Analysis of Temporal Variation of Soil Salinity during the Growing Season in a Flooded Rice Field of Thessaloniki Plain-Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Agricultural Practices
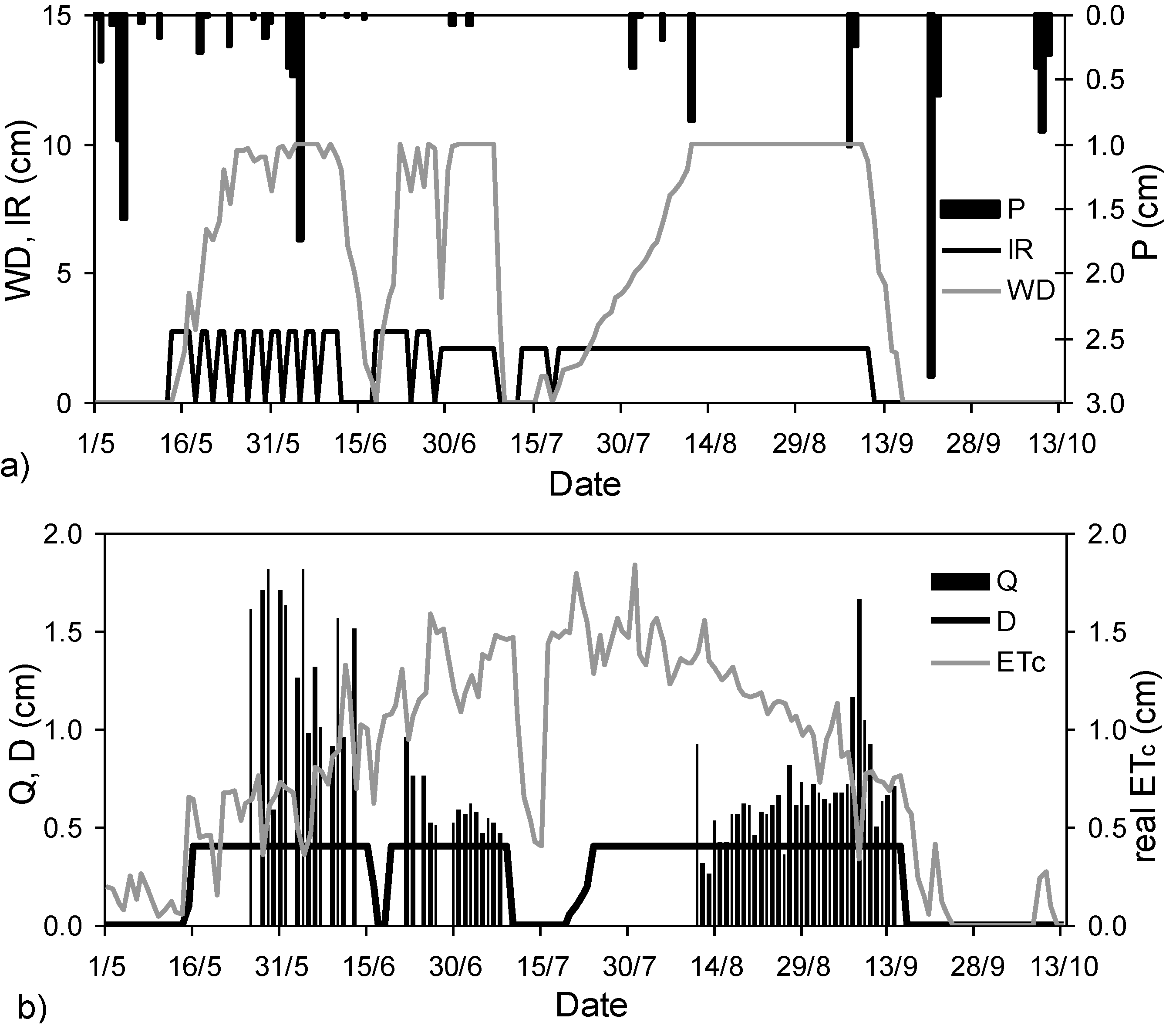
2.3. Water Balance Components
2.4. Measurements of Soil and Water Quality Parameters
| Soil Parameters | Soil Layer (cm) | |
|---|---|---|
| 15–20 cm | 35–40 cm | |
| Soil Texture | Silty Clay | Silty Clay Loam |
| CaCO3 (%) | 6.16 | 7.19 |
| Organic Matter (%) | 2.09 | 1.73 |
| 1 CEC (cmolc·kg−1) | 40.58 | 33.01 |
| θs (cm3·cm−3) | 0.655 | 0.538 |
| θr (cm3·cm−3) | 0.000 | 0.000 |
| a (cm−1) | 0.0085 | 0.00196 |
| n | 1.200 | 1.129 |
| m (m = 1 − 1/n) | 0.167 | 0.114 |
| ρb (g·cm−3) | 1.123 | 1.223 |
| Irrigation water parameters | Mean ± S.D. | |
| pH | 8.1 ± 0.3 | |
| EC (μS·cm−1) | 429 ± 83 | |
| Ca2+ 3(mmolc·L−1) | 2.31 ± 0.54 | |
| Mg2+ (mmolc·L−1) | 1.04 ± 0.12 | |
| Na+ (mmolc·L−1) | 0.65 ± 0.08 | |
| K+ (mmolc·L−1) | 0.03 ± 0.01 | |
| Cl− (mmolc·L−1) | 0.65 ± 0.48 | |
| HCO3− (mmolc·L−1) | 3.17 ± 0.61 | |
| 2 SAR (mmolc·L−1)0.5 | 0.50 ± 0.05 | |
2.5. Analysis and Statistics
3. Results and Discussion
3.1. Variations in Ca2+, Mg2+, K+, Na+, Cl−, HCO3−, EC and pH in Soil Solution, Irrigation and Floodwater
- the decrease of algae growth and uptake because LAI exceeded the value of 2 and algal photosynthesis was restricted [37];
- the continuous irrigation water inflow with no further intermissions by the middle of July (Figure 1a) which contributed to the increase of K+ in the flooding water; and
