L.; Song, X.; Wang, Y.-H.; Guo, T.-C. Measuring leaf nitrogen concentration in winter wheat using double-peak spectral reflection remote sensing data. Field Crops Res. 2014, 159, 43–52. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ben Ghozlen, N.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Goffart, J.P.; Olivier, M.; Frankinet, M. Potato crop nitrogen status assessment to improve n fertilization management and efficiency: Past-present-future. Potato Res. 2008, 51, 355–383. [Google Scholar] [CrossRef]
- Wu, L.; Ogawa, Y.; Tagawa, A. Electrical impedance spectroscopy analysis of eggplant pulp and effects of drying and freezing-thawing treatments on its impedance characteristics. J. Food Eng. 2008, 87, 274–280. [Google Scholar] [CrossRef]
- Miao, Y.X.; Mulla, D.; Randall, G.; Vetsch, J.; Vintila, R. Combining chlorophyll meter readings and high spatial resolution remote sensing images for in-season site-specific nitrogen management of corn. Precis. Agric. 2009, 10, 45–62. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L.; Zarco-Tejada, P. Airborne hyperspectral images and ground-level optical sensors as assessment tools for maize nitrogen fertilization. Remote. Sens. 2014, 6, 2940–2962. [Google Scholar] [CrossRef]
- Rodriguez, D.; Fitzgerald, G.J.; Belford, R.; Christensen, L.K. Detection of nitrogen deficiency in wheat from spectral reflectance indices and basic crop eco-physiological concepts. Aust. J. Agric. Res. 2006, 57, 781–789. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Hernandez, P.; Solis, I.; Zarco-Tejada, P.J. Using high-resolution hyperspectral and thermal airborne imagery to assess physiological condition in the context of wheat phenotyping. Remote. Sens. 2015, 7, 13586–13605. [Google Scholar] [CrossRef]
- Li, F.; Gnyp, M.L.; Jia, L.; Miao, Y.; Yu, Z.; Koppe, W.; Bareth, G.; Chen, X.; Zhang, F. Estimating N status of winter wheat using a handheld spectrometer in the north China plain. Field Crops Res. 2008, 106, 77–85. [Google Scholar] [CrossRef]
- Tomkiewicz, D.; Piskier, T. A plant based sensing method for nutrition stress monitoring. Precis. Agric. 2012, 13, 370–383. [Google Scholar] [CrossRef]
- Goffart, J.P.; Olivier, M.; Frankinet, M. Crop nitrogen status assessment tools in a decision support system for nitrogen fertilization management of potato crops. HortTechnology 2011, 21, 282–286. [Google Scholar]
- Fernandez-Jaramillo, A.A.; Duarte-Galvan, C.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Romero-Troncoso, R.J.; Guevara-Gonzalez, R.G.; Millan-Almaraz, J.R. Instrumentation in developing chlorophyll fluorescence biosensing: A review. Sensors 2012, 12, 11853–11869. [Google Scholar] [CrossRef] [PubMed]
- Setiyono, T.D.; Yang, H.; Walters, D.T.; Dobermann, A.; Ferguson, R.B.; Roberts, D.F.; Lyon, D.J.; Clay, D.E.; Cassman, K.G. Maize-n: A decision tool for nitrogen management in maize. Agron. J. 2011, 103, 1276–1283. [Google Scholar] [CrossRef]
- Holzworth, D.P.; Huth, N.I.; deVoil, P.G.; Zurcher, E.J.; Herrmann, N.I.; McLean, G.; Chenu, K.; van Oosterom, E.J.; Snow, V.; Murphy, C.; et al. Apsim-evolution towards a new generation of agricultural systems simulation. Environ. Model. Softw. 2014, 62, 327–350. [Google Scholar] [CrossRef]
- Vymazal, J. Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecol. Eng. 2005, 25, 478–490. [Google Scholar] [CrossRef]
- Castelle, A.J.; Johnson, A.W.; Conolly, C. Wetland and stream buffer size requirements—A review. J. Environ. Qual. 1994, 23, 878–882. [Google Scholar] [CrossRef]
- Delgado, J.A.; Gagliardi, P.; Gross, C.M.; Lal, H.; McKinney, S.P.; Cover, H.; Hesketh, E.; Shaffer, M.J. A new GIS nitrogen trading tool concept for conservation and reduction of reactive nitrogen losses to the environment. Adv. Agron. 2010, 105, 117–171. [Google Scholar]