- evapotranspiration reaching maximum values after the middle of July (Figure 1b), which enhanced evapoconcentration.
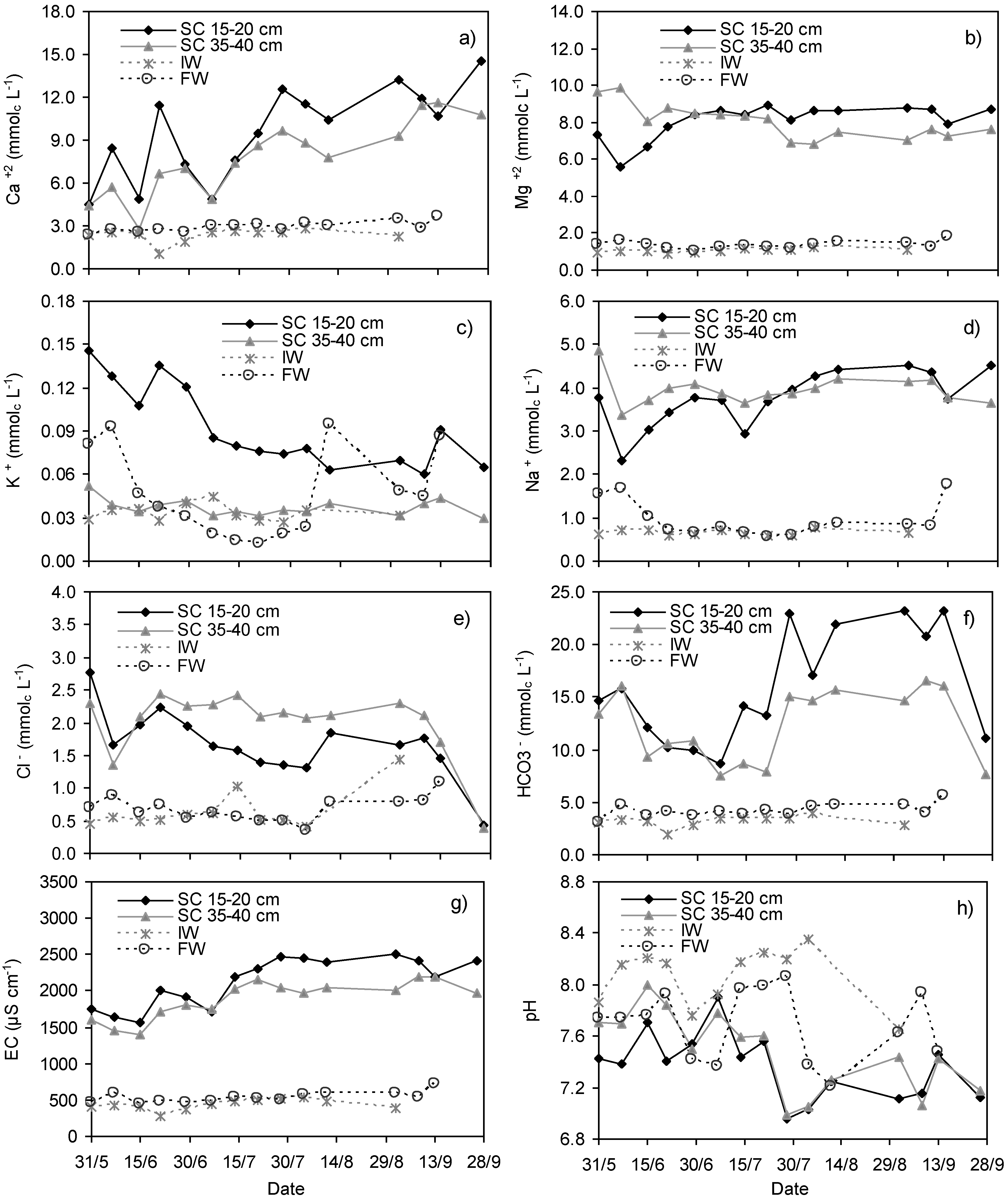
- the continuous water inflow by irrigation enhanced oxygenation; and
- when the plant reaches the tillering and internode elongation stages, the roots are at full development while stem aerenchyma starts to be fully active providing a low-resistance pathway for diffusion of O2 within the roots, enhancing soil oxygenation [67]. In flooded soils, where lowland rice is expected to rely solely on ammonium because of the ambient reducing conditions, it has been well established that the root-induced release of O2 in the rhizosphere, and the subsequent oxidation of the soil, can make a substantial, and sometimes major contribution to the observed rhizosphere acidification [68,69,70].
| Parameter | Soil Layer | 5 May | 6 October |
|---|---|---|---|
| Ca2+ (mmolc·L−1) | 15–20 cm | 5.58 ± 0.18a 1 | 4.45 ± 0.25b |
| Ca2+ (mmolc·L−1) | 35–40 cm | 3.69 ± 0.23a | 3.61 ± 0.33a |
| Mg2+ (mmolc·L−1) | 15–20 cm | 2.35 ± 0.02a | 2.1 ± 0.07b |
| Mg2+ (mmolc·L−1) | 35–40 cm | 1.76 ± 0.17a | 1.83 ± 0.15a |
| K+ (mmolc·L−1) | 15–20 cm | 0.13 ± 0.01a | 0.08 ± 0.01b |
| K+ (mmolc·L−1) | 35–40 cm | 0.05 ± 0.01a | 0.04 ± 0.01a |
| Na+ (mmolc·L−1) | 15–20 cm | 1.66 ± 0.13a | 1.96 ± 0.13a |
| Na+ (mmolc·L−1) | 35–40 cm | 1.85 ± 0.22a | 2.09 ± 0.23a |
| Cl− (mmolc·L−1) | 15–20 cm | 1.07 ± 0.13b | 2.40 ± 0.50a |
| Cl− (mmolc·L−1) | 35–40 cm | 1.13 ± 0.07b | 3.33 ± 0.13a |
| HCO3− (mmolc·L−1) | 15–20 cm | 5.67 ± 0.53a | 3.34 ± 0.07b |
| HCO3− (mmolc·L−1) | 35–40 cm | 3.56 ± 0.23a | 3.20 ± 0.01a |
| EC (μS·cm−1) | 15–20 cm | 1010 ± 32a | 832 ± 28b |
| EC (μS·cm−1) | 35–40 cm | 736 ± 42a | 751 ± 72a |
3.2. Variations in Exchangeable Cations Ca2+, Mg2+, K+, Na+ and ESP

| Parameter | Soil Layer | 5 May | 6 October |
|---|---|---|---|
| Ca2+ (cmolc·kg−1) | 15–20 cm | 32.04 ± 1.46a 2 | 30.69 ± 0.95a |
| Ca2+ (cmolc·kg−1) | 35–40 cm | 26.35 ± 1.59a | 26.13 ± 1.63a |
| Mg2+ (cmolc·kg−1) | 15–20 cm | 6.95 ± 0.45a | 8.03 ± 0.21a |
| Mg2+ (cmolc·kg−1) | 35–40 cm | 5.34 ± 0.77a | 5.87 ± 0.79a |
| K+ (cmolc·kg−1) | 15–20 cm | 0.38 ± 0.04a | 0.42 ± 0.04a |
| K+ (cmolc·kg−1) | 35–40 cm | 0.16 ± 0.03a | 0.17 ± 0.02a |
| Na+ (cmolc·kg−1) | 15–20 cm | 1.21 ± 0.07b | 1.67 ± 0.05a |
| Na+ (cmolc·kg−1) | 35–40 cm | 1.16 ± 0.15b | 1.47 ± 0.16a |
| 1 ESP (%) | 15–20 cm | 2.98 ± 0.07b | 4.09 ± 0.11a |
| 1 ESP (%) | 35–40 cm | 3.51 ± 0.32b | 4.36 ± 0.13a |
3.3. WMPs Effects on the Salinity of Rice Fields
- the continuous irrigation water inflow prevents oxygen depletion from the floodwater enhancing algae production which seems to play a crucial role on ions uptake. In the parallel experiments which were performed in the same experimental field, Aschonitis et al. [37] estimated the total final produced dry algal biomass at 1047 kg·ha−1 whereas algae uptake of Ca2+, Mg2+, Na+ and K+ was estimated to 62.61, 3.24, 3.35 and 39.58 kg·ha−1, respectively. Apart from their implication in salinity components regulation, algae remove a significant part of nutrients (nitrogen and phosphorus) from the floodwater [35,37]. On the other hand, the surface runoff removed 296.3, 133.1, 87.7 and 13.2 kg·ha−1 of Ca2+, Mg2+, Na+ and K+, respectively [37]. Furthermore, due to the continuous flooding and runoff, the quality characteristics of the runoff water are such, that it could be reused for irrigation of rice fields in this area at least once [5]. The significant contribution of controlled surface runoff in semi-arid environments has also been verified by Chen et al. [75]; and
- the continuous inflow of irrigation water and the attainment of a constant ponding depth in the rice-fields prevents further increase of the water temperature and evaporation [36], which lead to evapoconcentration and salt accumulation.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rhoades, J.D. Sustainability of irrigation: An overview of salinity problems and control strategies. In Footprints of Humanity: Reflections on 50 Years of Water Resource Developments. In Proceedings of the Annual Conference of the Canadian Water Resources Association, Lethbridge, AB, Canada, 3–6 June 1997.
- Szabolcs, I. Prospects of soil salinity for the 21st Century. In Proceedings of the 15th World Congress of Soil Science (ISSS), Acapulco, Mexico City, 10–16 July 1994; Volume 1, pp. 123–141.