- Zhang, W.F.; Dou, Z.X.; He, P.; Ju, X.T.; Powlson, D.; Chadwick, D.; Norse, D.; Lu, Y.L.; Zhang, Y.; Wu, L.; et al. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in china. Proc. Natl. Acad. Sci. USA. 2013, 110, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Van Grinsven, H.J.M.; ten Berge, H.F.M.; Dalgaard, T.; Fraters, B.; Durand, P.; Hart, A.; Hofman, G.; Jacobsen, B.H.; Lalor, S.T.J.; Lesschen, J.P.; et al. Management, regulation and environmental impacts of nitrogen fertilization in northwestern Europe under the nitrates directive: A benchmark study. Biogeosciences 2012, 9, 5143–5160. [Google Scholar] [CrossRef] [Green Version]
- Stevens, C.J.; Leach, A.M.; Dale, S.; Galloway, J.N. Personal nitrogen footprint tool for the United Kingdom. Environ. Sci. Proc. Impacts 2014, 16, 1563–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobbágy, E.G.; Sala, O.E. The imprint of crop choice on global nutrient needs. Environ. Res. Lett. 2014, 9, 084014. [Google Scholar] [CrossRef]
- Dimkpa, C.O. Can nanotechnology deliver the promised benefits without negatively impacting soil microbial life? J. Basic Microbiol. 2014, 54, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.J.W.; Perry, P.J.; Ciani, S.; Pandian, S.; Schmidt, W. Manganese deficiency alters the patterning and development of root hairs in Arabidopsis. J. Exp. Bot. 2008, 59, 3453–3464. [Google Scholar] [CrossRef] [PubMed]
- Riedell, W.E. Mineral-nutrient synergism and dilution responses to nitrogen fertilizer in field-grown maize. J. Plant Nutr. Soil Sci. 2010, 173, 869–874. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, X.; Li, H.; Li, H.; Cheng, L.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of NH4+-N plus p enhances zinc and iron accumulation in maize via modifying root traits and rhizosphere processes. Field Crops Res. 2014, 164, 107–116. [Google Scholar] [CrossRef]
- Matula, J. Determination of potassium, magnesium, phosphorus, manganese and cation exchange capacity for fertilizer recommendations used by Czech union of rapeseed growers. Commun. Soil Sci. Plant Anal. 1996, 27, 1679–1691. [Google Scholar] [CrossRef]
- Herrera, J.M.; Delgado, J.A. Integrated nitrogen management. In Advances in Nitrogen Management for Water Quality; Delgado, J.A., Follett, R.F., Eds.; Soil and Water Conservation Society: Ankeny, IA, USA, 2010. [Google Scholar]
- Vanlauwe, B.; Descheemaeker, K.; Giller, K.E.; Huising, J.; Merckx, R.; Nziguheba, G.; Wendt, J.; Zingore, S. Integrated soil fertility management in sub-Saharan Africa: Unravelling local adaptation. SOIL Discuss. 2014, 1, 1239–1286. [Google Scholar] [CrossRef]
| Crop | Agronomic Efficiency (kg grain/kg N) | N Recovery % | Reference |
|---|---|---|---|
| Barley | 9 | 63 | Delogu et al. [27] |
| Maize | 20–50 | 37 | Rimski-Korsakov et al. [28] |
| Oilseed rape | 17 | 50 | Rathke et al. [29] |
| Rice | 10–30 | 30–40 | Cassman et al. [20] |
| Grain sorghum | 5–12 | 55–65 | Varvel and Peterson [30] |
| Soybean | 14 | 50 | Varvel and Peterson [31] |
| Sunflower | 22 | 51 | Scheiner et al. [32] |
| Wheat | 33 | 35–45 | Cassman et al. [20] |
| Source | Nitrogen Content (%) |
|---|---|
| Anhydrous ammonia | 82 |
| Aqua ammonia | 20–25 |
| Ammonium nitrate | 33.5–34 |
| Ammonium nitrate sulfate | 26 |
| Ammonium nitrate/lime | 20.5 |
| Ammonium sulfate | 21 |
| Ammonium thiosulfate | 12 |
| Urea-ammonium nitrate solution | 28–32 |
| Ammonium chloride | 26 |
| Urea | 46 |
| Monoammonium phosphate | 10–11 |
| Diammonium phosphate | 18 |
| Sodium nitrate | 16 |
| Potassium nitrate | 13 |
| Calcium nitrate | 15.5 |
| Calcium ammonium nitrate | 21–27 |
| Sulfur-coated urea | 39 |
| Urea-formaldehyde | 38 |
| Time of Application | N Source | Method of Application |
|---|---|---|
| Pre-sowing | Urea | Incorporated |
| Ammonium nitrate | Broadcast on the surface | |