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinisation of Land and Water Resources; University of New South Wales Press Ltd.: Canberra, Australia, 1995. [Google Scholar]
- Playán, E.; Pérez-Coveta, O.; Martínez-Cob, A.; Herrero, J.; García-Navarro, P.; Latorre, B.; Brufau, P.; Garcés, J. Overland water and salt flows in a set of rice paddies. Agric. Water Manag. 2008, 95, 645–658. [Google Scholar] [CrossRef]
- Litskas, V.D.; Aschonitis, V.G.; Lekakis, E.H.; Antonopoulos, V.Z. Effects of land use and irrigation practices on Ca, Mg, K, Na loads in rice-based agricultural systems. Agric. Water Manag. 2014, 132, 30–36. [Google Scholar] [CrossRef]
- Hammecker, C.; van Asten, P.; Marlet, S.; Maeght, J.L.; Poss, R. Simulating the evolution of soil solutions in irrigated rice soils in the Sahel. Geoderma 2009, 150, 129–140. [Google Scholar] [CrossRef]
- Ceuppens, J.; Wopereis, M.C.S.; Miézan, K.M. Soil salinization processes in rice irrigation schemes in the Senegal River Delta. Soil Sci. Soc. Am. J. 1996, 61, 1122–1130. [Google Scholar] [CrossRef]
- Van Asten, P.J.A.; van’t Zelfde, J.A.; van der Zee, S.E.A.T.M.; Hammecker, C. The effect of irrigated rice cropping on the alkalinity of two alkaline rice soils in the Sahel. Geoderma 2004, 119, 233–247. [Google Scholar]
- Sharma, P.K.; de Datta, S.K. Effects of puddling on soil physical properties and processes. In Soil Physics and Rice; IRRI: Los Baños, Philippines, 1985; pp. 217–234. [Google Scholar]
- Aschonitis, V.G.; Kostopoulou, S.K.; Antonopoulos, V.Z. Methodology to assess the effects of rice cultivation under flooded conditions on van Genuchten’s model parameters and pore size distribution. Transp. Porous Med. 2012, 91, 861–876. [Google Scholar] [CrossRef]
- Wopereis, M.C.S.; Wösten, J.H.M.; Bouma, J.; Woodhead, T. Hydraulic resistance in puddled rice soils: Measurement and effects on water movement. Soil Till. Res. 1992, 24, 199–209. [Google Scholar] [CrossRef]
- Wopereis, M.C.S.; Bouman, B.A.M.; Kropff, M.J.; Ten Berge, H.F.M.; Maligaya, A.R. Water use efficiency of flooded rice fields. I. Validation of the soil-water balance model SAWAH. Agric. Water Manag. 1994, 26, 277–289. [Google Scholar] [CrossRef]
- Chen, S.K.; Liu, C.W.; Huang, H.C. Analysis of water movement in paddy rice fields (II) simulation studies. J. Hydrol. 2002, 268, 259–271. [Google Scholar] [CrossRef]
- Chen, S.K.; Liu, C.W. Analysis of water movement in paddy rice fields (I) experimental studies. J. Hydrol. 2002, 260, 206–215. [Google Scholar] [CrossRef]
- Boivin, P.; Favre, F.; Hammecker, C.; Maeght, J.L.; Delariviére, J.; Poussin, J.C.; Wopereis, M.C.S. Processes driving soil solution chemistry in a flooded rice-cropped vertisol: Analysis of long-time monitoring data. Geoderma 2002, 110, 87–107. [Google Scholar] [CrossRef]
- Wopereis, M.C.S.; Ceuppens, J.; Boivin, P.; N’Diaye, A.M.; Kane, A. Preserving soil quality under irrigation in the Senegal River Valley. Roy. Neth. Soc. Agric. Sci. 1998, 46, 97–107. [Google Scholar]
- Häfele, S.; Wopereis, M.C.S.; Boivin, P.; Diaye, A.M.N. Effect of puddling on soil desalinization and rice seedling survival in the Senegal River Delta. Soil Till. Res. 1999, 51, 35–46. [Google Scholar] [CrossRef]
- Cass, A.; Gusli, S.; MacLeod, D.A. Sustainability of soil structure quality in rice paddy-soybeen cropping systems in South Sulawesi, Indonesia. Soil Till. Res. 1994, 31, 339–352. [Google Scholar] [CrossRef]
- Favre, F.; Boivin, P.; Wopereis, M. Water movement and soil swelling in a dry, cracked vertisol. Geoderma 1997, 78, 113–123. [Google Scholar] [CrossRef]
- Phillips, I.R.; Greenway, M. Changes in water soluble and exchangeable ions, cation exchange capacity, and phosphorusmax in soils under alternating waterlogged and drying conditions. Commun. Soil Sci. Plant Anal. 1998, 29, 51–65. [Google Scholar] [CrossRef]
- Narteh, L.T.; Sahrawat, K.L. Influence of flooding on electrochemical and chemical properties of West African soils. Geoderma 1999, 87, 179–207. [Google Scholar] [CrossRef]
- Favre, F.; Tessier, D.; Abdelmoula, M.; Génin, J.M.; Gates, W.P.; Boivin, P. Iron reduction and changes in cation exchange capacity in intermittently waterlogged soil. Eur. J. Soil Sci. 2002, 53, 175–183. [Google Scholar] [CrossRef]
- Lu, S.G.; Tang, C.; Rengel, Z. Combined effects of waterlogging and salinity on electrochemistry, water-soluble cations and water dispersible clay in soils with various salinity levels. Plant Soil 2004, 264, 231–245. [Google Scholar] [CrossRef]
- Boivin, P.; Saejiew, A.; Grunberger, O.; Arunin, S. Formation of soils with contrasting textures by translocation of clays rather ferrolysis in flooded rice fields in Northeast Thailand. Eur. J. Soil Sci. 2004, 55, 713–724. [Google Scholar] [CrossRef]
- Inao, K.; Kitamura, Y. Pesticide paddy field model (PADDY) for predicting pesticide concentrations in water and soil in paddy fields. Pest Sci. 1999, 55, 38–46. [Google Scholar] [CrossRef]
- Singh, K.B.; Gajri, P.R.; Arora, V.K. Modelling the effects of soil and water management practices on the water balance and performance of rice. Agric. Water Manag. 2001, 49, 77–95. [Google Scholar] [CrossRef]
- Liu, C.W.; Chen, S.K.; Jang, C.S. Modelling water infiltration in cracked paddy field soil. Hydrol. Process 2004, 18, 2503–2513. [Google Scholar] [CrossRef]
- Chung, S.O.; Kim, H.S.; Kim, J.S. Model development for nutrient loading from paddy rice fields. Agric. Water Manag. 2003, 62, 1–17. [Google Scholar] [CrossRef]
- Miao, Z.; Cheplick, M.J.; Williams, M.W.; Trevisan, M.; Padovani, L.; Gennari, M.; Ferrero, A.; Vidotto, F.; Capri, E. Simulating pesticide leaching and runoff in rice paddies with the RICEWQ-VADOFT model. J. Environ. Qual. 2003, 32, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, V.M.; Rao, N.H.; Sarma, P.B.S. A coupled soil water and nitrogen balance model for flooded rice fields in India. Agrc. Ecosyst. Environ. 2004, 103, 425–441. [Google Scholar] [CrossRef]
- Watanabe, H.; Takagi, K.; Vu, S.H. Simulation of mefenacet concentrations in paddy field by improved PCPF-1 model. Pest Manag. Sci. 2005, 62, 20–29. [Google Scholar] [CrossRef]
- Tournebize, J.; Watanabe, H.; Takagi, K.; Nishimura, T. The development of a coupled model (PCPF-SWMS) to simulate water flow and pollutant transport in Japanese paddy fields. Paddy Water Environ. 2006, 4, 39–51. [Google Scholar] [CrossRef]
- Jeon, J.H.; Yoon, C.G.; Ham, J.H.; Jung, K.W. Model development for surface drainage loading estimates from paddy rice fields. Paddy Water Environ. 2005, 3, 93–101. [Google Scholar] [CrossRef]
- Antonopoulos, V.Z. Modelling of water and nitrogen balance in the ponded water of rice fields. Paddy Water Environ. 2008, 6, 387–395. [Google Scholar] [CrossRef]
- Antonopoulos, V.Z. Modelling of water and nitrogen balances in the ponded water and soil profile of rice fields in Northern Greece. Agric. Water Manag. 2010, 98, 321–330. [Google Scholar] [CrossRef]
- Aschonitis, V.G.; Antonopoulos, V.Z. Evaluation of the water balance and the soil and ponding water temperature in paddy-rice fields with the modified GLEAMS model. In Proceedings of the Agricultural Engineering Conference, Crete, Greece, 23–25 June 2008.