| Anhydrous ammonia | Subsurface injection | |
| N solution | Sprayed or dripped on the surface | |
| At sowing or pre-emergence | All sources | In the row with the seed |
| All sources | Banded beside seed | |
| Anhydrous ammonia | Subsurface injection | |
| N solution | Sprayed or dripped on the surface | |
| Post-emergence | All sources | In the inter-row (side-dress) in bands |
| Anhydrous ammonia | In the inter-row (side-dress), subsurface injection | |
| Anhydrous ammonia | Subsurface injection | |
| N solution | Foliar, sprayed on the leaves |
| Management Practice | Goal(s) as Related to N Nutrition |
|---|---|
| Crop and crop rotation | Increased uptake and utilization of soil available N by using N efficient crops. Reduction of N losses by minimizing fallow frequency and accessing deeper N pools with deep rooted crops. Increased N supply from mineralizable N. Increased N demand by reducing the incidence of pest and diseases. |
| Cover and inter-cropping | Reduction of N losses by minimizing fallow frequency and accessing deeper N pools with deep rooted crops. Increased N supply from mineralizable N. |
| Management of crop residues | Control of N mineralization. |
| Genotype | Increased uptake and utilization of soil available N by using N efficient genotypes. |
| Irrigation and crop protection | Increased uptake and utilization of soil available N by maximizing crop N demand and use. |
| Adequate nutrition of other nutrients | Increased uptake and utilization of soil available N by maximizing crop N demand and use. |
| Accurate prediction of N need | Increased uptake and utilization of soil available N by avoiding over/under application of N fertilizer. |
| N source | Avoidance of N losses caused by specific N transformations in the soil. Increased N physiological efficiency (yield/N uptake) due to the metabolism of N forms (NO3/NH4) |
| Timing of N application | Reduction of N losses and increased N agronomic efficiency (yield/N supply) |
| N application method (placement) | Reduction of N losses and immobilization. Improved spatial availability of soil mineral N. |
| Timing, intensity, and depth of tillage | Control of soil mineral N. |
| Type of Method | Level of Analysis | Method | Reference |
|---|---|---|---|
| Destructive | Tissue assessment | Kjeldahl wet digestion | |
| Dumas combustion | |||
| Nitrate ion-selective electrodes | Tomkiewicz and Piskier [113] | ||
| Non-Destructive | Leaf assessment | Leaf-light transmittance (chlorophyll meter, SPAD) | Miao et al. [108] |
| Polyphenols-dualex (Chlorophyll fluorescence) | Goffart et al. [114] | ||
| Multiplex (Chlorophyll fluorescence) | Fernandez-Jaramillo et al. [115] | ||
| Canopy assessment | Satellite or aerial assessment (normalized vegetation index (NDVI), spectrometry) | ||
| Passive sensors (Greenseeker®, Yara-N-sensor®, N-tester®, Crop circle, digital imaging) | Muñoz-Huerta [99] | ||
| Active sensors (FieldSpec-spectroradiometer, CropScan) | Muñoz-Huerta [99] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, J.M.; Rubio, G.; Häner, L.L.; Delgado, J.A.; Lucho-Constantino, C.A.; Islas-Valdez, S.; Pellet, D. Emerging and Established Technologies to Increase Nitrogen Use Efficiency of Cereals. Agronomy 2016, 6, 25. https://doi.org/10.3390/agronomy6020025
Herrera JM, Rubio G, Häner LL, Delgado JA, Lucho-Constantino CA, Islas-Valdez S, Pellet D. Emerging and Established Technologies to Increase Nitrogen Use Efficiency of Cereals. Agronomy. 2016; 6(2):25. https://doi.org/10.3390/agronomy6020025
Chicago/Turabian StyleHerrera, Juan M., Gerardo Rubio, Lilia Levy Häner, Jorge A. Delgado, Carlos A. Lucho-Constantino, Samira Islas-Valdez, and Didier Pellet. 2016. "Emerging and Established Technologies to Increase Nitrogen Use Efficiency of Cereals" Agronomy 6, no. 2: 25. https://doi.org/10.3390/agronomy6020025