- Aschonitis, V.G.; Lekakis, E.H.; Petridou, Ν.C.; Koukouli, S.G.; Pavlatou-Ve, A. Nutrients fixation by Algae and limiting factors of algal growth in flooded rice fields under semi-arid Mediterranean conditions—Case study in Thessaloniki Plain in Greece. Nutr. Cycl. Agroecosys. 2013, 96, 1–13. [Google Scholar] [CrossRef]
- Phogat, V.; Yadav, A.K.; Malik, R.S.; Kumar, S.; Cox, J. Simulation of salt and water movement and estimation of water productivity of rice crop irrigated with saline water. Paddy Water Environ. 2010, 8, 333–346. [Google Scholar] [CrossRef]
- Pochai, N.; Pongnoo, N. A numerical treatment of a mathematical model of ground water flow in rice field near marine shrimp aquaculture farm. Proc. Eng. 2012, 32, 1191–1197. [Google Scholar] [CrossRef]
- Singh, A.; Panda, S.N. Integrated salt and water balance modeling for the management of waterlogging and salinization. II: Application of SAHYSMOD. J. Irrig. Drain. Eng. ASCE 2012, 138, 964–971. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Yao, R.; Yu, S. Irrigation regime and salt dynamics for rice with brackish water irrigation in coastal region of North Jiangsu Province. Trans. Chin. Soc. Agric. Eng. 2014, 30, 54–63. [Google Scholar]
- N.AG.RE.F. (National Agriculture Research Foundation-Institute of Soil Science). Soil Map of Thessaloniki Region-Area of Gallikos and Axios Rivers; Institute of Soil Science: Thessaloniki, Greece, 2003; pp. 137–138. [Google Scholar]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; US Department of Agriculture-Soil Conservation Service: Washington, DC, USA, 1975. [Google Scholar]
- Litskas, V.D.; Aschonitis, V.G.; Antonopoulos, V.Z. Water quality in irrigation and drainage networks of Thessaloniki plain in Greece related to land use, water management, and agroecosystem protection. Environ. Monit. Assess. 2010, 163, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Aschonitis, V.G. Modeling of Evapotranspiration, Physical Soil Properties and Water, Nitrogen and Phosphorus Balance in Flooded Rice Fields. Ph.D. Thesis, Aristotle University, School of Agriculture, Thessaloniki, Greece, 2012. [Google Scholar]
- Aschonitis, V.G.; Papamichail, D.M.; Lithourgidis, A.; Fano, E.A. Estimation of leaf area index and foliage area index of rice using an indirect gravimetric method. Commun. Soil Sci. Plant Anal. 2014, 45, 1726–1740. [Google Scholar] [CrossRef]
- Allen, R.G.; Walter, I.A.; Elliott, R.; Howell, T.; Itenfisu, D.; Jensen, M.; Snyder, R.L. The ASCE Standardized Reference Evapotranspiration Equation; Final Report (ASCE-EWRI); ASCE: Columbus, OH, USA, 2005. [Google Scholar]
- Tuller, M.; Islam, M.R. Field methods for monitoring solute transport. In Soil-Water-Solute Process Characterization, an Integrated Approach; Benedí, A., Muñoz-Carpena, J.R., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2005; pp. 309–355. [Google Scholar]
- McDonald, J.D.; Bélanger, N.; Sauvé, S.; Courchesne, F.; Hendershot, W.H. Collection and characterization of soil solutions. In Soil Sampling and Methods of Analysis, 2nd ed.; Canadian Society of Soil Science, Carter, M.R., Gregorich, E.G., Eds.; CRC Press LLC, Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 179–196. [Google Scholar]
- Tacker, P.; Vories, E.; Wilson, C., Jr.; Slaton, N. Water management. In Rice Production Handbook; Miscellaneous Publication 192; Slaton, N.A., Ed.; Cooperative Extension Service, University of Arkansas: Little Rock, AR, USA, 2001; pp. 75–86. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Irrigation and Drainage Paper 29; FAO, U.N.: Rome, Italy, 1994. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Allison, L.E.; Moodie, C.D. Carbonates. In Methods of Soil Analysis. Part 2; Black, C.A., Ed.; Agronomy Monograph; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1379–1400. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Eds.; APHA, AWWA, WEF: Washington, DC, USA, 2005. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis. Part 3. Chemical Methods; Soil Science Society of America Book Series 5.3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI., USA, 1996; pp. 417–436. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; University of California Division of Agricultural Science: Riverside, CA, USA, 1961. [Google Scholar]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis. Part 2, Chemical and Microbiological Properties, 2nd ed.; Klute, A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Dane, J.H.; Hopmans, J.W. Water retention and storage. In Methods of Soil Analysis. Part 1. Physical Methods, 3rd ed.; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 671–720. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Van Genuchten, M.T.; Leij, F.J.; Yates, S.R.M. The RETC Code for Quantifying the Hydraulic Functions of Unsaturated Soils; USDA, Agricultural Research Service: Riverside, CA, USA, 1991. [Google Scholar]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-induced pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Jones, C.; Brown, B.D.; Engel, R.; Horneck, D.; Orson-Rutz, K. Factors Affecting Nitrogen Fertilizer Volatilization; USDA, Montana State University and Montana State University Extension Bulletin: Bozeman, MT, USA, 2013; p. 8. [Google Scholar]
- Mahrous, F.N.; Mikkelsen, D.S.; Hafez, A.A. Effect of soil salinity on the electro-chemical and chemical kinetics of some plant nutrients in submerged soils. Plant Soil 1983, 75, 455–472. [Google Scholar] [CrossRef]
- Larson, K.D.; Graetz, D.A.; Schaffer, B. Flood-induced chemical transformations in calcareous agricultural soils of South Florida. Soil Sci. 1991, 152, 33–40. [Google Scholar] [CrossRef]
- Chorom, M.; Rengasamy, P. Carbonate chemistry, pH, and physical properties of an alkaline sodic soil as affected by various amendments. Austral. J. Soil Res. 1997, 35, 149–162. [Google Scholar] [CrossRef]
- Armstrong, W. Aeration in higher plants. Adv. Botan. Res. 1979, 7, 225–232. [Google Scholar]
- Begg, C.B.M.; Kirk, G.J.D.; MacKenzie, A.F.; Neue, H.U. Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytol. 1994, 128, 469–477. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; van Du, L.E. Changes in rice root architecture, porosity, and oxygen and proton release under phosphorus deficiency. New Phytol. 1997, 135, 191–200. [Google Scholar] [CrossRef]
- Kirk, G. The Biogeochemistry of Submerged Soils; John Wiley and Sons Ltd.: Chichester, West Sussex, UK, 2004; p. 297. [Google Scholar]
- Hinsinger, P. Plant-induced changes in soil processes and properties. In Soil Conditions and Plant Growth; Gregory, P.J., Nortcliff, S., Eds.; John Wiley and Sons Ltd.: Chichester, West Sussex, UK, 2013; pp. 323–365. [Google Scholar]
- Fillery, I.R.P.; Simpson, J.R.; de Datta, S.K. Influence of field environment and fertilizer management on ammonia loss from flooded rice. Soil Sci. Soc. Am. J. 1984, 48, 914–920. [Google Scholar] [CrossRef]
- De Datta, S.K. Principles and Practices of Rice Production; John Wiley and Sons, Inc.: Singapore, Singapore, 1981; p. 640. [Google Scholar]
- Abrol, I.P.; Yadav, J.S.P.; Massoud, F.I. Salt-Affected Soils and Their Management; FAO Soils Bulletin: Rome, Italy, 1988. [Google Scholar]
- Chen, Y.; Zhang, G.; Xu, Y.J.; Huang, Z. Influence of irrigation water discharge frequency on soil salt removal and rice yield in a semi-arid and saline-sodic area. Water 2013, 5, 578–592. [Google Scholar] [CrossRef]
- Ntanos, D.A. Strategies for rice production and research in Greece. Cah. Opt. Méditerr. 2001, 50, 115–122. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekakis, E.; Aschonitis, V.; Pavlatou-Ve, A.; Papadopoulos, A.; Antonopoulos, V. Analysis of Temporal Variation of Soil Salinity during the Growing Season in a Flooded Rice Field of Thessaloniki Plain-Greece. Agronomy 2015, 5, 35-54. https://doi.org/10.3390/agronomy5010035
Lekakis E, Aschonitis V, Pavlatou-Ve A, Papadopoulos A, Antonopoulos V. Analysis of Temporal Variation of Soil Salinity during the Growing Season in a Flooded Rice Field of Thessaloniki Plain-Greece. Agronomy. 2015; 5(1):35-54. https://doi.org/10.3390/agronomy5010035
Chicago/Turabian StyleLekakis, Emanuel, Vassilis Aschonitis, Athina Pavlatou-Ve, Aristotelis Papadopoulos, and Vassilis Antonopoulos. 2015. "Analysis of Temporal Variation of Soil Salinity during the Growing Season in a Flooded Rice Field of Thessaloniki Plain-Greece" Agronomy 5, no. 1: 35-54. https://doi.org/10.3390/agronomy5010035






